Microwave-Assisted Isolation of Essential oil of Cinnamomum iners Reinw. ex Bl.: Comparison with Conventional Hydrodistillation
Abstract
:Introduction
Results and Discussion
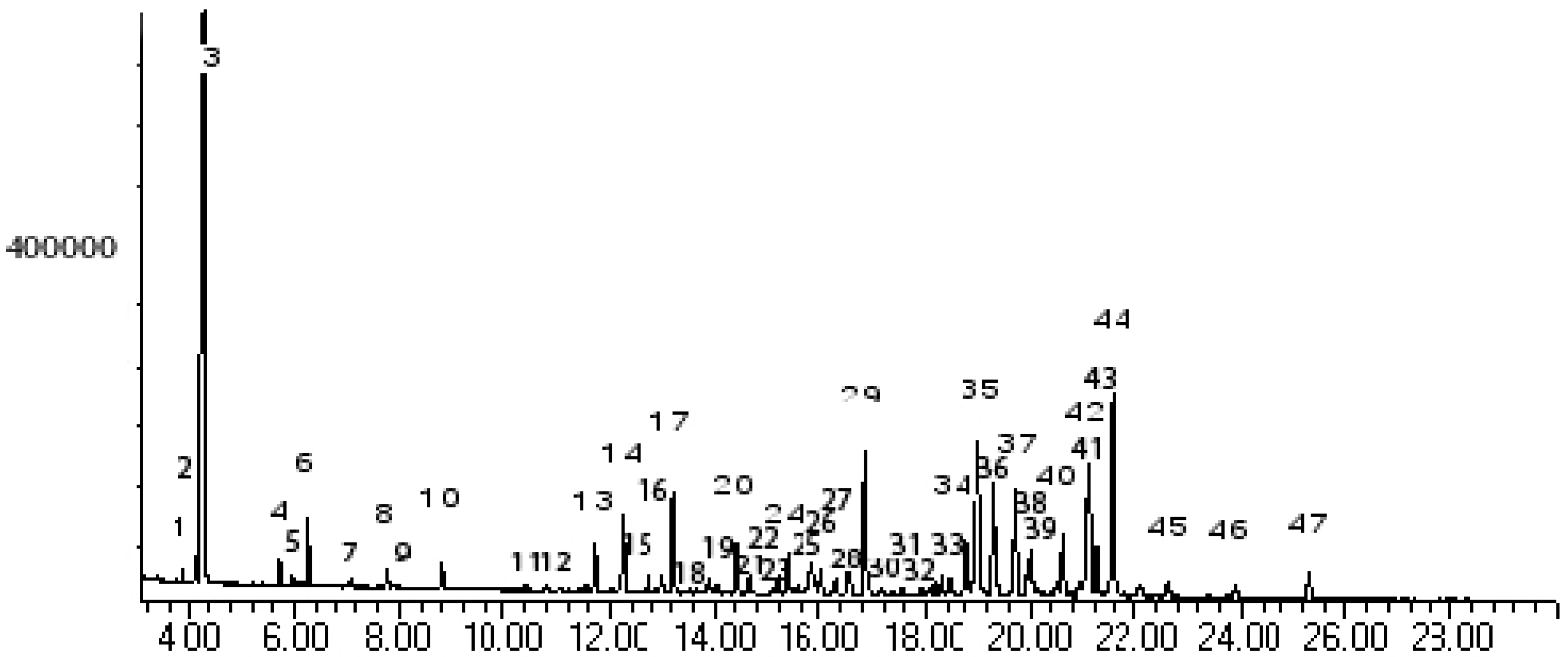
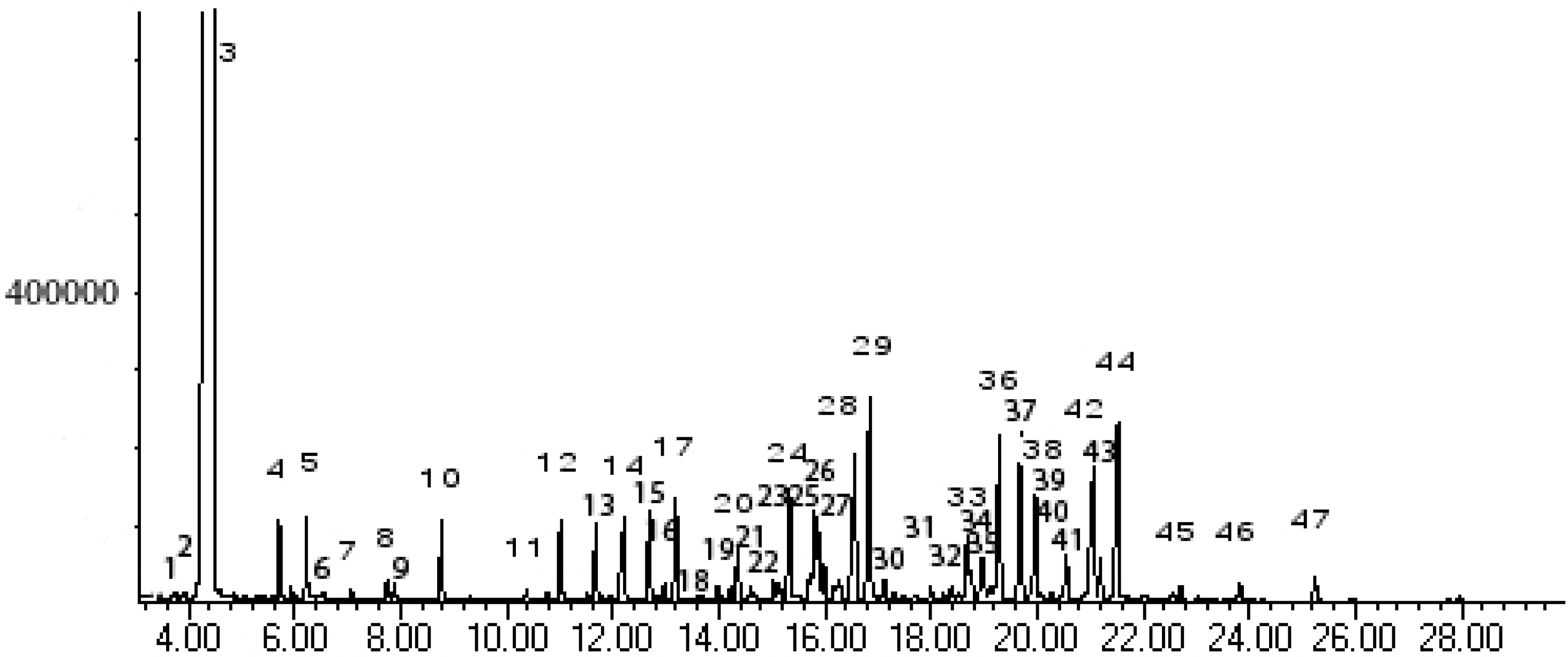
| Peak No. | Kovát Retention Index | Compounds | % Area | |
|---|---|---|---|---|
| Hydrodistillation | Microwave-assisted | |||
| 1 | - | cis-linalool oxide | 0.22 | - |
| 2 | - | trans-linaool oxide | 0.53 | - |
| 3 | 1103 | linalool | 36.90 | 55.27 |
| 4 | 1176 | borneol L | 0.52 | 1.02 |
| 5 | 1186 | terpinen-4-ol | 0.19 | 0.14 |
| 6 | 1198 | alpha-terpineol | 1.50 | 0.91 |
| 7 | 1232 | 2,6-octadien-1-ol | - | 0.12 |
| 8 | 1258 | geraniol | 0.37 | 0.25 |
| 9 | 1261 | propanoic acid | - | 0.17 |
| 10 | 1291 | (-)-bornyl acetate | 0.60 | 1.08 |
| 11 | 1343 | cyclohexene | - | 0.17 |
| 12 | 1362 | 3-allyl-6-methoxyphenol | - | 1.29 |
| 13 | 1382 | alpha-copaene | 1.32 | 1.19 |
| 14 | 1396 | beta-elemene | 2.32 | 1.82 |
| 15 | 1410 | dodecanal | 0.82 | 1.51 |
| 16 | 1420 | cis-alpha-bergamotene | 0.46 | 0.30 |
| 17 | 1427 | caryophyllene | 2.94 | 1.69 |
| 18 | 1446 | (+)-aromadendrene | 0.45 | - |
| 19 | 1448 | 2-propen-1-ol | - | 0.22 |
| 20 | 1460 | alpha-humulene | 1.63 | 0.97 |
| 21 | 1465 | aromadendrene | - | 0.12 |
| 22 | 1466 | alloaromadendrene | 0.52 | - |
| 23 | 1481 | alpha-amorphene | 0.61 | - |
| 24 | 1485 | germacrene-D | 1.38 | 1.87 |
| 25 | 1490 | beta-selinene | 0.34 | - |
| 26 | 1503 | alpha-muurolene | 0.75 | 0.60 |
| 27 | 1511 | beta-bisabolene | 0.58 | 0.53 |
| 28 | 1519 | alpha-cubebene | 1.22 | 3.30 |
| 29 | 1527 | delta-cadinene | 4.53 | 3.88 |
| 30 | 1564 | epiglobulol | 0.38 | - |
| 31 | 1566 | nerolidol | 0.53 | - |
| 32 | 1571 | palustrol | 0.67 | - |
| 33 | 1577 | unidentified | - | 1.26 |
| 34 | 1581 | spathulenol | 2.04 | 0.46 |
| 35 | 1585 | caryophyllene oxide | 6.85 | 0.95 |
| 36 | 1594 | viridiflorol | 4.01 | 3.36 |
| 37 | 1608 | unidentified | 3.63 | 3.26 |
| 38 | 1617 | tetradecanal | 1.38 | 2.09 |
| 39 | 1639 | calarene | 0.43 | - |
| 40 | 1643 | naphthalene, 1,2,3,4,4a,7-hexahydro | 2.18 | 0.93 |
| 41 | 1656 | isospathulenol | 0.50 | - |
| 42 | 1662 | alpha-cadinol | 6.10 | 3.78 |
| 43 | 1667 | alpha-copaene | 1.71 | 0.72 |
| 44 | 1679 | cadinol | 7.03 | 3.70 |
| 45 | 1712 | alpha-longipinene | 0.58 | 0.19 |
| 46 | 1742 | unidentified | 0.48 | 0.38 |
| 47 | 1774 | benzyl benzoate | 0.80 | 0.51 |
| DPPH method IC50 (μg/mL) | ABTS method TEAC (mM/mg) | ||
| Hydrodistillation | Microwave-assisted | Hydrodistillation | Microwave-assisted |
| 218.88 | 216.66 | 0.176 | 0.175 |
| FRAP method EC1 (μM) | Lipid peroxidation method AA (%) | ||
| Hydrodistillation | Microwave-assisted | Hydrodistillation | Microwave-assisted |
| 387.77 | 387.41 | 13.22 | 13.28 |
Conclusions
Experimental Section
Materials
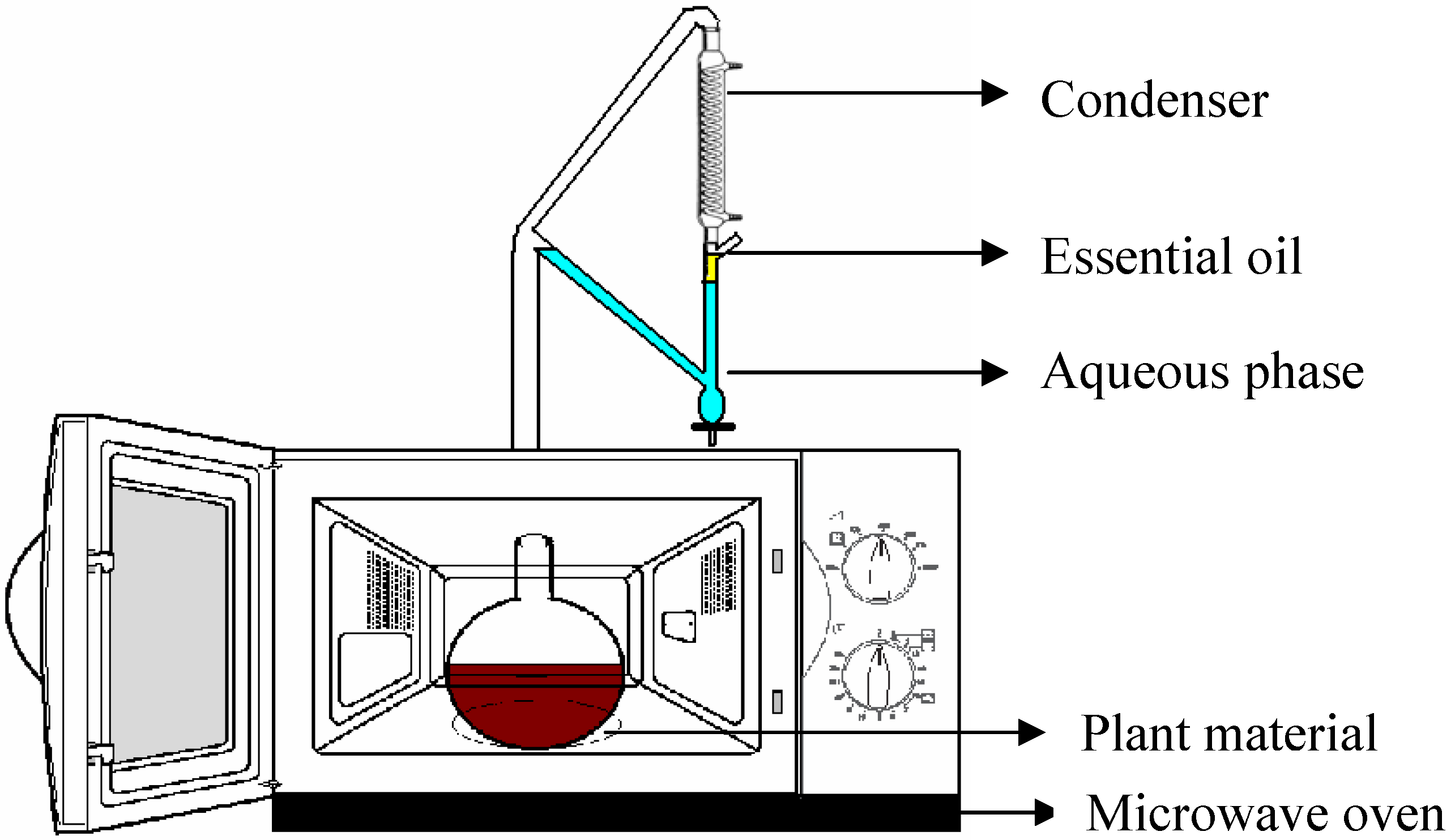
Isolation and Analysis of the Essential Oil
Antioxidant activity: DPPH method [26]
ABTS Method [27]
Ferric Reducing Antioxidant Power (FRAP) [28]
Lipid Peroxidation (β-Carotene Bleaching Method) [29]

Acknowledgments
References and Notes
- Phutdhawong, W.; Buddhasukh, D.; Pyne, S.; Rujiwatra, A.; Pakawatchai, C. Microwave-assisted facile synthesis and crystal structure of cis-9,10,11,15-tetrahydro-9,10[3',4']-furanoanthracene-12,14-dione. Synth. Comm. 2006, 36, 881–883. [Google Scholar] [CrossRef]
- Phutdhawong, W.; Buddhasukh, D. Facile microwave-assisted synthesis of 9,10-dihydro-9,10-ethanoanthracene-11-carboxylic acid methyl ester. Molecules 2005, 10, 1409–1412. [Google Scholar] [CrossRef]
- Bougrin, K.; Loupy, A.; Soufiaoui, M. Microwave-assisted solvent-free heterocyclic synthesis. J. Photochem. Photobiol. C: Photochem. Rev. 2005, 6, 139–167. [Google Scholar] [CrossRef]
- Venkatesh, M. S.; Raghavan, G. S. V. An Overview of Microwave Processing and Dielectric Properties of Agri-food Materials. Biosyst. Eng. 2004, 88, 1–18. [Google Scholar] [CrossRef]
- Wang, L.; Sun, D.-W. Recent developments in numerical modelling of heating and cooling processes in the food industry-a review. Trends Food Sci. Tech. 2003, 14, 408–423. [Google Scholar] [CrossRef]
- Li, B.; Sun, D.-W. Novel methods for rapid freezing and thawing of foods - a review. J. Food Eng. 2002, 54, 175–182. [Google Scholar]
- Lidstrom, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis-a review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Mujumdar, A. S.; Wang, S. Trends in microwave-related drying of fruits and vegetables. Trends Food Sci. Tech. 2006, 17, 524–534. [Google Scholar] [CrossRef]
- Lucchesi, M. E.; Smadja, J.; Bradshaw, S.; Louw, W.; Chemat, F. Solvent free microwave extraction of Elletaria cardamomum L.: A multivariate study of a new technique for the extraction of essential oil. J. Food Eng. 2007, 79, 1079–1086. [Google Scholar] [CrossRef]
- Ferhat, M. A.; Meklati, B. Y.; Smadja, J.; Chemat, F. An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. J. Chromatogr. A 2006, 1112, 121–126. [Google Scholar] [CrossRef]
- Deng, C.; Xu, X.; Yao, N.; Li, N.; Zhang, X. Rapid determination of essential oil compounds in Artemisia Selengensis Turcz by gas chromatography-mass spectrometry with microwave distillation and simultaneous solid-phase microextraction. Anal. Chim. Acta 2006, 556, 289–294. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, L.; Li, T.; Zhou, X.; Wang, L.; Zhang, H.; Liu, L.; Li, Y.; Liu, Z.; Wang, H.; Zeng, H.; He, H. Improved solvent-free microwave extraction of essential oil from dried Cuminum cyminum L. and Zanthoxylum bungeanum Maxim. J. Chromatogr. A 2006, 1102, 11–17. [Google Scholar] [CrossRef]
- Chemat, F.; Lucchesi, M. E.; Smadja, J.; Favretto, L.; Colnaghi, G.; Visinoni, F. Microwave accelerated steam distillation of essential oil from lavender: A rapid, clean and environmentally friendly approach. Anal. Chim. Acta 2006, 555, 157–160. [Google Scholar] [CrossRef]
- Tigrine-Kordjani, N.; Meklati, B. Y.; Chemat, F. Microwave 'dry' distillation as a useful tool for extraction of edible essential oils. Int. J. Aromather. 2006, 16, 141–147. [Google Scholar] [CrossRef]
- Lucchesi, M. E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: comparison with conventional hydro-distillation. J. Chromatogr A 2004, 1043, 323–327. [Google Scholar]
- Wannissorn, B.; Jarikasem, S.; Siriwangchai, T.; Thubthimthed, S. Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia 2005, 76, 233–236. [Google Scholar] [CrossRef]
- Pormjit, S. Pharmacognosy; R. D. P. Press: BKK, 1989; pp. 86–87. [Google Scholar]
- Mastura, M.; Azah, M. A. N.; Khozirah, S.; Mawardi, R.; Manaf, A. A. Anticandidal and antidermatophytic activity of Cinnamomum species essential oils. Cytobios 1999, 98, 17–23. [Google Scholar]
- Iida, N.; Ishii, R.; Hakamata, J.; Myamoto, S.; Oozeki, H. Amylase inhibitors for food and pharmaceutical. Jpn. Kokai Tokkyo Koho. Patent No. JP 09040572, 1997. [Google Scholar]
- Bin Jantan, I.; Goh, S. H. Essential oils of Cinnamomum species from Peninsular Malaysia. J. Essent. Oil Res. 1992, 4, 161–171. [Google Scholar] [CrossRef]
- Baruah, A.; Nath, S. C.; Hazarika, A. K. Stem bark oil of Cinnamomum iners Reinw. Indian Perfumer 2001, 45, 261–263. [Google Scholar]
- Adams, R. P. Identification of Essential Oil Components by GC/MS; Allured Publishing Corporation: Carol Stream, ILL, USA, 1995. [Google Scholar]
- Davies, N. W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Pelle, L.; Jason, T.; Bernard, W.; Jacob, W. Microwave Assisted Organic Synthesis_a Review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Bryliakov, K. P.; Talsi, E. P.; Stas’ko, S. N.; Kholdeeva, O. A.; Popov, S. A.; Tkachev, A. V. Stereoselective oxidation of linalool with tert-butyl hydroperoxide, catalyzed by a vanadium(V) complex with a chiral terpenoid ligand. J. Mol. Catal. A: Chem. 2003, 194, 79–88. [Google Scholar] [CrossRef]
- Phutdhawong, W.; Donchai, A.; Korth, J.; Pyne, S. G.; Picha, P.; Ngamkham, J.; Buddhasukh, D. The components and anticancer activity of the volatile oil from Streblus asper. Flav. Frag. J. 2004, 19, 445–447. [Google Scholar] [CrossRef]
- Roberta, R.; Nicoletta, P.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical catin decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I. F. F.; Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Jiang, P.; Burczynski, F.; Campbell, C.; Pierce, G.; Austria, J. A.; Briggs, C. J. Rutin and flavonoid contents in three buckwheat species Fagopyrum esculentum, F. tataricum, and F. homotropicum and their protective effects against lipid peroxidation. Food Res. Int. 2007, 40, 356–364. [Google Scholar] [CrossRef]
- Miller, N. J. A simplified method for the evaluation of antioxidants. J. Am. Oil. Chem. Soc. 1971, 48, 91. [Google Scholar] [CrossRef]
- Sample Availability: Samples of (–)-linalool are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Phutdhawong, W.; Kawaree, R.; Sanjaiya, S.; Sengpracha, W.; Buddhasukh, D. Microwave-Assisted Isolation of Essential oil of Cinnamomum iners Reinw. ex Bl.: Comparison with Conventional Hydrodistillation. Molecules 2007, 12, 868-877. https://doi.org/10.3390/12040868
Phutdhawong W, Kawaree R, Sanjaiya S, Sengpracha W, Buddhasukh D. Microwave-Assisted Isolation of Essential oil of Cinnamomum iners Reinw. ex Bl.: Comparison with Conventional Hydrodistillation. Molecules. 2007; 12(4):868-877. https://doi.org/10.3390/12040868
Chicago/Turabian StylePhutdhawong, Weerachai, Rungthip Kawaree, Samart Sanjaiya, Waya Sengpracha, and Duang Buddhasukh. 2007. "Microwave-Assisted Isolation of Essential oil of Cinnamomum iners Reinw. ex Bl.: Comparison with Conventional Hydrodistillation" Molecules 12, no. 4: 868-877. https://doi.org/10.3390/12040868




