Cancer Preventive Mechanismsof the Green Tea Polyphenol (-)-Epigallocatechin-3-gallate
Abstract
:1. Introduction
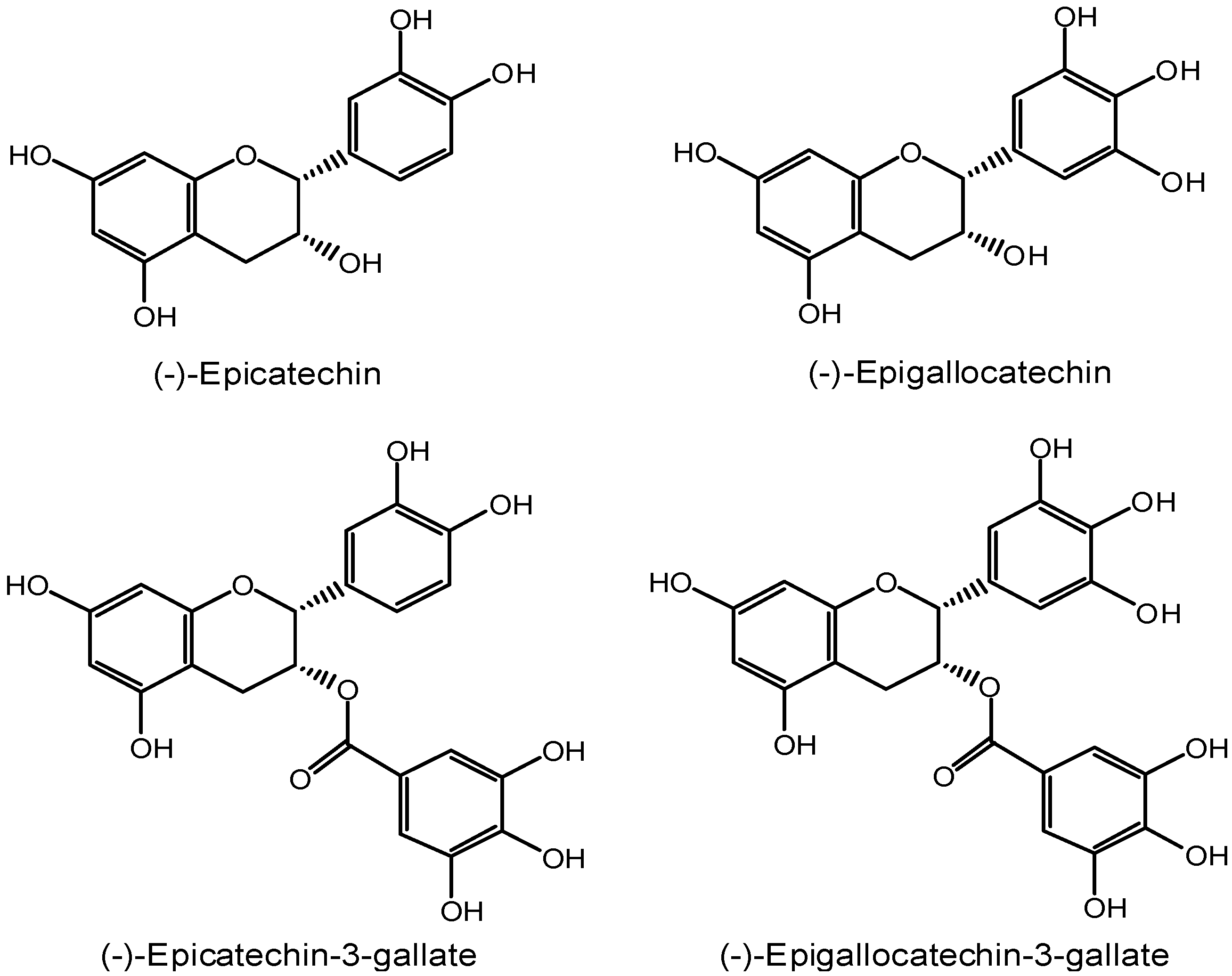
2. Inhibition of the cancer initiation stage by EGCG
3. Inhibition of the cancer promotion stage by EGCG
3a. Interference with intracellular signaling pathway (Figure 2).

3b. Cell cycle modulation
4. Inhibition of the cancer progression stage by EGCG
5. Conclusions
Acknowledgments
References and Notes
- Fujiki, H.; Suganuma, M.; Imai, K.; Nakachi, K. Green tea: Cancer preventive beverage and/or drug. Cancer Lett. 2002, 188, 9–13. [Google Scholar] [CrossRef]
- Imai, K.; Suga, K.; Nakachi, K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev. Med. 1997, 26, 769–775. [Google Scholar] [CrossRef]
- Inoue, M.; Tajima, K.; Hirose, K.; Hamajima, N.; Takezake, T.; Kuroishi, T.; Tominaga, S. Tea and coffee consumption and risk of digestive tract cancers: data from a comparative case-referent study in Japan. Cancer Causes Control 1998, 9, 209–216. [Google Scholar]
- Ji, B. T.; Chow, W. H.; Hsing, A. W.; McLaughlin, J. K.; Dai, Q.; Gao, Y. T.; Blot, W. J.; Fraumeni, J. F. Green tea consumption and the risk of pancreatic and colorectal cancers. Int. J. Cancer 1999, 70, 255–258. [Google Scholar]
- Nakachi, K.; Suemasu, K.; Suga, T.; Takeo, K.; Imai, K.; Higashi, Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn. J. Cancer Res. 1998, 89, 254–261. [Google Scholar] [CrossRef]
- Arts, I. C.; Jacobs, D. R.; Gross, M.; Harnack, L. J.; Folsom, A. R. Dietary catechins and cancer incidence among postmenopausal women: the Iowa Women’s Health Study (United States). Cancer Cause Control 2002, 13, 373–382. [Google Scholar] [CrossRef]
- Mimoto, J.; Kiura, K.; Matsuo, K.; Yoshino, T.; Takata, I.; Ueoka, H.; Kataoka, M.; Harada, M. (-) -Epigallocatechin gallate can prevent cisplatin-induced lung tumorigenesis in A/J mice. Carcinogenesis 2000, 21, 915–919. [Google Scholar] [CrossRef]
- Chen, J. J.; Ye, Z. Q.; Koo, M. W. Growth inhibition and cell cycle arrest effects of epigallocatechin gallate in the NBT-II bladder tumour cell line. BJU Int. 2004, 93, 1082–1086. [Google Scholar] [CrossRef]
- Mantena, S. K.; Roy, A. M.; Katiyar, S. K. Epigallocatechin-3-gallate inhibits photocarcinogenesis through inhibition of angiogenic factors and activation of CD8+ T cells in tumors. Photochem. Photobiol. 2005, 81, 1174–1179. [Google Scholar] [CrossRef]
- Stuart, E. C.; Scandlyn, M. J.; Rosengren, R. J. Role of epigallocatechin gallate (EGCG) in the treatment of breast and prostate cancer. Life Sci. 2006, 79, 2329–2336. [Google Scholar]
- Thangapazham, R. L.; Singh, A. K.; Sharma, A.; Warren, J.; Gaddipati, J. P.; Maheshwari, R. K. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007, 245, 232–241. [Google Scholar] [CrossRef]
- Wang, Z. Y.; Das, M.; Bickers, D. R.; Mukhtar, H. Interaction of Epicatechins Derived from Green Tea with Hepatic CytochromP-450. Drug Metab. Dispos. 1988, 16, 98–103. [Google Scholar]
- Mukhtar, H.; Wang, Z. Y.; Katiqan, S. K.; Agarwal, R. Tea components: antimutagenic and antigagenic effects. Prev. Med. 1992, 21, 351–360. [Google Scholar] [CrossRef]
- Zhao, B. L.; Li, X. J.; He, R. G.; Cheng, S. J.; Xin, W. J. Scavenging effect of extracts of green tea and natural antioxidants on active oxygen radicals. Cell Biophys. 1989, 14, 175–185. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, B.; Li, M.; Shen, S.; Xin, W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim. Biophys. Acta. 1996, 1304, 210–222. [Google Scholar] [CrossRef]
- Zhang, G.; Miura, Y.; Yagasaki, K. Suppression of adhesion and invasion of hepatoma cells in culture by tea compounds through antioxidative activity. Cancer Lett. 2000, 159, 169–173. [Google Scholar] [CrossRef]
- Okabe, S.; Suganuma, M.; Hayashi, M.; Sueoka, E.; Komori, A.; Fujiki, H. Mechanisms of growth inhibition of human lung cancer cell line, PC-9, by tea polyphenols. Jpn. J. Cancer Res. 1997, 88, 639–643. [Google Scholar] [CrossRef]
- Katiyar, S. K.; Afaq, F.; Azizuddin, K.; Mukhtar, H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (-)-epigallocatechin-3-gallate. Toxicol. Appl. Pharmacol. 2001, 176, 110–117. [Google Scholar] [CrossRef]
- Maeda-Yamamoto, M.; Suzuki, N.; Sawai, Y.; Miyase, T.; Sano, M.; Hashimoto-Ohta, A.; Isemura, M. Association of suppression of extracellular signal-regulated kinase phosphorylation by epigallocatechin gallate with the reduction of matrix metalloproteinase activities in human fibrosarcoma HT1080 cells. J. Agric. Food Chem. 2003, 51, 1858–1863. [Google Scholar] [CrossRef]
- Karin, M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995, 270, 16483–16486. [Google Scholar] [CrossRef]
- Whitmarsh, A. J.; Davis, R. J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996, 74, 589–607. [Google Scholar] [CrossRef]
- Lin, A.; Karin, M. NF-kappaB in cancer: a marked target. Semin. Cancer Biol. 2003, 13, 107–114. [Google Scholar]
- Afaq, F.; Adhami, V. M.; Ahmad, N.; Mukhtar, H. Inhibition of ultraviolet B-mediated activation of nuclear factor-κB in normal human epidermal keratinocytes by green tea constituent (-)-epigallocatechin-3-gallate. Oncogene 2003, 22, 1035–1044. [Google Scholar] [CrossRef]
- Gupta, S.; Hastak, K.; Afaq, F.; Ahmad, N.; Mukhtar, H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor-κB and induction of apoptosis. Oncogene 2004, 23, 2507–2522. [Google Scholar] [CrossRef]
- Surh, Y. J.; Chun, K. S.; Cha, H. H. Molecular mechanisms underlying chemoprevention activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappaB activation. Mutat. Res. 2001, 480-481, 243–268. [Google Scholar] [CrossRef]
- Karin, M.; Liu, Z. G.; Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997, 9, 240–246. [Google Scholar] [CrossRef]
- Angel, P.; Karin, M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1991, 1072, 129–157. [Google Scholar]
- Dong, Z.; Ma, W.; Huang, C.; Yang, C. S. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (-)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997, 57, 4414–4419. [Google Scholar]
- Huang, C.; Ma, W. Y.; Hanenberger, D.; Cleary, M. P.; Bowden, G. T.; Dong, Z. Inhibition of ultraviolet B-induced activator protein-1 (AP-1) activity by aspirin in AP-1-luciferase transgenic mice. J. Biol. Chem. 1997, 272, 26325–26331. [Google Scholar]
- Shimizu, M.; Deguchi, A.; Lim, J. T.; Moriwaki, H.; Kopelovich, L.; Weinstein, I. B. (-)-Epigallocatechin gallate and polyphenol E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 2005, 11, 2735–2746. [Google Scholar]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar] [CrossRef]
- Jung, Y. D.; Kim, M. S.; Shin, B. A.; Chay, K. O.; Ahn, B. W.; Liu, W.; Bucana, C. D.; Gallick, G. E.; Ellis, L. M. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br. J. Cancer 2001, 84, 844–850. [Google Scholar] [CrossRef]
- Nihal, M.; Ahmad, N.; Mukhtar, H.; Wood, G. S. Anti-proliferative and proapoptotic effects of (-) -epigallocatechin-3-gallate on human melanoma: Possible implications for the chemoprevention of melanoma. Int. J. Cancer 2005, 11, 513–521. [Google Scholar]
- Kavanagh, K. T.; Hafer, L. J.; Kim, D. W.; Mann, K. K.; Sherr, D. H.; Rogers, A. E.; Sonenshein, G. E. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J. Cell Biochem. 2001, 82, 387–398. [Google Scholar] [CrossRef]
- Hastak, K.; Agarwal, M. K.; Mukhtar, H.; Agarwal, M. L. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB J. 2005, 19, 789–791. [Google Scholar]
- Berger, S. J.; Gupta, S.; Belfi, C. A.; Gosky, D. M.; Mukhtar, H. Green tea constituent (-)-epigallocatechin-3-gallate inhibits topoisomerase I activity in human colon carcinoma cells. Biochem. Biophys. Res. Commun. 2001, 288, 101–105. [Google Scholar] [CrossRef]
- Bodnar, A. G.; Ouellette, M.; Frolkis, M.; Holt, S. E.; Chiu, C. P.; Morin, G. B.; Harley, C. B.; Shay, J. W.; Lichtsteiner, S.; Wright, W. E. Extension of life-span by introduction of telomerase into normal human cells. Science (Wash. DC) 1998, 279, 349–352. [Google Scholar] [CrossRef]
- Yang, J.; Chang, E.; Cherry, A. M.; Bangs, C. D.; Oei, Y.; Bodnar, A.; Bronstein, A.; Chiu, C. P.; Herron, G. S. Human endothelial cell life extension by telomerase expression. J. Biol. Chem. 1999, 274, 26141–26148. [Google Scholar] [CrossRef]
- Naasani, I.; Oh-hashi, F.; Oh-hara, T.; Feng, W. Y.; Johnston, J.; Chan, K.; Tsuruo, T. Blocking telomerase by dietary polyphenols is a major mechanism for limiting the growth of human cancer cells in vitro and in vivo. Cancer Res. 2003, 63, 824–830. [Google Scholar]
- Naasani, I.; Seimiya, H.; Tsuruo, T. Telomerase inhibition, telomerase shortening, and senescence of cancer cells by tea catechin. Biochem. Biophys. Res. Comm. 1998, 249, 391–396. [Google Scholar] [CrossRef]
- Hwang, J. T.; Ha, J.; Park, In-Ja.; Lee, S. K.; Baik, H. W.; Kim, Y. M.; Park, O. J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007, 247, 115–121. [Google Scholar] [CrossRef]
- Nishikawa, T.; Nakajima, T.; Moriguchi, M.; Jo, M.; Sekoguchi, S.; Ishii, M.; Takashima, H.; Katagishi, T.; Kimura, H.; Minami, M.; Itoh, Y.; Kagawa, K.; Okanoue, T. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. J. Hepatol. 2006, 44, 1074–1082. [Google Scholar] [CrossRef]
- Lin, J.; Della-Fera, M. A.; Baile, C. A. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obes. Res. 2005, 13, 982–990. [Google Scholar] [CrossRef]
- Islam, S.; Islam, N.; Kermode, T.; Johnstone, B.; Mukhtar, H.; Moskowitz, R. W.; Goldberg, V. M.; Malemud, C. J.; Haqqi, T. M. Involvement of caspase-3 in epigallocatechin-3-gallate-mediated apoptosis of human chondrosarcoma cells. Biochem. Biophys. Res. Commun. 2000, 270, 793–797. [Google Scholar] [CrossRef]
- Qin, J.; Xie, L. P.; Zheng, X. Y.; Wang, Y. B.; Bai, Y.; Shen, H. F.; Li, L. C.; Dahiya, R. A component of green tea, (-)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochem. Biophys. Res. Commun. 2007, 354, 852–857. [Google Scholar] [CrossRef]
- Amin, A. R.; Thakur, V. S.; Paul, R. K.; Feng, G. S.; Qu, C. K.; Mukhtar, H.; Agarwal, M. L. SHP-2 tyrosine phosphatase inhibits p73-dependent apoptosis and expression of a subset of p53 target genes induced by EGCG. Proc. Natl. Acad. Sci. USA 2007, 104, 5419–5424. [Google Scholar]
- Manna, S.; Banerjee, S.; Mukherjee, S.; Das, S.; Panda, C. K. Epigallocatechin gallate induced apoptosis in Sarcoma180 cells in vivo: mediated by p53 pathway and inhibition in U1B, U4-U6 UsnRNAs expression. Apoptosis 2006, 11, 2267–2276. [Google Scholar] [CrossRef]
- Hsu, S.; Lewis, J. B.; Borke, J. L.; Singh, B.; Dickinson, D. P.; Caughman, G. B.; Athar, M.; Drake, L.; Aiken, A. C.; Huynh, C. T.; Das, B. R.; Osaki, T.; Schuster, G. S. Chemopreventive effects of green tea polyphenols correlate with reversible induction of p57 expression. Anticancer Res. 2001, 21, 3743–3848. [Google Scholar]
- Hsu, S.; Singh, B. B.; Lewis, J. B.; Borke, J. L.; Dickinson, D. P.; Drake, L.; Caughman, G. B.; Schuster, G. S. Chemoprevention of oral cancer by green tea. Gen. Dent. 2002, 50, 140–146. [Google Scholar]
- Liotta, L. A. Tumor invasion and metastases: role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986, 46, 1–7. [Google Scholar] [CrossRef]
- Conese, M.; Blasi, F. The urokinase/urokinase-receptor system and cancer invasion. Baillieres Clin. Haematol. 1995, 8, 365–389. [Google Scholar] [CrossRef]
- Jankun, J.; Keck, R. W.; Skrzypczak-Jankun, E.; Swiercz, R. Inhibitors of urokinase reduce size of prostate cancer xenografts in severe combined immunodeficient mice. Cancer Res. 1997, 57, 559–563. [Google Scholar]
- Jankun, J.; Selman, S. H.; Swiercz, R.; Skrzypczak-Jankun, E. Why drinking green tea could prevent cancer. Nature 1997, 387, 561. [Google Scholar] [CrossRef]
- Kim, M. H.; Jung, M. A.; Hwang, Y. S.; Jeong, M.; Kim, S. M.; Ahn, S. J.; Shin, B. A.; Ahn, B. W.; Jung, Y. D. Regulation of urokinase plasminogen activator by epigallocatechin-3-gallate in human fibrosarcoma cells. Eur. J. Pharmacol. 2004, 487, 1–6. [Google Scholar] [CrossRef]
- Nagase, H.; Woessner, J. F. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef]
- Foda, H. D.; Zucker, S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov. Today 2001, 6, 478–482. [Google Scholar] [CrossRef]
- Maeta, H.; Ohgi, S.; Terada, T. Protein expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinase 1 and 2 in papillary thyroid carcinomas. Virchows Arch. 2001, 438, 121–128. [Google Scholar] [CrossRef]
- Adhami, V. M.; Siddiqui, I. A.; Ahmad, N.; Gupta, S.; Mukhtar, H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004, 6, 8715–8722. [Google Scholar]
- Fassina, G.; Vene, R.; Morini, M. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin. Cancer Res. 2004, 10, 4865–4873. [Google Scholar] [CrossRef]
- Annabi, B.; Lachambre, M. P.; Bousquet-Gagnon, N.; Page, M.; Gingras, D.; Beliveau, R. Green tea polyphenol (-)-epigallocatechin-3-gallate inhibits MMP-2 secretion and MT1-MMP-driven migration in glioblastoma cells. Biochim. Biophys. Acta. 2002, 1542, 209–220. [Google Scholar] [CrossRef]
- Kuzuhara, T.; Sei, Y.; Yamaguchi, K.; Suganuma, M.; Fujiki, H. DNA and RNA as new binding targets of green tea catechins. J. Biol. Chem. 2006, 281, 17446–17456. [Google Scholar] [CrossRef] [Green Version]
- Sample Availability: Not applicable.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Chen, L.; Zhang, H.-Y. Cancer Preventive Mechanismsof the Green Tea Polyphenol (-)-Epigallocatechin-3-gallate. Molecules 2007, 12, 946-957. https://doi.org/10.3390/12050946
Chen L, Zhang H-Y. Cancer Preventive Mechanismsof the Green Tea Polyphenol (-)-Epigallocatechin-3-gallate. Molecules. 2007; 12(5):946-957. https://doi.org/10.3390/12050946
Chicago/Turabian StyleChen, Lei, and Hong-Yu Zhang. 2007. "Cancer Preventive Mechanismsof the Green Tea Polyphenol (-)-Epigallocatechin-3-gallate" Molecules 12, no. 5: 946-957. https://doi.org/10.3390/12050946



