Solar Ultraviolet-B Radiation Increases Phenolic Content and Ferric Reducing Antioxidant Power in Avena sativa
Abstract
:Introduction
Results
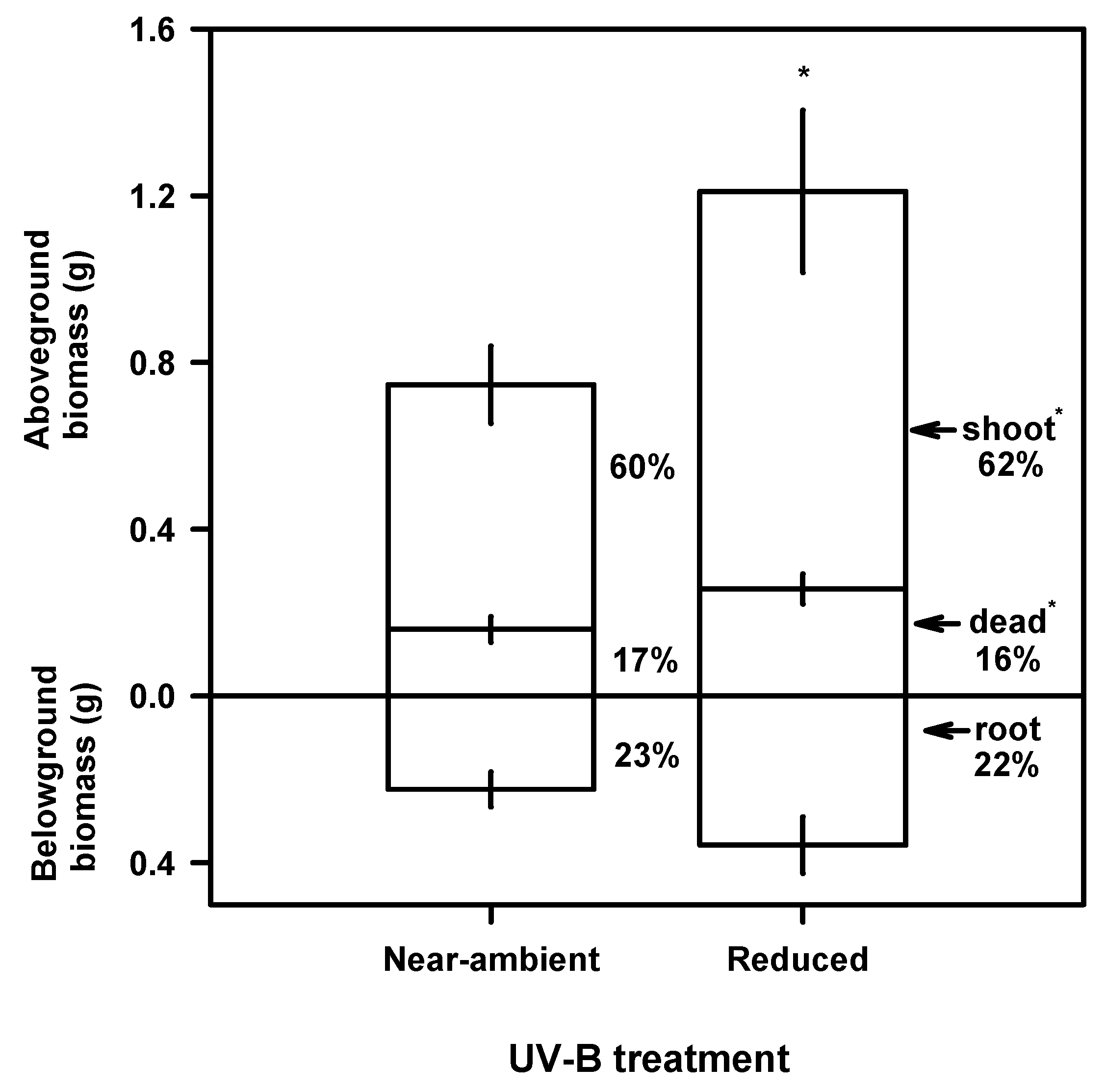
Growth and Biomass
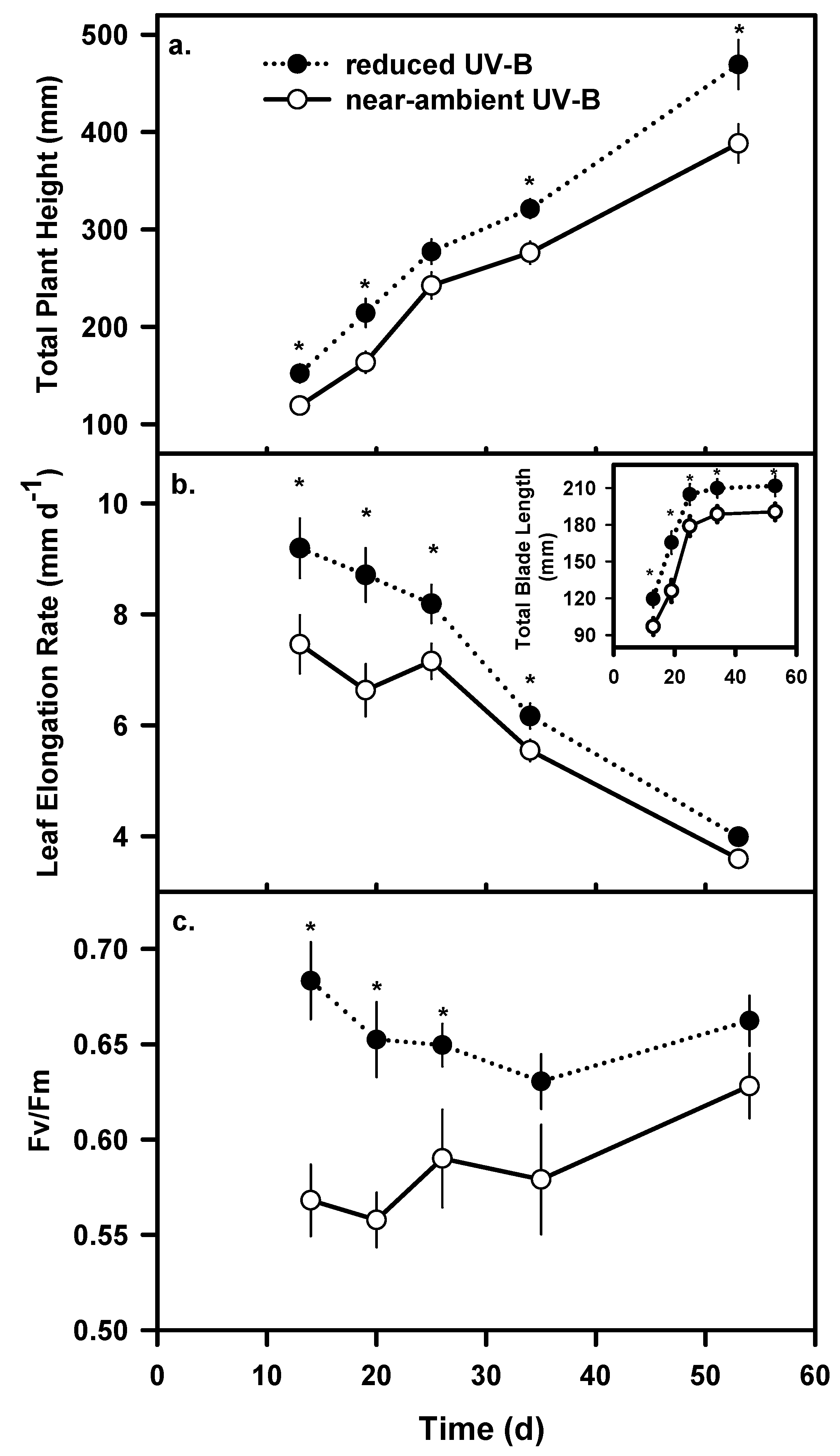
Chlorophyll fluorescence
Leaf Chlorophyll, Carotenoids and Bulk-soluble UV-B-absorbing Phenolics
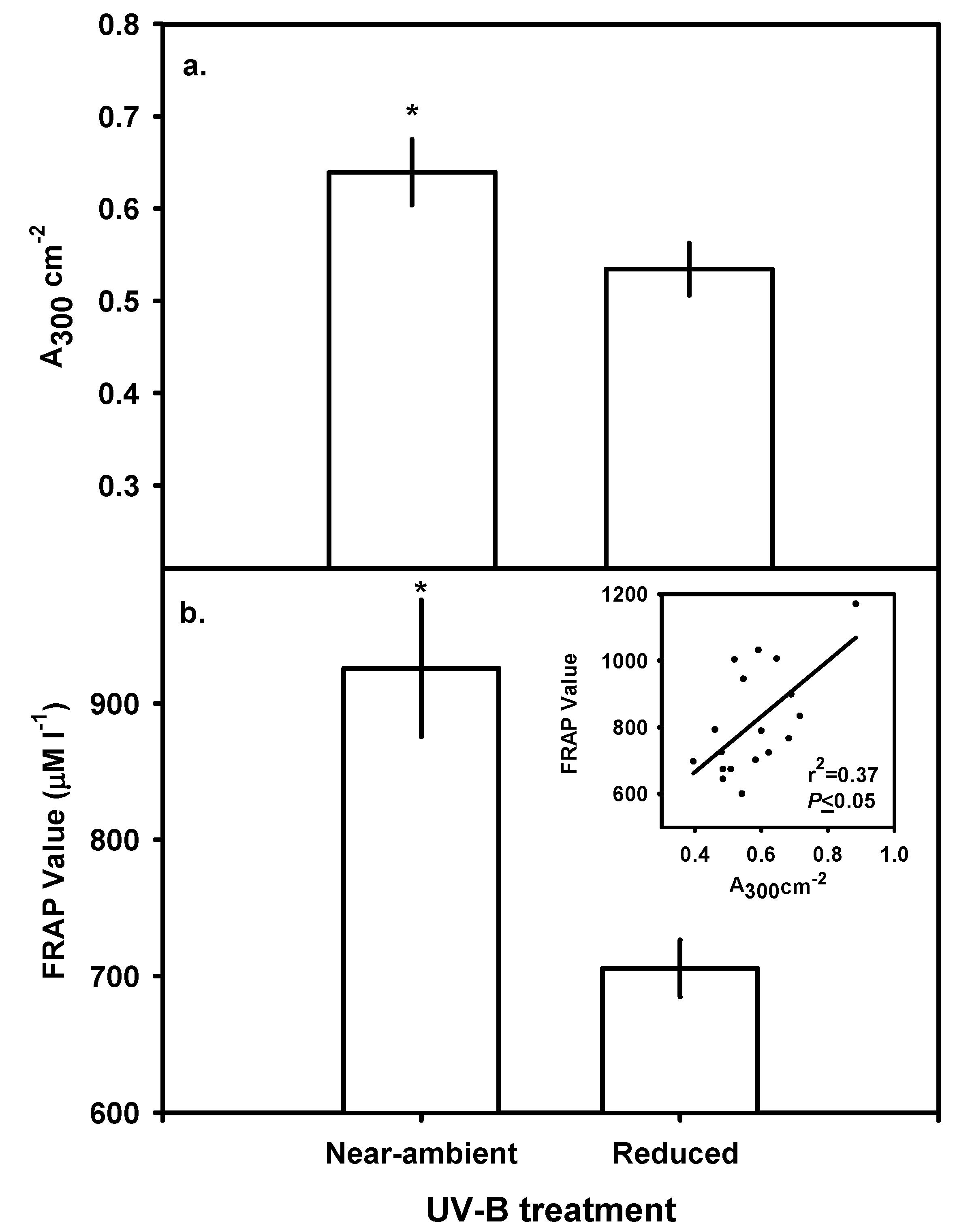
Ferric Reducing Antioxidant Power (FRAP) assay
Discussion
Experimental
Study Site and Field Manipulations
Growth and Biomass
Chlorophyll Fluorescence
Leaf Chlorophyll, Carotenoids and Bulk-soluble UV-B-absorbing Phenolics
Ferric Reducing Antioxidant Power (FRAP) assay
Acknowledgments
References and Notes
- Day, T.A.; Neale, P.J. Effects of UV-B radiation on terrestrial and aquatic primary producers. Ann. Rev. Ecol. Syst. 2002, 33, 371–396. [Google Scholar] [CrossRef]
- Mazza, C.A.; Battista, D.; Zima, A.M.; Szwarcberg-Bracchitta, M.; Giordano, C.V.; Acevedo, A.; Scopel, A.L.; Ballaré, C.L. The effects of solar ultraviolet-B radiation on the growth and yield of barely are accompanied by increased DNA damage and antioxidant response. Plant Cell Environ. 1999, 22, 61–70. [Google Scholar] [CrossRef]
- Furness, M.H.; Upadhyaya, M.K.; Ormond, D.P. Seedling growth and leaf surface morphological responses of three rangeland weeds to ultraviolet-B radiation. Weed Sci. 1999, 47, 427–434. [Google Scholar]
- Hunt, J.E.; McNeil, D.L. The influence of present-day levels of ultraviolet-B radiation on seedlings of two Southern Hemisphere temperate tree species. Plant Ecol. 1999, 143, 39–50. [Google Scholar] [CrossRef]
- Pfündel, E.E. Action of visible and UV radiation on chlorophyll fluorescence from dark adapted grape leaves (Vitis vinifera L.). Photosyn. Res. 2003, 75, 29–39. [Google Scholar] [CrossRef]
- Albert, K.R.; Mikkelsen, T.N.; Ro-Poulsen, H. Effect of ambient versus reduced UV-B radiation on high arctic Salix arctica assessed by measurements and calculations of chlorophyll a fluorescence parameters from fluorescence transients. Physiol. Plant. 2005, 124, 208–226. [Google Scholar] [CrossRef]
- Krizek, D.T.; Mirecki, R.M.; Britz, S.J. Inhibitory effects of ambient levels of solar UV-A and UV-B radiation on growth of cucumber. Physiol. Plant. 1997, 100, 189–198. [Google Scholar]
- Krizek, D.T.; Britz, S.J.; Mirecki, R.M. Inhibitory effects of ambient levels of solar UV-A and UV-B radiation on growth of cv. New Red Fire lettuce. Physiol. Plant. 1998, 103, 1–7. [Google Scholar]
- Xiong, F.S.; Day, T.A. Effect of solar ultraviolet-B radiation during springtime ozone depletion on photosynthesis and biomass production of Antarctic vascular plants. Plant Physiol. 2001, 125, 738–751. [Google Scholar] [CrossRef]
- Xiong, F.S.; Ruhland, C.T.; Day, T.A. Effect of solar ultraviolet-B radiation on growth of Colobanthus quitensis at Palmer Station, Antarctica. Global Change Biol. 2002, 8, 1146–1155. [Google Scholar] [CrossRef]
- Ruhland, C.T.; Xiong, F.S.; Clark, W.D.; Day, T.A. The influence of ultraviolet-B radiation on growth, hydroxycinnamic acids and flavonoids of Deschampsia antarctica during springtime ozone depletion in Antarctica. Photochem. Photobiol. 2005, 81, 1086–1093. [Google Scholar] [CrossRef]
- Day, T.A. Ecosystems and ultraviolet-B radiation; Cockell, C., Blaustein, A.R., Eds.; Springer-Verlag: New York, 2001; Chapter 4; pp. 80–117. [Google Scholar]
- Searles, P.S.; Flint, S.D.; Caldwell, M.M. A meta-analysis of plant field studies simulating stratospheric ozone depletion. Oecologia 2001, 127, 1–10. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Robberecht, R.; Flint, S.D. Internal filters: Prospects for UV-acclimation in higher plants. Physiol. Plant. 1983, 58, 445–450. [Google Scholar] [CrossRef]
- Day, T.A. Relating UV-B radiation screening effectiveness of foliage to absorbing-compound concentration and anatomical differences in a diverse group of plants. Oecologia 1993, 95, 542–550. [Google Scholar]
- Day, T.A.; Howells, B.W.; Rice, W.J. Ultraviolet absorption and epidermal-transmittance spectra in foliage. Physiol. Plant. 1994, 92, 207–218. [Google Scholar]
- Bornman, J.F.; Reuber, S.; Cen, Y.P.; Weissenböck, G. Ultraviolet radiation as a stress factor and the role of protective pigments; Lumsden, P., Ed.; Cambridge University Press: New York, 1997; pp. 157–168. [Google Scholar]
- Heideg, E.; Mano, J.; Ohno, C.; Asada, K. Increased levels of monodehydroascorbate radical in UV-B-irradiated broad bean leaves. Plant Cell Physiol. 1997, 38, 684–690. [Google Scholar] [CrossRef]
- Heideg, E.; Rosenavist, E.; Váradj, G.; Bornman, J.; Vincze, É. A comparison of UV-B induced stress responses in three barley cultivars. Func. Plant Biol. 2006, 33, 77–90. [Google Scholar]
- Galatro, A.; Simontacchi, M.; Puntarulo, S. Free radical generation and antioxidant content in chloroplasts from soybean exposed to ultraviolet-B. Physiol. Plant. 2001, 113, 564–570. [Google Scholar] [CrossRef]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Kramer, G.F.; Norman, H.A.; Krizek, D.T.; Mireki, R.M. Influence of UV-B radiation on polyamines, lipid peroxidation and membrane lipids in cucumber. Phytochemistry 1991, 22, 2101–2108. [Google Scholar]
- Malanga, G.; Puntarulo, S. Oxidative responses and antioxidant content in Chlorella vulgaris after exposure to ultraviolet-B radiation. Physiol. Plant 1995, 22, 672–679. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Fukumoto, R.; Kasahara, H.; Sakaki, T.; Kato, M. Peroxidation of lipids and growth inhibition induced by UV-B radiation. Plant Cell Rep. 1995, 22, 566–670. [Google Scholar]
- Davies, K.J.A. Protein damage and degradation by oxygen radicals. 1. General aspects. J. Biol. Chem. 1987, 262, 9895–9901. [Google Scholar]
- Salin, M.L. Toxic oxygen species and protective systems of the chloroplast. Physiol. Plant 1987, 72, 681–689. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free radicals in biology and medicine. Clarendon Press: Oxford, U.K., 1999. [Google Scholar]
- Elstner, E.F. Active oxygen/oxidative stress and plant metabolism; Pell, E.J., Steffen, L.K., Eds.; American Society of Plant Physiology: Rockville, MD, 1991; pp. 13–25. [Google Scholar]
- Rao, M.V.; Paliyath, G.; Ormrod, DP. Changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar]
- Kliebenstein, D.J.; Monde, R.A.; Last, R.L. Superoxide dismutase in Arabidopsis: An eclectic enzyme family with disparate regulation and protein localization. Plant Physiol. 1998, 118, 637–650. [Google Scholar]
- Santos, I.; Almeida, J.; Salema, R. The influence of UV-B radiation on the superoxide dismutase of maize, potato, sorghum and wheat leaves. Can. J. Bot. 1999, 77, 70–76. [Google Scholar]
- Boldt, R.; Scandalios, J.G. Influence of UV-light on the expression of the Cat2 and Cat3 catalase genes in maize. Free Rad. Biol. Med. 1997, 23, 505–514. [Google Scholar] [CrossRef]
- Smirnoff, N. The function and metabolism of ascorbic acid in plants. Ann. Bot. 1996, 78, 661–669. [Google Scholar] [CrossRef]
- Rennenberg, H. Glutathione metabolism and possible biological roles in higher plants. Phytochem 1982, 21, 2771–2781. [Google Scholar] [CrossRef]
- Hausladen, A.; Alscher, R.G. Antioxidants in higher plants; Alscher, R.G., Hess, J.K., Eds.; CRC Press: Boca Raton, FL, 1993; pp. 1–30. [Google Scholar]
- Hess, J.L. Antioxidants in higher plants; Alscher, R.G., Hess, J.K., Eds.; CRC Press: Boca Raton, FL, 1993; pp. 111–134. [Google Scholar]
- Burton, G.W.; Ingold, K.U. β-carotene: an unusual type of lipid antioxidant. Science 1984, 224, 569–573. [Google Scholar]
- Demming-Adams, B.; Adams, W.W. Antioxidants in higher plants; Alscher, R.G., Hess, J.K., Eds.; CRC Press: Boca Raton FL, 1993; pp. 59–90. [Google Scholar]
- Shirley, B.W. Flavonoids in seeds and grains: physiological function, agronomic importance and the genetics of biosynthesis. Seed Sci. Res. 1998, 8, 415–422. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Szeto, T.Y.; Strain, J.J.; Tomlinson, J. Consumption of green tea causes rapid increase in plasma antioxidant power in humans. Nutr. Cancer 1999, 34, 83–87. [Google Scholar] [CrossRef]
- Dixon, R.A.; Steele, C.L. Flavonoids and isoflavonoids- a gold mine for metabolic engineering. Trend. Plant Sci. 1999, 4, 394–400. [Google Scholar] [CrossRef]
- Duthie, G.C.; Duthie, S.J.; Kyle, J.A.M. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef]
- Hagerman, A.E. Polymeric phenolics as antioxidants. Polyphenols Actualites. 2001, 18–23. [Google Scholar]
- Peterson, D.M. Oat antioxidants. J. Cereal Sci. 2001, 33, 115–129. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma as a measure of “antioxidant power”: The FRAP Assay. Analyt. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Bornman, J.F. Target sites of UV-B radiation in photosynthesis of higher plants. J. Photochem. Photobiol. B: Biol. 1989, 4, 145–156. [Google Scholar]
- Melis, A.; Nemson, J.A.; Harrison, M.A. Damage to functional components and partial degradation of photosystem II reaction center proteins upon chloroplast exposure to ultraviolet-B radiation. Biochim. Biophys. Acta. 1992, 1100, 312–320. [Google Scholar]
- Day, T.A.; Howells, B.W.; Ruhland, C.T. Changes in growth and pigment concentrations with leaf age in pea under modulated UV-B field treatments. Plant Cell Environ. 1996, 19, 101–108. [Google Scholar] [CrossRef]
- Ruhland, C.T.; Day, T.A. Changes in UV-B radiation screening effectiveness with leaf age in Rhododendron maximum. Plant Cell Environ. 1996, 19, 740–746. [Google Scholar] [CrossRef]
- Li, J.Y.; Oulee, T.M.; Raba, R.; Amundons, R.G.; Last, R.L. Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell. 1993, 5, 171–179. [Google Scholar] [CrossRef]
- Liu, L.; McClure, J.W. Effects of UV-B on activities of enzymes of secondary phenolic metabolism in barley primary leaves. Physiol. Plant. 1995, 93, 734–739. [Google Scholar]
- Strid, Å. Alterations in expression of defence genes in Pisum sativum after exposure to supplementary ultraviolet-B radiation. Plant Cell Physiol. 1993, 34, 949–953. [Google Scholar]
- Jordan, B.R.; James, P.E.; Strid, Å; Anthony, R.G. The effect of ultraviolet-B radiation on gene expression and pigment composition in etiolated and green pea leaf tissue: UV-B-induced changes are gene specific and dependent on developmental stage. Plant Cell Environ. 1994, 17, 45–54. [Google Scholar]
- Caldwell, M.M. Photophysiology; Geise, A.C., Ed.; Academic Press: New York, 1971; Vol. 6, pp. 131–177. [Google Scholar]
- Ruhland, C.T.; Day, T.A. Effects of ultraviolet-B radiation on leaf elongation, production and phenylpropanoid concentrations of Deschampsia antarctica and Colobanthus quitensis in Antarctica. Physiol. Plant. 2000, 109, 244–251. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Stratil, P.; Bořivoj, K.; Kubán, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables - Evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar]
- Ruhland, C.T.; Day, T.A. Size and longevity of seed banks in Antarctica and the influence of ultraviolet-B radiation on survivorship, growth and pigment concentrations of Colobanthus quitensis seedlings. Env. Exp. Bot. 2001, 45, 143–154. [Google Scholar] [CrossRef]
- Coleman, R.S.; Day, T.A. Response of cotton and sorghum to several levels of subambient solar UV-B radiation: a test of the saturation hypothesis. Physiol. Plant. 2004, 122, 362–372. [Google Scholar] [CrossRef]
- Bilger, W.; Rolland, M.; Nybakken, L. UV screening in higher plants induced by low temperature in the absence of UV-B radiation. Photochem. Photobiol. Sci. 2007, 6, 190–195. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Day, T.A.; Ruhland, C.T.; Grobe, C.W.; Xiong, F.S. Growth and reproduction of Antarctic vascular plants in response to warming and UV radiation reductions in the field. Oecologia 1999, 119, 24–35. [Google Scholar] [CrossRef]
- Levizou, E.; Manetas, Y. Spectrophotometric assessment of leaf UV-B absorbing compounds and chemically determined total phenolic levels are strongly correlated. Can. J. Bot. 2002, 80, 690–694. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ruhland, C.T.; Fogal, M.J.; Buyarski, C.R.; Krna, M.A. Solar Ultraviolet-B Radiation Increases Phenolic Content and Ferric Reducing Antioxidant Power in Avena sativa. Molecules 2007, 12, 1220-1232. https://doi.org/10.3390/12061220
Ruhland CT, Fogal MJ, Buyarski CR, Krna MA. Solar Ultraviolet-B Radiation Increases Phenolic Content and Ferric Reducing Antioxidant Power in Avena sativa. Molecules. 2007; 12(6):1220-1232. https://doi.org/10.3390/12061220
Chicago/Turabian StyleRuhland, Christopher T., Mitchell J. Fogal, Christopher R. Buyarski, and Matthew A. Krna. 2007. "Solar Ultraviolet-B Radiation Increases Phenolic Content and Ferric Reducing Antioxidant Power in Avena sativa" Molecules 12, no. 6: 1220-1232. https://doi.org/10.3390/12061220



