Structural Characterisation by ESI-MS of Feruloylated Arabino-oligosaccharides Synthesised by Chemoenzymatic Esterification
Abstract
:Introduction
Results and Discussion
ESI-MSn analysis of feruloylated products
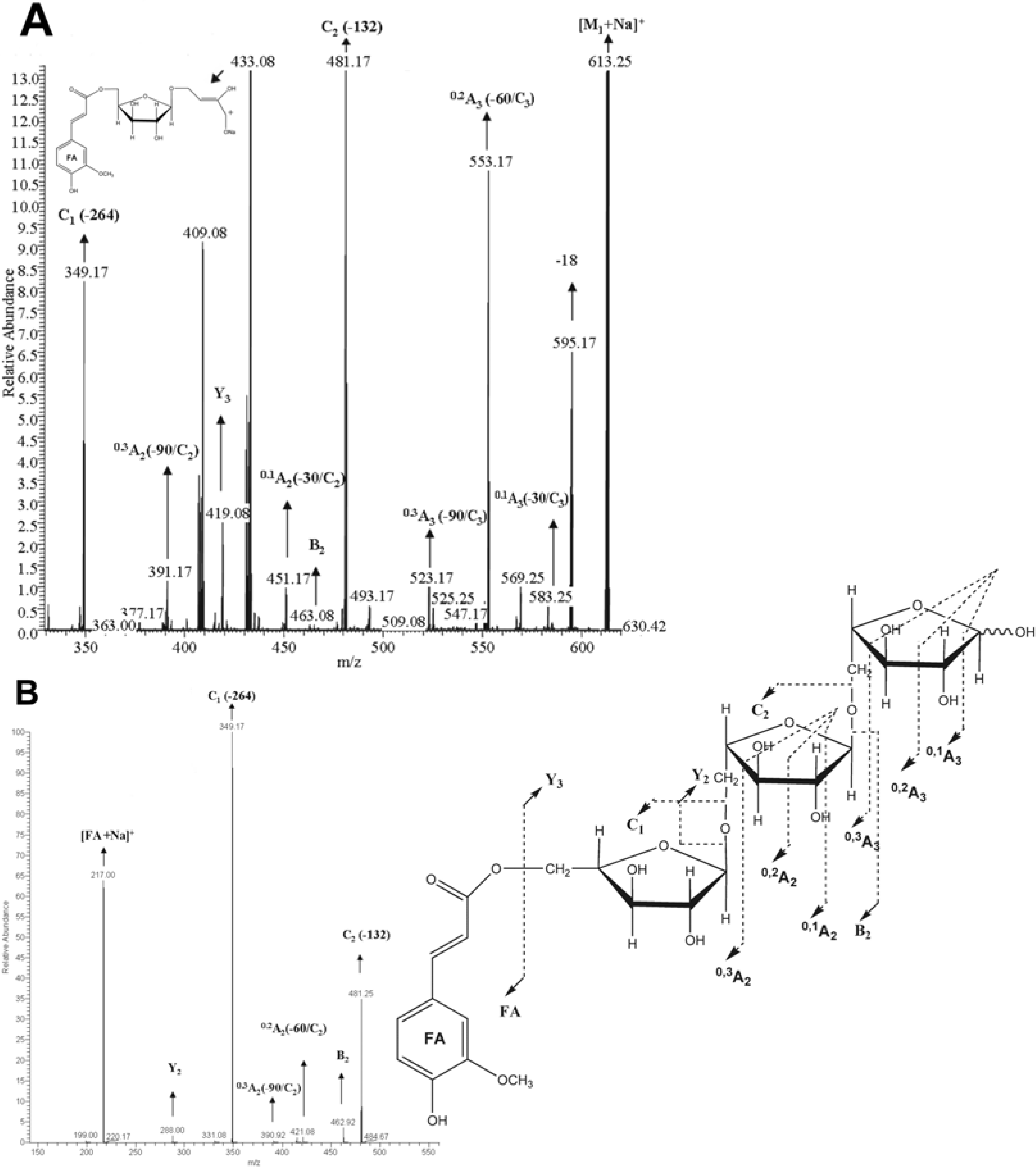
Identification of feruloylated arabinotriose 1
Identification of feruloylated arabinotetraose 2
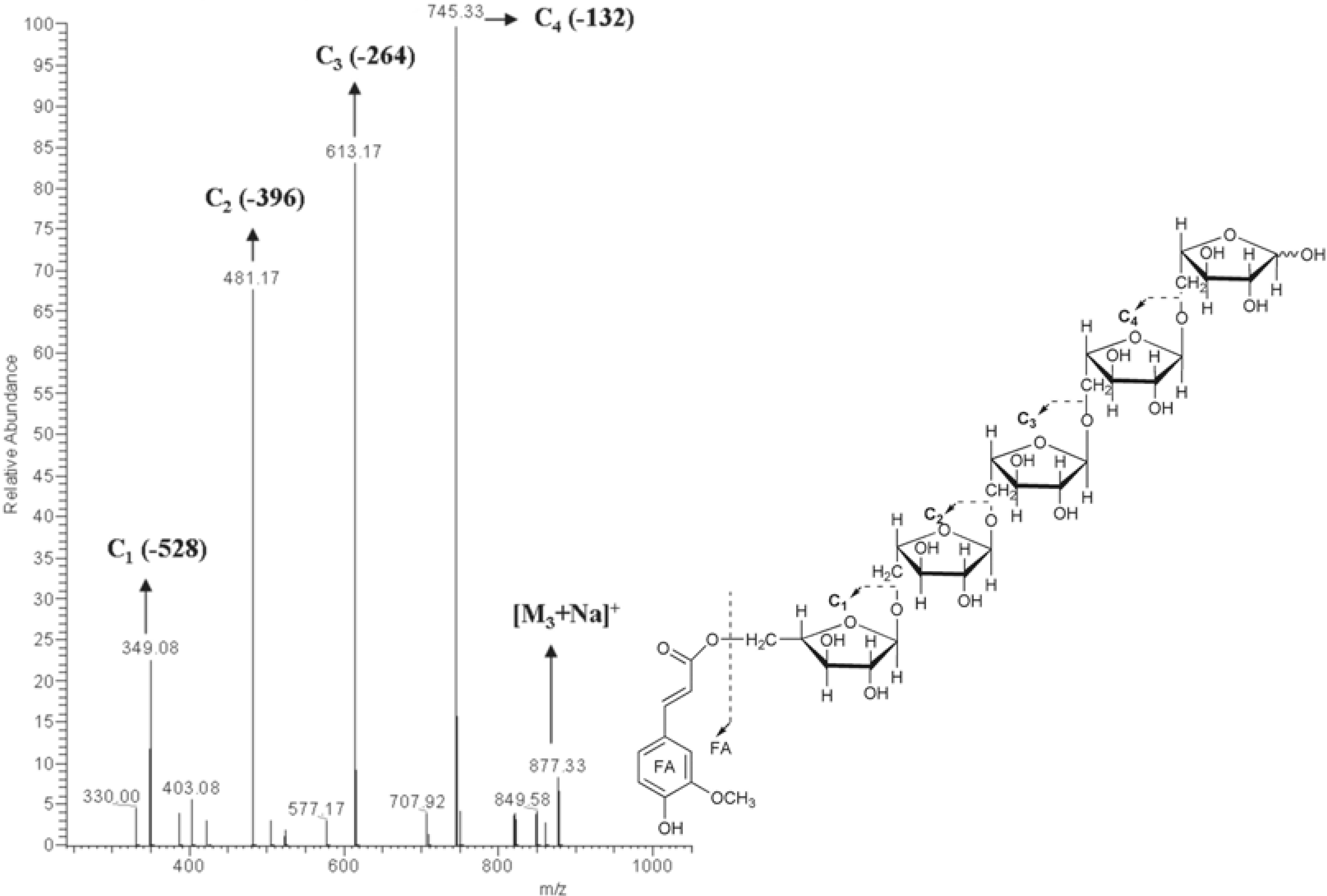
Identification of feruloylated arabinopentaose (3) and feruloylated arabinohexaose 4

Conclusions

Experimental
General
Reactions conditions
Thin Layer Chromatography
Electrospray Ionization Mass Spectrometry (ESI-MSn)
Acknowledgments
References
- Crepin, V.F.; Faulds, C.B.; Connerton, I.F. Functional classification of the microbial feruloyl esterases. Appl. Microbiol. Biotechnol. 2004, 63, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Shroeter, H.; Kataeva, I.A.; Blum, D.L.; Shah, A.K.; Ljungahl, L.G.; Rose, J.P.; Wang, B.C. Phenolic antioxidants attenuate neuronal cell death following uptake of oxidized low-density lipoprotein. Free Rad. Biol. Med. 2000, 29, 1222–1233. [Google Scholar] [PubMed]
- Davidson, P.M.; Branen, A.L. Anti-microbial activity of non-halogenated phenolic compounds. J. Food. Prot. 1981, 44, 597–603. [Google Scholar]
- Topakas, E.; Stamatis, H.; Biely, P.; Kekos, D.; Macris, B.J.; Christakopoulos, P. Purification and characterization of a feruloyl esterase (FAE-II) from Fusarium oxysporum catalysing esterification of phenolic acids in ternary water-organic solvent mixtures. J. Biotechnol. 2003, 102, 33–44. [Google Scholar]
- Topakas, E.; Vafiadi, C.; Stamatis, H.; Christakopoulos, P. Sporotrichum thermophile type C feruloyl esterase (StFaeC): Purification characterization, and its use for phenolic acid (sugar) ester synthesis. Enzyme Microb. Technol. 2005, 36, 729–736. [Google Scholar] [CrossRef]
- Vafiadi, C.; Topakas, E.; Christakopoulos, P. Regioselective esterase-catalyzed feruloylation of L-arabinobiose. Carbohydr. Res. 2006, 341, 1992–1997. [Google Scholar] [CrossRef] [PubMed]
- Vafiadi, C.; Topakas, E.; Wong, K.K.Y.; Suckling, I.D.; Christakopoulos, P. Mapping the hydrolytic and synthetic selectivity of a type C feruloyl esterase (StFaeC) from Sporotrichum thermophile using alkyl ferulates. Tetrahedron: Asymmetry 2005, 16, 373–379. [Google Scholar]
- Mastihubova, M.; Mastihuba, V.; Bilanicova, D.; Borekova, M. Commercial enzyme preparations catalyse feruloylation of glycosides. J. Mol. Cata.l B-Enzym. 2006, 38, 54–57. [Google Scholar]
- Figueroa-Espinoza, M.-C.; Villeneuve, P. Phenolic acids enzymatic lipophilization. J. Agric. Food Chem. 2005, 53, 2779–2787. [Google Scholar]
- Tawata, S.; Taira, S.; Kobamoto, N.; Zhu, J.; Ishihara, M.; Toyama, S. Synthesis and antifungal activity of cinnamic acid esters. Biosci. Biotechnol. Biochem. 1996, 60, 909–910. [Google Scholar]
- Chen, X.; Martin, B.D.; Neubauer, T.K.; Linhardt, R.F.; Dordick, J.S.; Rethwisch, D.G. Enzymatic and chemoenzymatic approaches to synthesis of sugar-based polymer and hydrogels. Carbohyd. Polym. 1995, 28, 15–21. [Google Scholar] [CrossRef]
- Patil, N.S.; Dordich, J.S.; Retwisch, D.G. Macroporous poly(sucrose acrylate) hydrogel for controlled release of macromolecules. Biomaterials 1996, 17, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Katapodis, P.; Vardakou, M.; Kalogeris, M.; Kekos, D.; Macris, B.J.; Christakopoulos, P. Enzymic production of a feruloylated oligosaccharide with antioxidant activity from wheat flour arabinoxylan. Eur. J. Nutr. 2003, 42, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Yao, H. Feruloyl oligosaccharides stimulate the growth of Bifidobacterium bifidum. Anaerobe 2005, 11, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Li, A.; Yang, A. A study on the synthesis of starch ferulate and its biological properties. Food Chem. 2001, 74, 91–95. [Google Scholar] [CrossRef]
- Corke, H.; Beta, T. Effect of ferulic acid and catechin on sorghum and maize starch pasting properties. Cereal Chem. 2004, 81, 418–422. [Google Scholar] [CrossRef]
- Domon, D.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB/MS/MS spectra of glycoconjugates. Glycoconjugate J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Sample availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Vafiadi, C.; Topakas, E.; Bakx, E.J.; Schols, H.A.; Christakopoulos, P. Structural Characterisation by ESI-MS of Feruloylated Arabino-oligosaccharides Synthesised by Chemoenzymatic Esterification. Molecules 2007, 12, 1367-1375. https://doi.org/10.3390/12071367
Vafiadi C, Topakas E, Bakx EJ, Schols HA, Christakopoulos P. Structural Characterisation by ESI-MS of Feruloylated Arabino-oligosaccharides Synthesised by Chemoenzymatic Esterification. Molecules. 2007; 12(7):1367-1375. https://doi.org/10.3390/12071367
Chicago/Turabian StyleVafiadi, Christina, Evangelos Topakas, Edwin J Bakx, Henk A Schols, and Paul Christakopoulos. 2007. "Structural Characterisation by ESI-MS of Feruloylated Arabino-oligosaccharides Synthesised by Chemoenzymatic Esterification" Molecules 12, no. 7: 1367-1375. https://doi.org/10.3390/12071367



