Comparison of the Biological Properties of Several Marine Sponge-Derived Sesquiterpenoid Quinones
Abstract
:Introduction
Results and Discussion
Herbicidal activity
| Name | Drimane rearranged decalin ring | X | R1 | R2 |
| (1) [1, 2] Dysidea avara |  |  | H | H |
| (2) [3, 4] Rhopaloeides sp. |  | OH | OCH3 | |
| (3) [22] Smenospongia sp. | OH | OH | ||
| (4) [22, 23] Smenospongia sp. | OH | OCH2CH3 | ||
| (5) [22] Smenospongia sp. | OH | NH(CH2)2C6H5 | ||
| (6) [22] Smenospongia sp. | OH | NH(CH2)2CH(CH3)2 | ||
| (7) [22] Smenospongia sp. | OH | NHCH2CH(CH3)2 | ||
| (8), [24] Rhopaloeides sp. |  | - | - | |
| (9) [25] Rhopaloeides sp. |  |  | - | - |
| (10) [26] Dysidea sp. |  |  | OH | OCH3 |
 where IC50 is the concentration that causes 50 % inhibition of PPDK, X is the concentration of the compound, h is the Hill slope and curve fitting is constrained between 0 and 100% inhibition.
where IC50 is the concentration that causes 50 % inhibition of PPDK, X is the concentration of the compound, h is the Hill slope and curve fitting is constrained between 0 and 100% inhibition.
 where IC50 is the concentration that causes 50 % inhibition of PPDK, X is the concentration of the compound, h is the Hill slope and curve fitting is constrained between 0 and 100% inhibition.
where IC50 is the concentration that causes 50 % inhibition of PPDK, X is the concentration of the compound, h is the Hill slope and curve fitting is constrained between 0 and 100% inhibition.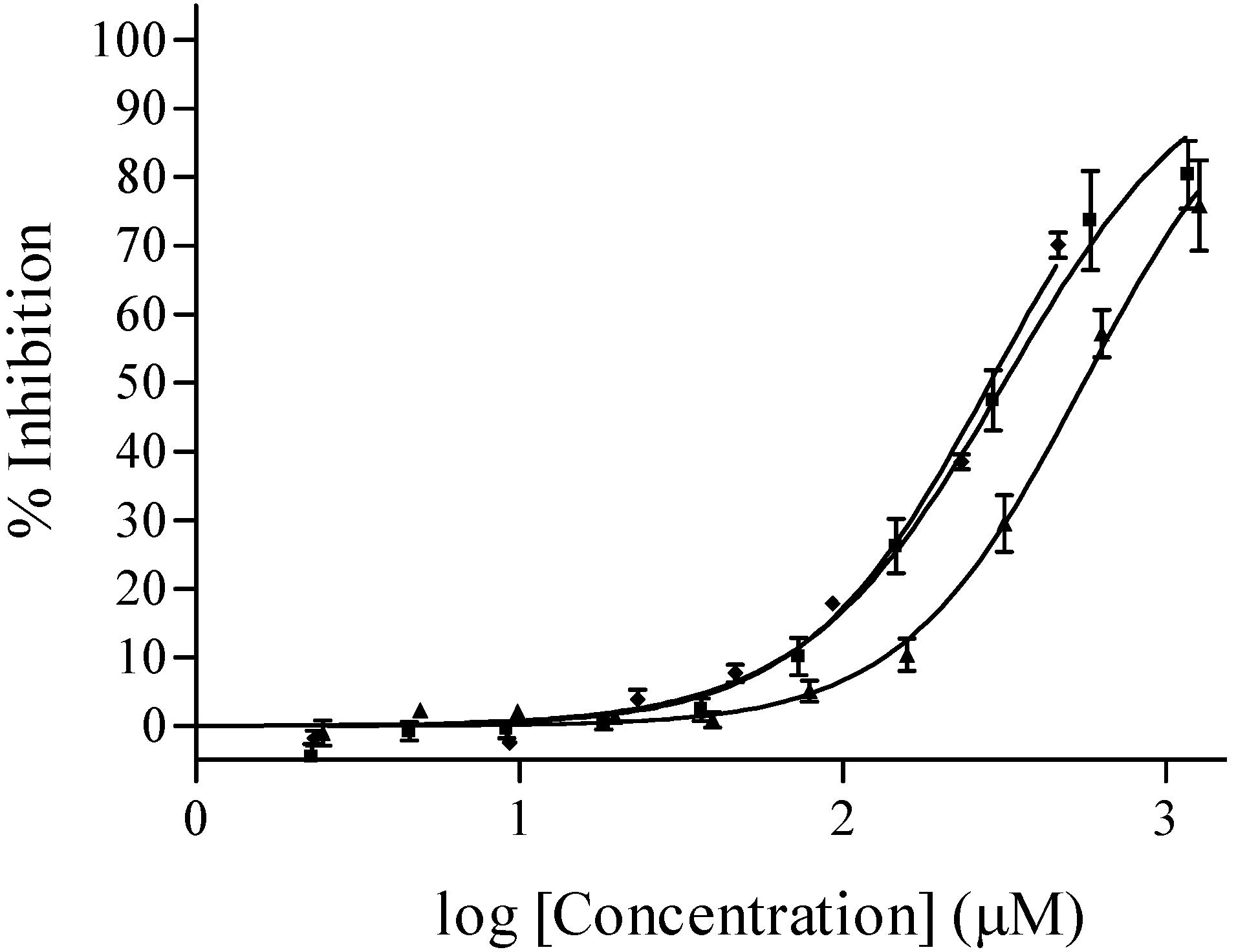
Theoretical chemical property calculations
| Molecular mass | clog P | H-bond donors | H-bond acceptors | Rot. Bonds | PSA (Å2) | SMV(Å3) | PPDK IC50 (μM) | C4 phytotoxicity d | % inhibitionof PLA2 | |||
| 24h | 48h | 72h | ||||||||||
| Desired Tice a | 150≤x ≤500 | ≤5.0 | ≤3 | 2≤x≤12 | ≤12 | 50-60 | 6 | 6 | 6 | |||
| Lipinski b | ≤500 | ≤5.0 | ≤5 | ≤10 | ||||||||
| (2) | 358.2 | 6.0 | 1 | 4 | 3 | 63.6 | 351.4 | 285.4 | 4 | 4 | 4 | 36.4 ± 8.2 at 279 µM |
| (3) | 344.2 | 5.9 | 2 | 4 | 2 | 74.6 | 333.8 | 556.0 | 2 | 3 | 3 | - |
| (4) | 372.3 | 6.4 | 1 | 4 | 4 | 63.6 | 368.2 | 316.2 | 2 | 3 | 3 | 73.2 ± 4.8 at 269 µM |
| (5) | 447.3 | 8.9 | 2 | 4 | 6 | 66.4 | 443.2 | Inactive c | - | - | - | 41.0 ± 0.6 at 224 µM |
| (6) | 413.6 | 8.2 | 2 | 4 | 6 | 66.4 | 421.8 | Inactive c | - | - | - | 61.5 ± 6.1 at 242 µM |
| (7) | 399.6 | 7.7 | 2 | 4 | 5 | 63.6 | 405.0 | Inactive c | - | - | - | Inactive at 251 µM |
| (8) | 372.5 | 7.7 | 2 | 4 | 4 | 66.8 | 367.8 | Inactive c | - | - | - | Inactive at 269 µM |
| (9) | 372.5 | 7.7 | 2 | 4 | 4 | 66.8 | 367.3 | Inactive c | - | - | - | - |
Anti-inflammatory activity
Conclusions
Experimental
General
Naturally Occurring Compounds
PPDK, PEPC, NAD-MDH enzyme-coupled assay

Determination of C4 plant phytotoxicity
Determination of PLA2 inhibition
Theoretical chemical property calculations
Acknowledgments
References
- Minale, L.; Riccio, R.; Sodano, G. Avarol, a novel sesquiterpenoid hydroquinone with a rearranged drimane skeleton from the sponge Dysidea avara. Tetrahedron Lett. 1974, 38, 3401–3404. [Google Scholar] [CrossRef]
- de Rosa, S.; Minale, L.; Riccio, R.; Sodano, G. The absolute configuration of avarol, a rearranged sesquiterpenoid hydroquinone from a marine sponge. J. Chem. Soc., Perkin Trans. I 1976, 1408–1414. [Google Scholar]
- Luibrand, R. T.; Erdman, T. R.; Vollmer, J. J.; Scheuer, P. J.; Finer, J.; Clardy, J. Ilimaquinone, a sesquiterpenoid quinone from a marine sponge. Tetrahedron 1979, 35, 609–612. [Google Scholar] [CrossRef]
- Capon, R. J.; MacLeod, J. K. A revision of the absolute stereochemistry of ilimaquinone. J. Org. Chem. 1987, 52, 5059–5060. [Google Scholar] [CrossRef]
- Loya, S.; Tal, R.; Kashman, Y.; Hizi, A. Ilimaquinone, a selective inhibitor of the RNase H activity of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 1990, 34, 2009–2012. [Google Scholar]
- Bourguet-Kondracki, M.-L.; Longeon, A.; Morel, E.; Guyot, M. Sesquiterpene quinones as immunomodulating agents. Int. J. Immunopharm. 1991, 13, 393–399. [Google Scholar] [CrossRef]
- Graillet, C.; Pesando, D.; Girard, J. P. Cellular mechanisms of the cytotoxicity of natural substances derived from the sea, studied during fertilization and egg cleavage in the sea-urchin. Oceanus 1991, 17, 229–230. [Google Scholar]
- El Sayed, K. A.; Dunbar, D. C.; Goins, D. K.; Cordova, C. R.; Perry, T. L.; Wesson, K. J.; Sanders, S. C.; Janus, S. A.; Hamann, M. T. The marine environment: a resource for prototype antimalarial agents. J. Nat. Toxins 1996, 5, 261–285. [Google Scholar]
- Popov, A. M.; Stekhova, S. I.; Utkina, N. K.; Rebachuk, N. M. Antimicrobial and cytotoxic activity of sesquiterpenequinones and brominated diphenyl esters isolated from marine sponges. Pharm. Chem. J. 1999, 33, 71–73. [Google Scholar] [CrossRef]
- Radeke, H. S.; Digits, C. A.; Bruner, S. D.; Snapper, M. L. New tools for studying vesicular-mediated protein trafficking: synthesis and evaluation of ilimaquinone analogs in a non-radioisotope-based antisecretory assay. J. Org. Chem. 1997, 62, 2823–2831. [Google Scholar] [CrossRef]
- Radeke, H. S.; Digits, C. A.; Casaubon, R. L.; Snapper, M. L. Interactions of (-)-ilimaquinone with methylation enzymes: implications for vesicular-mediated secretion. Chem. Biol. 1999, 6, 639–647. [Google Scholar]
- El Sayed, K.; Dunbar, D. C.; Bartyzel, P.; Zjawiony, J. K.; Day, W.; Hamann, M. T. Marine natural products as leads to develop new drugs and insecticides in biologically active natural products; CRC Press: Boca Raton, 1999; pp. 233–252. [Google Scholar]
- De Clercq, E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med. Res. Rev. 2000, 20, 323–349. [Google Scholar] [CrossRef]
- Takizawa, P. A.; Yucel, J. K.; Veit, B.; Faulkner, D. J.; Deerinck, T.; Soto, G.; Ellisman, M.; Malhotra, V. Complete vesiculation of Golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone. Cell 1993, 73, 1079–1090. [Google Scholar] [CrossRef]
- Tziveleka, L. A.; Vagias, C.; Roussis, V. Natural products with anti-HIV activity from marine organisms. Curr. Top. Med. Chem. 2003, 31, 512–535. [Google Scholar]
- Veit, B.; Yucel, J. K.; Malhotra, V. Microtubule independent vesiculation of Golgi membranes and the reassembly of vesicles into Golgi stacks. J. Cell. Biol. 1993, 122, 1197–1206. [Google Scholar] [CrossRef]
- Lu, P. H.; Chueh, S. C.; Kung, F. L.; Pan, S. L.; Shen, Y. C.; Guh, J. H. Ilimaquinone, a marine sponge metabolite, displays anticancer activity via GADD153-mediated pathway. Eur. J. Pharmacol. 2007, 556, 45–54. [Google Scholar] [CrossRef]
- Potts, B. C. M.; Faulkner, D. J. Phospholipase A2 inhibitors from marine organisms. J. Nat. Prod. 1992, 55, 1701–1717. [Google Scholar] [CrossRef]
- Ling, T.; Poupon, E.; Rueden, E. J.; Kim, S. H.; Theodorakis, E. A. Unified synthesis of quinone sesquiterpenes based on a radical decarboxylation and quinone addition reaction. J. Am. Chem. Soc. 2002, 124, 12261–12267. [Google Scholar] [CrossRef]
- Doyle, J. R.; Burnell, J. N.; Haines, D. S.; Llewellyn, L. E.; Motti, C. A.; Tapiolas, D. M. A rapid screening method to detect specific inhibitors of pyruvate, orthophosphate dikinase as leads for C4 plant-selective herbicides. J. Biomolec. Screen. 2005, 10, 67–75. [Google Scholar] [CrossRef]
- Edwards, G. E.; Nakamoto, H.; Burnell, J. N.; Hatch, M. D. Pyruvate,Pi dikinase and NADP-malate dehydrogenase in C4 photosynthesis: Properties and mechanism of light/dark regulation. Ann. Rev. Plant Physiol. 1985, 36, 255–286. [Google Scholar] [CrossRef]
- Kondracki, M.-L.; Guyot, M. Biologically active quinone and hydroquinone sesquiterpenoids from the sponge Smenospongia sp. Tetrahedron 1989, 45, 1995–2004. [Google Scholar] [CrossRef]
- Urban, S.; Capon, R. J. 5-epi-Isospongiaquinone, a new sesquiterpene/quinone antibiotic from an Australian marine sponge Spongia hispida. J. Nat. Prod. 1992, 55, 1638–1642. [Google Scholar] [CrossRef]
- Kondracki, M.-L.; Davoust, D.; Guyot, M. Smenospondiol, a biologically active hydroquinone from the sponge Smenospongia sp. J. Chem. Res. Synop. 1989, 3, 74–75. [Google Scholar]
- Salmoun, M.; Devijver, C.; Daloze, D.; Braekman, J. C.; Gomez, R.; de Kluijver, M.; Van Soest, R. W. M. New sesquiterpene/quinones from two sponges of the genus Hyrtios. J. Nat. Prod. 2000, 63, 452–456. [Google Scholar] [CrossRef]
- Lucas, R.; Giannini, C.; D’auria, M. V.; Pay´a, M. Modulatory effect of bolinaquinone, a marine sesquiterpenoid, on acute and chronic inflammatory processes. J. Pharmacol. Exp. Ther. 2003, 304, 1172–1180. [Google Scholar]
- Kirkwood, R. C. Recent developments in our understanding of the plant cuticle as a barrier to the foliar uptake of pesticides. Pestic. Sci. 1999, 55, 69–77. [Google Scholar] [CrossRef]
- Tice, C. M. Selecting the right compounds for screening: does Lipinski's Rule of 5 for pharmaceuticals apply to agrochemicals. Pest Manag. Sci. 2001, 57, 3–16. [Google Scholar] [CrossRef]
- Lipinski, C. A.; Lombardo, F.; Dominy, B. W.; Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Haines, D. S.; Burnell, J. N.; Doyle, J. R.; Llewellyn, L. E.; Motti, C. A.; Tapiolas, D. M. Translation of in vitro inhibition by marine natural products of the C4 acid cycle enzyme pyruvate P(i) dikinase to in vivo C4 plant tissue death. J. Agric. Food Chem. 2005, 53, 3856–3862. [Google Scholar]
- Ghose, A. K.; Crippen, G. M. Atomic physicochemical parameters for three dimensional structure-directed quantitative structure-activity relationships. I. Partition coefficients as a measure of hydrophobicity. J. Comput. Chem. 1986, 7, 565–577. [Google Scholar] [CrossRef]
- Medić-Šarić, M.; Mornar, A.; Jasprica, I. Lipophilicity study of salicylamide. Acta Pharm. 2004, 54, 91–101. [Google Scholar]
- Ferrándiz, M. L.; Sanz, M. J.; Bustos, G.; Payá, M.; Alcaraz, M. J.; De Rosa, S. Avarol and avarone, two new anti-inflammatory agents of marine origin. Eur. J. Pharmacol. 1994, 253, 75–82. [Google Scholar] [CrossRef]
- Escrig, V.; Ubeda, A.; Ferrandiz, M. L.; Darias, J.; Sanchez, J. M.; Alcaraz, M. J.; Paya, M. Variabilin: A dual inhibitor of human secretory and cytosolic phospholipase A2 with anti-inflammatory activity. J. Pharmacol. Exp. Ther. 1997, 282, 123–131. [Google Scholar]
- Aoki, S.; Kong, D.; Matsui, K.; Rachmat, R.; Kobayashi, M. Sesquiterpene aminoquinones, from a marine sponge, induce erythroid differentiation in human chronic myelogenous leukaemia, K562 cells. Chem. Pharm. Bull. 2004, 52, 935–937. [Google Scholar] [CrossRef]
- Veber, D. F; Johnson, S. R; Cheng, H. -Y.; Smith, B. R.; Ward, K. W.; Kopple, K. D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Peng, J.; Shen, X.; El Sayed, K.; Dunbar, D.; Perry, T.; Wilkins, S.; Hamann, M. Marine natural products as prototype agrochemical agents. J. Agric. Food Chem. 2003, 51, 2246–2252. [Google Scholar] [CrossRef]
- Sladic, D.; Gasic, M. J. Reactivity and biological activity of the marine sesquiterpene hydroquinone avarol and related compounds from sponges of the order Dictyoceratida. Molecules 2006, 11, 1–33. [Google Scholar] [CrossRef]
- Bolton, J. L.; Trush, M. A.; Penning, T. M.; Dryhurst, G.; Monks, T. J. Role of Quinones in Toxicology. Chem. Res. Tox. 2000, 13, 135–160. [Google Scholar] [CrossRef]
- Molinari, A.; Oliva, A.; Aguilera, N.; Miguel del Corral, J. M.; Castro, M. A.; Gordaliza, M.; Garcia-Cravalos, M. D.; San Feliciano, A. New antineoplastic prenylhydroquinones. Synthesis and evaluation. Bioorg. Med. Chem. 2000, 8, 1027–1032. [Google Scholar] [CrossRef]
- Ashton, R.; Burnell, J. N.; Furbank, R. T.; Jenkins, C. L. D.; Hatch, M. D. Enzymes of C4 photosynthesis. Meth. Plant Biochem. 1990, 3, 39–71. [Google Scholar] [CrossRef]
- Lobo de Araujo, A.; Radvanyi, F. Determination of phospholipase A2 activity by a colorimetric assay using a pH indicator. Toxicon 1987, 25, 1181–1188. [Google Scholar] [CrossRef]
- Leo, A. J. Calculating log POCT from structures. Chem. Rev. 1993, 93, 1281–1304. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Sample Availability: contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Motti, C.A.; Bourguet-Kondracki, M.-L.; Longeon, A.; Doyle, J.R.; Llewellyn, L.E.; Tapiolas, D.M.; Yin, P. Comparison of the Biological Properties of Several Marine Sponge-Derived Sesquiterpenoid Quinones. Molecules 2007, 12, 1376-1388. https://doi.org/10.3390/12071376
Motti CA, Bourguet-Kondracki M-L, Longeon A, Doyle JR, Llewellyn LE, Tapiolas DM, Yin P. Comparison of the Biological Properties of Several Marine Sponge-Derived Sesquiterpenoid Quinones. Molecules. 2007; 12(7):1376-1388. https://doi.org/10.3390/12071376
Chicago/Turabian StyleMotti, Cherie A, Marie-Lise Bourguet-Kondracki, Arlette Longeon, Jason R Doyle, Lyndon E Llewellyn, Dianne M Tapiolas, and Ping Yin. 2007. "Comparison of the Biological Properties of Several Marine Sponge-Derived Sesquiterpenoid Quinones" Molecules 12, no. 7: 1376-1388. https://doi.org/10.3390/12071376




