An Efficient and Rapid Synthetic Route to Biologically Interesting Pyranochalcone Natural Products
Abstract
:Introduction
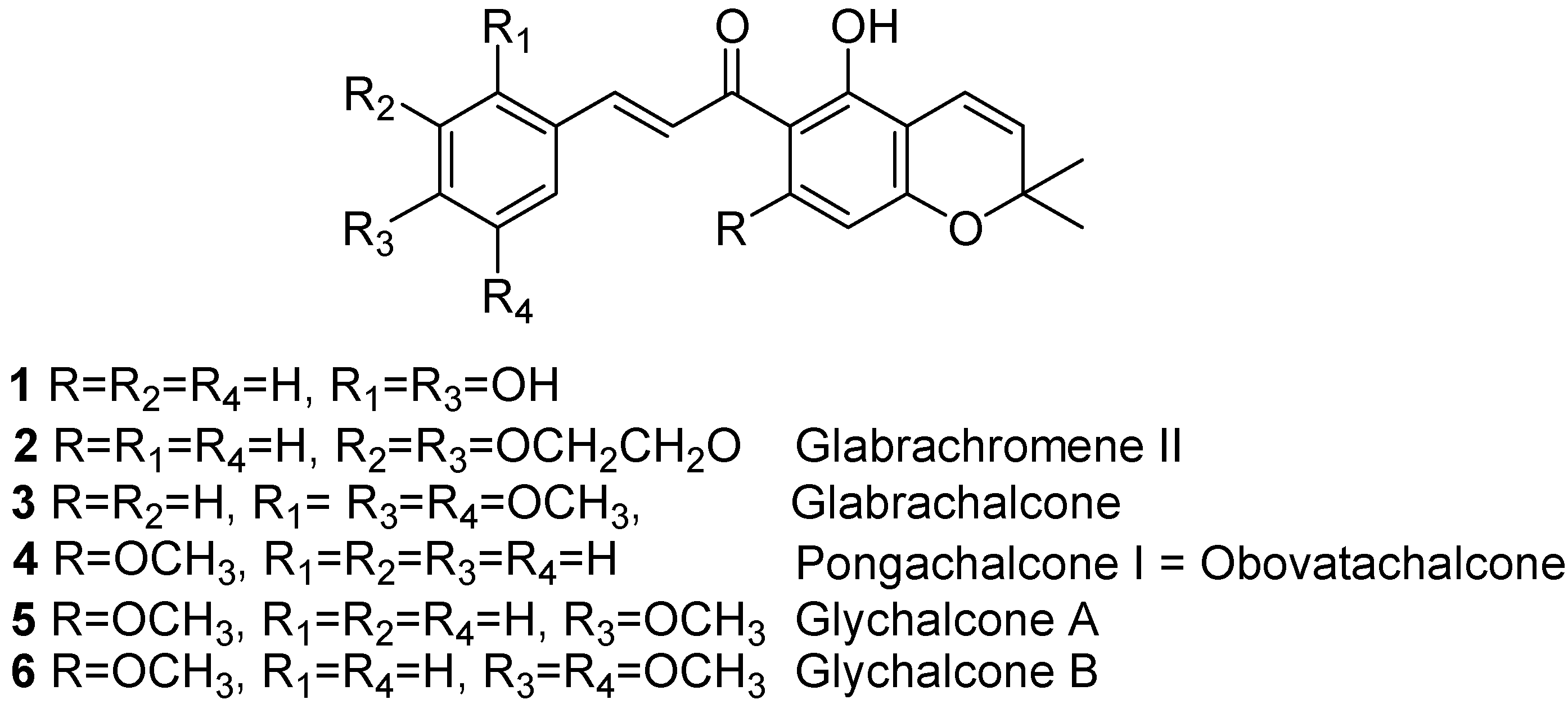
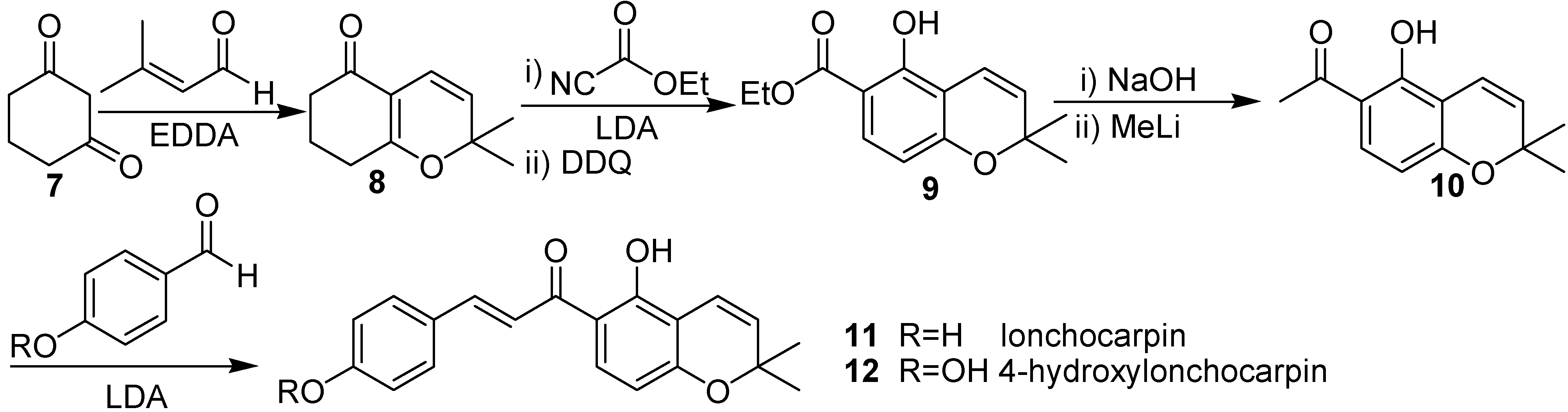
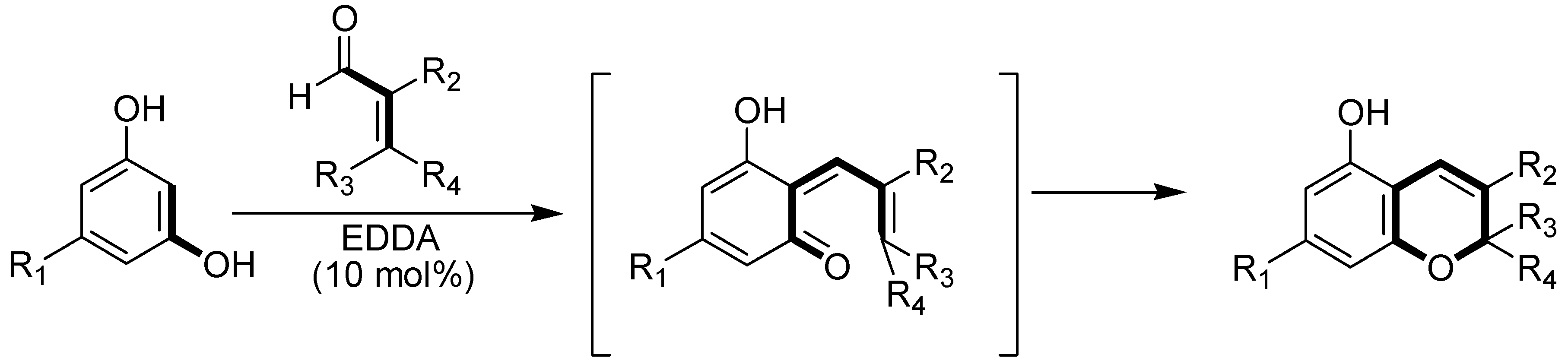
Results and Discussion
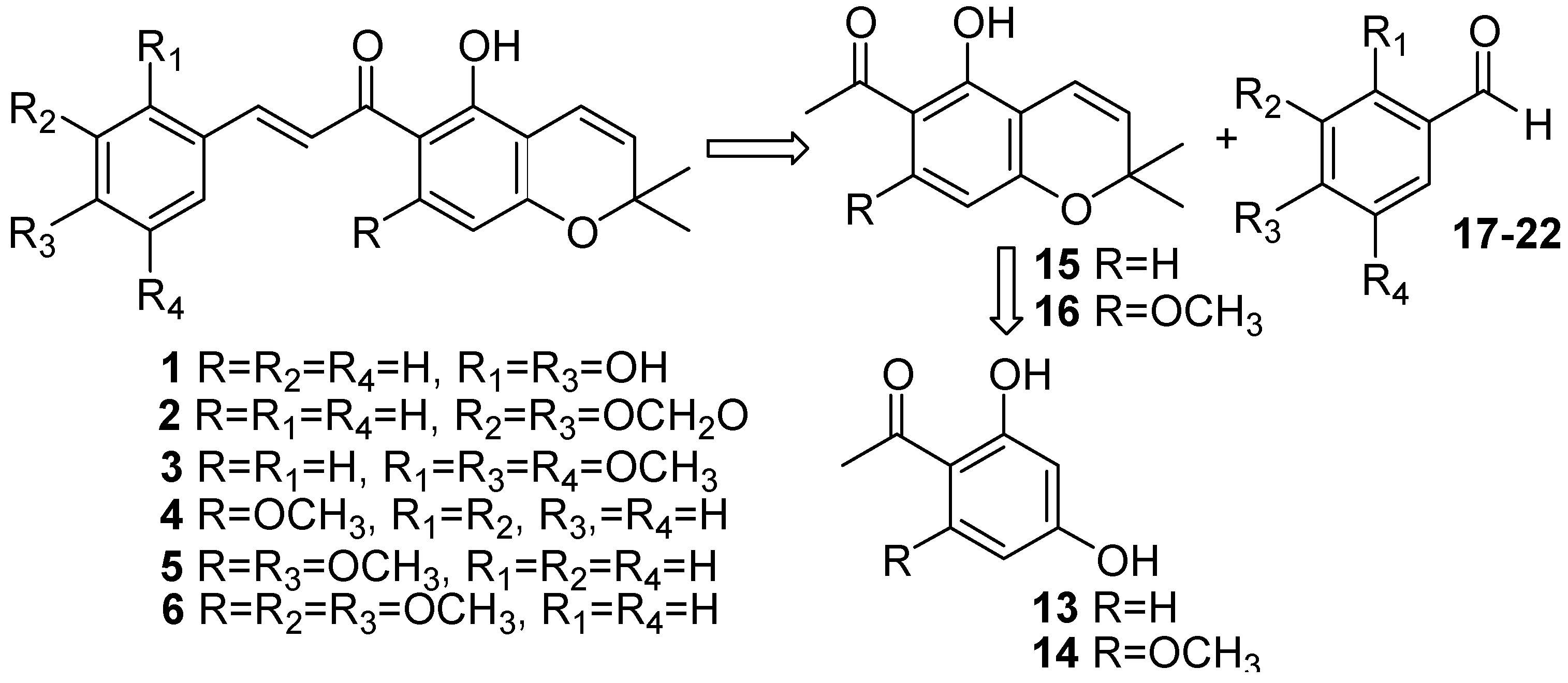
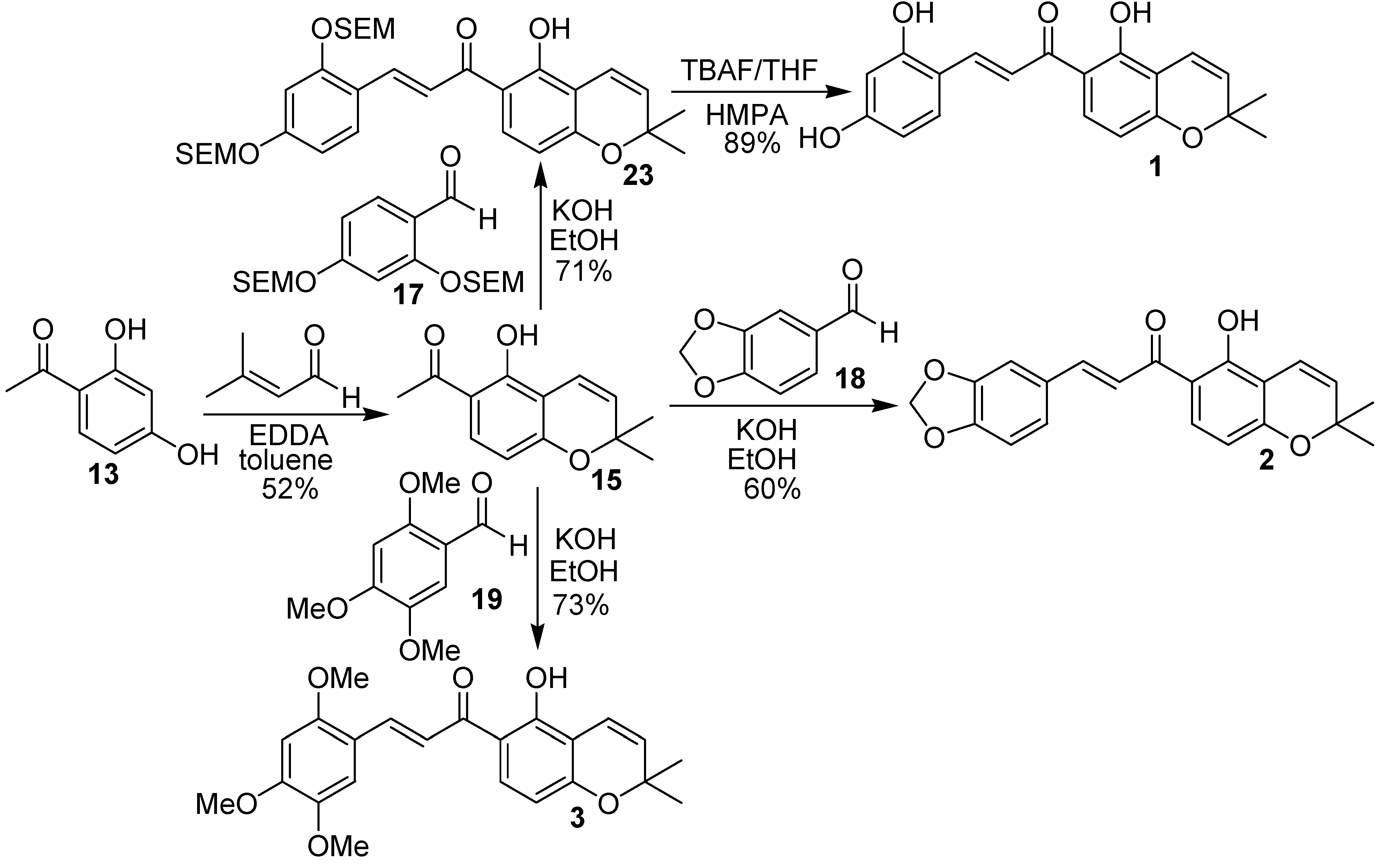
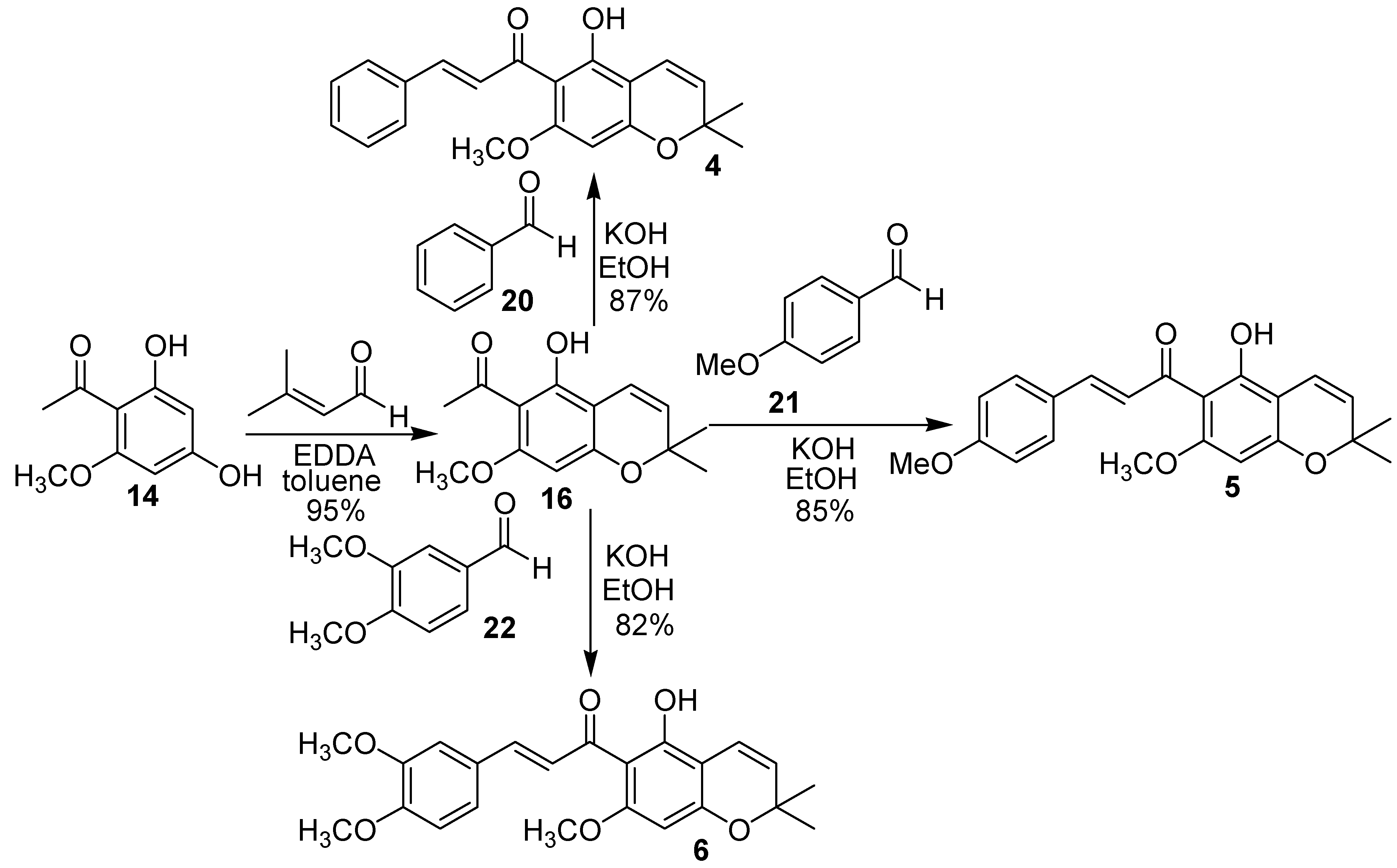
Conclusions
Experimental
General
Desmethylisoencecalin (15) [16]
3-[2,4-Bis-(2-trimethylsilanylethoxy)phenyl]-1-(5-hydroxy-2,2,dimethyl-2H-chromen-6-yl)propenone (23)
3”,3”-Dimethylpyrano[3’,4’]-2,4,2’-trihydroxychalcone (1) [4]
Glabrachromene II (2) [7]
Glabrachalcone (3) [8]
Isoevodionol (16) [18]
Pongachalcone I (4) [9]
Glychalcone A (5) [11]
Glychalcone B (6) [11]
Acknowledgments
References and Notes
- Wagner, H.; Farkas, L. The Flavonoids; Harorne, J. B., Mabry, T. J., Mabry, H., Eds.; Academic Press: New York, 1975; p. 127. [Google Scholar] Gripenberg, J. The Chemistry of Flavonoid Compounds; Geissman, T. A., Ed.; MacMillan: New York, 1962; p. 409. [Google Scholar] Wollenweber, E. Flavones and flavonols; Chapman & Hall: London, 1994; pp. 259–335. [Google Scholar] Harborne, J. B.; Baxter, H. The Handbook of Natural Flavonoids; Wiley: Chichester, 1999; vol. 2, p. 1. [Google Scholar] Saini, T. R.; Pathak, V. P.; Khanna, R. N. Glabrachromene II, a minor constituent of seeds of Pongamia glabra. J. Nat. Prod. 1983, 46, 936. [Google Scholar]
- Magalhães, A. F.; Azevedo Tozzi, A. M. G.; Noronha Sales, B. H. L.; Magalhães, E. G. Twenty-three flavonoids from Lonchocarpus subglaucescens. Phytochemistry 1996, 42, 1459–1471. [Google Scholar] Fang, N.; Casida, J. E. New bioactive flavonoids and stilbenes in Cube resin Insecticide. J. Nat. Prod. 1999, 62, 205–210. [Google Scholar]
- Miranda, C. L.; Stevens, J. F.; Helmrich, A.; Henderson, M. C.; Rodriguez, R. J.; Yang, Y.-H.; Deinzer, M. L.; Barnes, D. W.; Buhler, D. R. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem. Toxicol. 1999, 37, 271–285. [Google Scholar] Demizu, S.; Kajiyama, K.; Hiraga, Y.; Kinoshida, T.; Koyama, K.; Takahashi, K.; Tamura, Y.; Okada, K.; Kinoshida, T. Prenylated dibenzoylmethane derivatives from the root of Glycyrrhiza inflata (Xinjiang licorice). Chem. Pharm. Bull. 1992, 40, 392–395. [Google Scholar]
- Han, A.-R.; Kang, Y.-J.; Windono, T.; Lee, S. K.; Seo, E.-K. Prenylated Flavonoids from the Heartwood of Artocarpus communis with Inhibitory Activity on Lipopolysaccharide-Induced Nitric Oxide Production. J. Nat. Prod. 2006, 69, 719–721. [Google Scholar] [CrossRef]
- Fukai, T.; Satoh, K.; Nomura, T.; Sakagami, H. Antinephritis and radical scavenging activity of prenylflavonoids. Fitoterapia 2003, 74, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Koshihara, Y.; Fujimoto, Y.; Inoue, H. A new 5-lipoxygenase selective inhibitor derived from Artocarpus communis strongly inhibits arachidonic acid-induced ear edema. Biochem. Pharmacol. 1988, 37, 2161–2165. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, K.; Madhusudhana Rao, V.; Jagannadha Rao, K. V. Chemical examination of the heartwood of Pongamia glabra Vent.: isolation of chromenochalcones and synthesis of pongachalcones I and II. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1997, 15, 12–15. [Google Scholar]
- Pathak, V. P.; Saini, T. R.; Khanna, R. N. Glabrachalcone, a chromenochalcone from Pongamia glabra seeds. Phytochemistry 1983, 22, 1303–1304. [Google Scholar] Singhai, A. K.; Barua, N. C.; Sharma, R. P.; Baruah, J. N. A chalcone and an isoflavone from Millettia pachycarpa seeds. Phytochemistry 1983, 22, 1005–1006. [Google Scholar]
- Kare, M.; Kone, M. E. K.; Boulanger, A.; Niassy, B.; Lenouen, D.; Muckensturm, B.; Nongonierma, A. Isolation, identification and antibacterial tests of chalcones and rotenoids of Tephrosia deflexa Baker. J. Soc. ouest-afr. chim. 2006, 11, 41–45. [Google Scholar]
- Andrei, C. C.; Ferreira, D. T.; Faccione, M.; de Moraes, L. A. B.; de Carvalho, M. G.; Braz-Filho, R. C-prenylflavonoids from roots of Tephrosia tunicata. Phythochemistry 2000, 55, 799–804. [Google Scholar] [CrossRef]
- Wu, T.-S.; Chang, F.-C.; Wu, P.-L. Flavonoids, amidosulfoxides and an alkaloid from the leaves of Glycosmis citrifolia. Phytochemistry 1995, 39, 1453–1457. [Google Scholar] [CrossRef]
- Nicolaou, K. C.; Pfefferkorn, J. A.; Cao, G.-Q. Selenium-based solid-phase synthesis of benzopyrans I: applications to combinatorial synthesis of natural products. Angew. Chem., Int. Ed. 2000, 39, 734–739. [Google Scholar] Nicolaou, K. C.; Pfefferkorn, J. A.; Roecker, A. J.; Cao, G.-Q.; Barleenga, S.; Mitchell, H. J. Natural Product-like Combinatorial Libraries Based on Privileged Structures. 1. General Principles and Solid-Phase Synthesis of Benzopyrans. J. Am. Chem. Soc. 2000, 122, 9939–9953. [Google Scholar] Narender, T.; Gupta, S. A convenient and biogenetic type synthesis of few naturally occurring chromeno dihydrochalcones and their in vitro antileishmanial activity. Bioorg. Med. Chem. Lett. 2004, 14, 3913–3916. [Google Scholar]
- Subramanian, M.; Kumaraswami, K.; Rajendra Prasad, K. J. A new synthesis of pongachalcone I, glabrachromene, mixtecacin, candidin, atalantoflavone dimethylether, racemo-flavone dimethylether, and dihydropyranoaurones, and their analogs. J. Nat. Prod. 1992, 55, 1213–1229. [Google Scholar] Pathak, V. P.; Saini, T. R.; Kaanna, R. N. Glabrachalcone, a chromenochalcone from Pongamia glabra seeds. Phytochemistry 1983, 22, 1303–1304. [Google Scholar] Tan, W.-F.; Li, W.-D. Z.; Li, Y.-L. First total synthesis of (±) -glyflavanone-A. Synth. Commun. 2002, 32, 1077–1083. [Google Scholar]
- Lee, Y. R.; Kim, D. H. A new route to the synthesis of pyranoflavone and pyranochalcone natural products and their derivatives. Synthesis 2006, 603–608. [Google Scholar]
- Lee, Y. R.; Choi, J. H.; Yoon, S. H. Efficient and general method for the synthesis of benzopyrans by ethylenediamine diacetate-catalyzed reactions of resorcinols with α ,β -unsaturated aldehydes. One step synthesis of biologically active (±)-confluentin and (±)-daurichromenic acid. Tetrahedron Lett. 2005, 46, 7539–7543. [Google Scholar]
- Kulkarni, M. M.; Nagasampagi, B. A.; Deshpande, S. G.; Sharma, R. N. Five chromenes from Blepharispermum subsessile. Phytochemistry 1987, 26, 2969–2971. [Google Scholar] [CrossRef]
- Agarwal, S. K.; Verma, S.; Singh, S. S.; Tripathi, A. K.; Khan, Z. K.; Kumar, S. J. Antifeedant and antifungal activity of chromene compounds isolated from Blepharispermum subsessile. Ethnopharm. 2000, 71, 231–234. [Google Scholar] [CrossRef]
- Siani, A. C.; Silva, A. M. P.; Nakamura, M. J.; De Carvalho, M. V.; Henriques, M. G. M. O.; Ramos, M. F. S.; Kaiser, C. R. Chemical composition and anti-inflammatory activity of the hydrodistillate from Mariscus pedunculatus. J. Braz. Chem. Soc. 2001, 12, 354–359. [Google Scholar] Li, G.-L.; Zeng, J.-F.; Song, C.-Q.; Zhu, D. Y. Chromenes from Evodia lepta. Phytochemistry 1997, 44, 1175–1177. [Google Scholar]
- Stevens, J. F.; Ivacic, M.; Hsu, V. L.; Deinzer, M. L. Prenylflavonoids from Humulus lupulus Phytochemistry. Phytochemistry. 1997, 44, 1575–1585. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Lee, Y.R.; Wang, X.; Xia, L. An Efficient and Rapid Synthetic Route to Biologically Interesting Pyranochalcone Natural Products. Molecules 2007, 12, 1420-1429. https://doi.org/10.3390/12071420
Lee YR, Wang X, Xia L. An Efficient and Rapid Synthetic Route to Biologically Interesting Pyranochalcone Natural Products. Molecules. 2007; 12(7):1420-1429. https://doi.org/10.3390/12071420
Chicago/Turabian StyleLee, Yong Rok, Xue Wang, and Likai Xia. 2007. "An Efficient and Rapid Synthetic Route to Biologically Interesting Pyranochalcone Natural Products" Molecules 12, no. 7: 1420-1429. https://doi.org/10.3390/12071420



