A Facile, Mild And Efficient One-Pot Synthesis of 2-Substituted Indole Derivatives Catalyzed By Pd(PPh3)2Cl2
Abstract
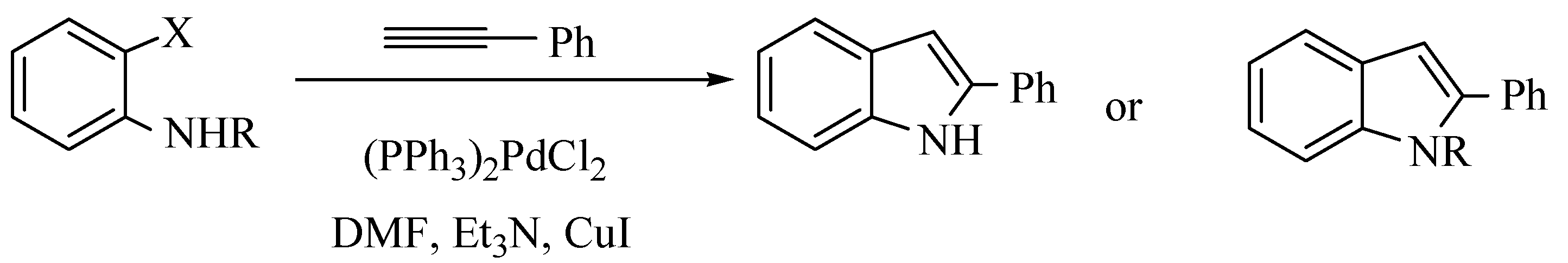
:Introduction
Results and Discussion
| Entry | Aryl halide | Product | Yield% |
|---|---|---|---|
| 1 |  |  | 72 |
| 2 |  | ″ | 69 |
| 3 |  | ″ | 78 |
| 4 |  | ″ | 75 |
| 5 |  |  | 82 |
| 6 |  | ″ | 80 |
| 7 |  |  | 74 |
| 8 |  | ″ | 76 |
| 9 |  |  | 68 |
| 10 |  | ″ | 70 |
| 11 |  |  | 68 |

Conclusions
Experimental
General
Preparation of Pd(PPh3)2Cl2
General procedure for the synthesis of 2-substituted-1H-indoles with Pd(PPh3)2Cl2
Synthesis of 1-methyl-2-phenylindole; (Table 1, entries 5, 6)
Synthesis of 1-benzyl-2-phenylindole (Table 1, entries 7, 8)
Synthesis of 2-phenyl-1-(toluene-4- sulfonyl)indole (Table 1, entries 9, 10)
Synthesis of 2-pentyl-1H-indole (Table 1, entry 11)
Acknowledgments
References and Notes
- Gribble, G.W. Pyrroles and benzo derivatives: applications. In Comprehensive Heterocyclic Chemistry II; Katritzky, A. R., Rees, C. W., Scriven, E. F. V., Eds.; Pergamon Press: New York; Oxford, 1996; Vol. 2, pp. 207–256, and references cited therein. [Google Scholar]
- Gribble, G. W. Recent developments in indole ring synthesis-methodology and applications. J. Chem. Soc. Perkin Trans. 1 2000, 1045–1047. [Google Scholar] [CrossRef]
- Von Angerer, E.; Knebel, N.; Kager, M.; Ganss, B. 1-(Aminoalkyl)-2-Phenylindoles As Novel Pure Estrogen Antagonists. J. Med. Chem. 1990, 33, 2635–2640. [Google Scholar] Ismail, M. F.; Shmeiss, N. A. M. M.; El-Diwani, H. I.; Arbid, M. S. Synthesis and pharmacological activity of some 2,3-diphenylindole derivatives. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1997, 36B, 288–292. [Google Scholar] Biberger, C.; Von Angerer, E. 1-benzyl-2-phenylindole- and 1,2-diphenylindole-based antiestrogens. Estimation of agonist and antagonist activities in transfection assays. J. Steroid Biochem. Mol. Biol. 1998, 64, 277–281. [Google Scholar] Medarde, M.; Ramos, A. C.; Caballero, E.; De Clairac, R. P. L.; Lopez, J. L.; Gravalos, D. G.; Feliciano, A. S. Synthesis and inotropic activity of hydroindene derivatives. Bioorg. Med. Chem. Lett. 1999, 9, 2303–2308. [Google Scholar] Stevenson, G. I.; Smith, A. L.; Lewis, S.; Michie, S. G.; Neduvelil, J. G.; Patel, S.; Marwood, R.; Castro, J. L. Solid-phase synthesis of 2,3-disubstituted indoles: Discovery of a novel, high-affinity, selective h5-HT2A antagonist. Bioorg. Med. Chem. Lett. 2000, 10, 2697–2699. [Google Scholar] Colletti, S. L.; Li, C.; Fisher, M. H.; Wyvratt, M. J.; Meinke, P. T. Tryptophan-replacement and indole-modifed apicidins: synthesis of potent and selective antiprotozoal agents. Tetrahedron Lett. 2000, 41, 7825–7827. [Google Scholar]
- Newcastle, G. W.; Katritzky, A. R. Generation And Reactions Of Sp(2)-Carbanionic Centers In The Vicinity Of Heterocyclic Nitrogen-Atoms. Adv. Heterocycl. Chem. 1993, 56, 155. [Google Scholar] Hudkins, R. L.; Diebold, J. L.; Marsh, F. D. Synthesis Of 2-Aryl-1H-Indoles And 2-Vinyl-1H-Indoles Via Palladium-Catalyzed Cross-Coupling Of Aryl And Vinyl Halides With 1-Carboxy-2-(Tributylstannyl)Indole. J. Org. Chem. 1995, 60, 6218–6220. [Google Scholar] Amat, M.; Hadida, S.; Pshenichnyi, G.; Bosch, J. Palladium(0)-catalyzed heteroarylation of 2- and 3-indolylzinc derivatives. An efficient general method for the preparation of (2-pyridyl)indoles and their application to indole alkaloid synthesis. J. Org. Chem. 1997, 62, 3158–3162. [Google Scholar] Kawasaki, I.; Yamashita, M.; Ohta, S. Total synthesis of nortopsentins A-D, marine alkaloids. Chem. Pharm. Bull. 1996, 44, 1831–1837. [Google Scholar] Johnson, C. N.; Stemp, G.; Anand, N.; Stephen, S. C.; Gallagher, T. Palladium (0)-catalysed arylations using pyrrole and indole 2-boronic acids. Synlett 1998, 1025–1027. [Google Scholar]
- Sundberg, R. J. Pyrroles and benzo derivatives: synthesis. In Comprehensive Heterocyclic Chemistry II; Katritzky, A. R., Rees, C. W., Scriven, E. F. V., Eds.; Pergamon Press: New York; Oxford, 1996; vol. 2, pp. 119–206. [Google Scholar] Sundberg, R. J. Indoles; Academic Press: London, 1996. [Google Scholar]
- Li, J. J.; Gribble, G. W. Palladium in Heterocyclic Chemistry; Pergamon: Amsterdam, 2000. [Google Scholar] Suzuki, N.; Yasaki, S.; Yasuhara, A.; Sakamoto, T. Convenient indole synthesis from 2-iodoanilines and terminal alkynes by the sequential Sonogashira reaction and the cyclization reaction promoted by tetrabutylammonium fluoride (TBAF). Chem. Pharm. Bull. 2003, 51, 1170–1173. [Google Scholar] Yasuhara, M. C.; Kanamori, Y.; Kaeko, M.; Numata, A.; Kondo, Y.; Sakamoto, T. Convenient synthesis of 2-substituted indoles from 2-ethynylanilines with tetrabutylammonium fluoride. J. Chem. Soc., Perkin Trans. 1 1999, 529–534. [Google Scholar] Falco, J. L.; Pique, M.; Gonzalez, M.; Buira, I.; Mendez, E.; Terencio, J.; Perez, C.; Princep, M.; Palomer, A.; Guglietta, A. Synthesis, pharmacology and molecular modeling of N-substituted 2-phenyl-indoles and benzimidazoles as potent GABA(A) agonists. Eur. J. Med. Chem. 2006, 41, 985–990. [Google Scholar] Feuerstein, M.; Doucet, H.; Santelli, M. Sonogashira reaction of heteroaryl halides with alkynes catalysed by a palladium-tetraphosphine complex. J. Mol. Catal. A: Chem. 2006, 256, 75–84. [Google Scholar] Lu, B. Z.; Zhao, W.Y.; Wei, H. X.; Dufour, M.; Farina, V.; Senanayake, C. H. A practical mild, one-pot, regiospecific synthesis of 2,3-disubstituted indoles via consecutive Sonogashira and Cacchi reactions. Org. Lett. 2006, 8, 3271–3274. [Google Scholar] Tang, Z. Y.; Hu, Q. S. Efficient synthesis of 2-substituted indoles based on palladium(II) acetate/tri-tert-butylphosphine-catalyzed alkynylation/ amination of 1,2-dihalobenzenes. Adv. Synth. Catal. 2006, 348, 846–850. [Google Scholar] Yue, D. W.; Yao, T. L.; Larock, R. C. Synthesis of 3-iodoindoles by the Pd/Cu-catalyzed coupling of N,N-dialkyl-2-iodoanilines and terminal acetylenes, followed by electrophilic cyclization. J. Org. Chem. 2006, 71, 62–69. [Google Scholar] Pal, M.; Subramanian, V.; Batchu, V. R.; Dager, I. Synthesis of 2-substituted indoles via Pd/C-catalyzed reaction in water. Synlett 2004, 1965–1969. [Google Scholar]
- Arcadi, A.; Cacchi, S.; Marinelli, F. A Versatile Approach To 2,3-Disubstituted Indoles Through The Palladium-Catalyzed Cyclization Of Ortho-Alkynyltrifluoroacetanilides With Vinyl Triflates And Aryl Halides. Tetrahedron Lett. 1992, 33, 3915–3917. [Google Scholar] Zhang, H. C.; Ye, H.; Moretto, A. F.; Brumfield, K. K.; Maryanoff, B. E. Facile solid-phase construction of indole derivatives based on a traceless, activating sulfonyl linker. Org. Lett. 2000, 2, 89–90. [Google Scholar] Fagnola, M. C.; Candiani, I.; Visentin, G.; Cabri, W.; Zarini, F.; Mongelli, N.; Bedeschi, A. Solid-phase synthesis of indoles using the palladium-catalysed coupling of alkynes with iodoaniline derivatives. Tetrahedron Lett. 1997, 38, 2307–2309. [Google Scholar] Zhang, H. C.; Ye, H.; White, K. B.; Maryanoff, B. E. Efficient synthesis of 3-substituted 2-arylindoles via Suzuki coupling reactions on the solid phase. Tetrahedron Lett. 2001, 42, 4751–4753. [Google Scholar] Dai, W. M.; Guo, D. S.; Sun, L. P. Chemistry of aminophenols. Part 1: Remarkable additive effect on Sonogashira cross-coupling of 2-carboxamidoaryl triflates and application to novel synthesis of indoles. Tetrahedron Lett. 2001, 42, 5275–5277. [Google Scholar] Dai, W. M.; Sun, L. P.; Guo, D. S. Chemistry of aminophenols. Part 2: A general and efficient synthesis of indoles possessing a nitrogen substituent at the C4, C5, C6, and C7 positions. Tetrahedron Lett. 2002, 43, 7699–7701. [Google Scholar]
- Larock, R. C.; Yum, E. K. Synthesis Of Indoles Via Palladium-Catalyzed Heteroannulation Of Internal Alkynes. J. Am. Chem. Soc. 1991, 113, 6689–6690. [Google Scholar] Larock, R. C.; Yum, E. K.; Refvik, M. D. Synthesis of 2,3-disubstituted indoles via palladium-catalyzed annulation of internal alkynes. J. Org. Chem. 1998, 63, 7652–7662. [Google Scholar] Roesch, K. R.; Larock, R. C. Synthesis of isoindolo[2,1-a]indoles by the palladium-catalyzed annulation of internal alkynes. Org. Lett. 1999, 1, 1551–1553. [Google Scholar] Jeschke, T.; Wensbo, D.; Annby, U.; Gronowitz, S. A Novel-Approach To Bz-Substituted Tryptophans Via Pd-Catalyzed Coupling Annulation. Tetrahedron Lett. 1993, 34, 6471–4673. [Google Scholar] Chen, C. Y.; Lieberman, D. R.; Larsen, R. D.; Reamer, R. A.; Verhoven, T. R.; Reider, P. J.; Cottrell, I. F.; Houghton, P. G. Synthesis Of The 5-Ht1d Receptor Agonist Mk-0462 Via A Pd-Catalyzed Coupling Reaction. Tetrahedron Lett. 1994, 35, 6981–6983. [Google Scholar] Zhang, H.-C.; Brumfield, K. K.; Maryanoff, B. E. Synthesis of trisubstituted indoles on the solid phase via palladium-mediated heteroannulation of internal alkynes. Tetrahedron Lett. 1997, 38, 2439–2441. [Google Scholar]
- Kondo, Y.; Kojima, S.; Sakamoto, T. General and facile synthesis of indoles with oxygen-bearing substituents at the benzene moiety. J. Org. Chem. 1997, 62, 6507–6513. [Google Scholar] [CrossRef] Rodriguez, A. L.; Koradin, C.; Dohle, W.; Konchel, P. Versatile indole synthesis by a 5-endo-dig cyclization mediated by potassium or cesium bases. Angew. Chem. Int. Ed. 2000, 39, 2488–2494. [Google Scholar]
- Saulnier, M. G.; Fennesson, D. B.; Deshpande, M. S.; Vyas, D. M. Synthesis Of A Rebeccamycin-Related Indolo[2,3-A]Carbazole By Palladium(0) Catalyzed Polyannulation. Tetrahedron Lett. 1995, 36, 7841–7843. [Google Scholar] Hiroya, K.; Itoh, S.; Ozawa, M.; Kanamori, Y.; Sakamoto, T. Efficient construction of indole rings from 2-ethynylaniline derivatives catalyzed by copper(II) salts and its application to the tandem cyclization reactions. Tetrahedron Lett. 2002, 43, 1277–1279. [Google Scholar] Roshchin, A. I.; Bumagin, N. A. Synthesis of 2-phenylindole N-derivatives under conditions of catalysis by palladium complexes. Khim. Geterotsikl. Soedin. 1999, 2, 194–198. (in Russian). [Google Scholar] Sakai, N.; Annaka, K.; Konakahara, T. Palladium-Catalyzed Coupling Reaction of Terminal Alkynes with Aryl Iodides in the Presence of Indium Tribromide and Its Application to a One-Pot Synthesis of 2-Phenylindole. Org. Lett. 2004, 4, 1527–1530. [Google Scholar]
- Larock, R. C. Palladium-catalyzed annulation. J. Organomet. Chem. 1999, 576, 111–1124. [Google Scholar] Larock, R. C. Palladium-catalyzed annulation. Pure Appl. Chem. 1999, 71, 1435–1442. [Google Scholar]
- Heravi, M. M.; Bakavoli, M. Synthesis of a novel heterocyclic system, thiazolo[3,2-d][1,2,4]triazine. J. Chem. Res. 1995, 480–481. [Google Scholar] Heravi, M. M.; Aghapoor, K.; Nooshabadi, M. A.; Mojtahedi, M. G. Regioselective annelation of 3-(prop-2-ynylsulfanyl)-1,2,4-benzotriazine to thiazolo[2,3-c][1,2,4]benzotriazine. Monatsh. Chem. 1997, 128, 1143–1146. [Google Scholar] Heravi, M. M.; Keivanloo, A.; Rahimizadeh, M.; Bakavoli, M.; Ghassemzadeh, M. Heteroannulation through palladium catalysis: A novel cyclization leading to regioselective synthesis of 3-substituted thiazolo[3,2-C]1,2,4-triazin-5-ones. Phosphorus, Sulfur Silicon Relat. Elem. 2002, 177, 2491–2496. [Google Scholar] Heravi, M. M.; Keivanloo, A.; Rahimizadeh, M.; Bakavoli, M.; Ghassemzadeh, M. Pd-Cu catalyzed heterocyclization during Sonogashira coupling: synthesis of 3-benzylthiazolo[3,2-a]benzimidazole. Tetrahedron Lett. 2004, 45, 5747–5749. [Google Scholar] Heravi, M. M.; Keivanloo, A.; Rahimizadeh, M.; Bakavoli, M.; Ghassemzadeh, M. Regioselective synthesis of 6-benzylthiazolo[3,2-b]1,2,4-triazoles during Sonogashira coupling. Tetrahedron Lett. 2005, 46, 1607–1610. [Google Scholar]
- Hong, K. B.; Lee, W. C.; Yum, E. K. Synthesis of 2-substituted indoles by palladium-catalyzed heteroannulation with Pd-NaY zeolite catalysts. Tetrahedron Lett. 2004, 45, 693–694. [Google Scholar]
- Heck, R. F. Palladium Reagents in Organic Synthesis; Academic Press: London, 1985. [Google Scholar] Chen, M.-Y.; Fang, J.-M. J. Chin. Chem. Soc. 1989, 36, 469–472. Bernstein, R. B. Chemical Dynamics via Molecular Beam and Laser Techniques; Oxford: New York, 1982; Chapter 9. [Google Scholar] Talley, L. D.; Lin, M. C. Aspects of the Kinetics and Dynamics of Surface Reactions; Landman, U., Ed.; AIP Conf. Proceed., American Institute of Physics: New York, 1980; pp. 297–306. [Google Scholar] Huang, C.-M. M. S. Thesis, Fu Jen Catholic University, Taiwan, 1987.
- Moore, J. L.; Stephen, M.; Taylor, S. M.; Soloshonok, V. A. An efficient and operationally convenient general synthesis of tertiary amines by direct alkylation of secondary amines with alkyl halides in the presence of Huenig’s base. Arkivoc 2005, (vi), 287–292. [Google Scholar]
- Shriner, R. L.; Fuson, R. C.; Curtin, D. Y.; Morrill, T. S. Systematic Identification of Organic Compounds, 6th edn; Wiley: New York, 1980. [Google Scholar]
- Sample Availability: Available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Oskooie, H.A.; Heravi, M.M.; Behbahani, F.K. A Facile, Mild And Efficient One-Pot Synthesis of 2-Substituted Indole Derivatives Catalyzed By Pd(PPh3)2Cl2. Molecules 2007, 12, 1438-1446. https://doi.org/10.3390/12071438
Oskooie HA, Heravi MM, Behbahani FK. A Facile, Mild And Efficient One-Pot Synthesis of 2-Substituted Indole Derivatives Catalyzed By Pd(PPh3)2Cl2. Molecules. 2007; 12(7):1438-1446. https://doi.org/10.3390/12071438
Chicago/Turabian StyleOskooie, Hossien A., Majid M. Heravi, and Farahnaz K. Behbahani. 2007. "A Facile, Mild And Efficient One-Pot Synthesis of 2-Substituted Indole Derivatives Catalyzed By Pd(PPh3)2Cl2" Molecules 12, no. 7: 1438-1446. https://doi.org/10.3390/12071438




