Characterization of Phenolic Compounds in Pinus laricio Needles and Their Responses to Prescribed Burnings
Abstract
:Introduction
Results and Discussion
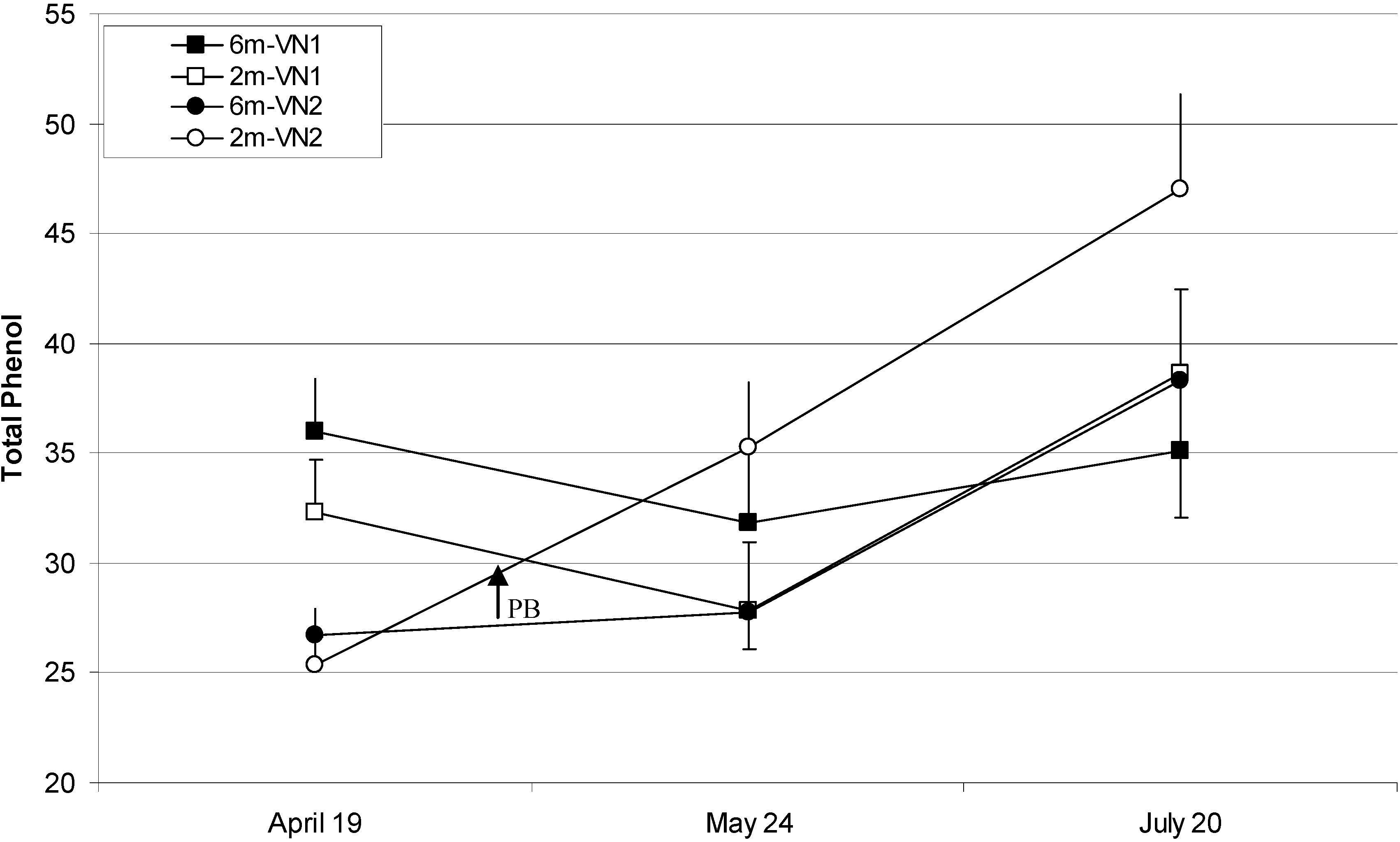
Total Phenolic Compounds
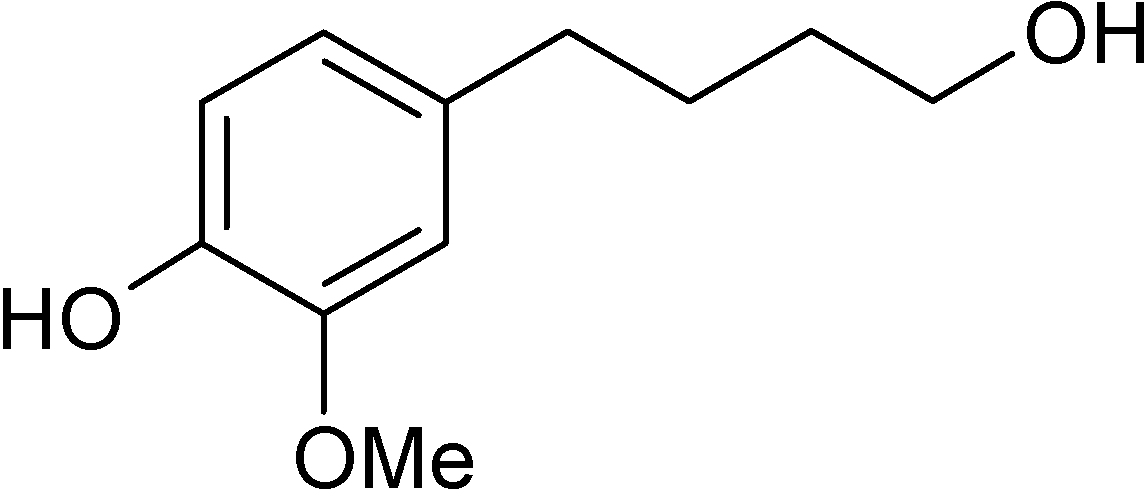
Simple phenolic compounds
| RI | Common Name of phenolic compounds | CAS N° | OSP1 | OSP2 | OSP3 | OSP4 |
|---|---|---|---|---|---|---|
| 1232 | Phenylethyl alcohol | 60-12-8 | 116.8 ± 43.0 | 130.8 ± 79.7 | 169.6 ± 40.8 | 63.6 ± 8.0 |
| 1251 | Benzoic acid | 65-85-0 | 70.5 ± 12.0 | 73.9 ± 20.2 | 158.5 ± 40.3 | 112.0 ± 49.6 |
| 1303 | Benzeneacetic acid | 103-82-2 | 112.4 ± 50.2 | 57.1 ± 19.7 | 63.7 ± 24.3 | 55.6 ± 8.0 |
| 1479 | Eugenol | 97-53-0 | 7.9 ± 4.9 | 7.6 ± 3.7 | 21.3 ± 5.1 | 24.2 ± 10.1 |
| 1522 | Salicylic acid | 69-72-7 | 9.7 ± 2.4 (a) | 6.6 ± 1.5 (a,b) | 3.0 ± 3.0 (a,b) | 1.8 ± 1.8 (b) |
| 1542 | Vanillin | 121-33-5 | 65.2 ± 9.7 (a,b) | 63.7 ± 7.5 (a,b) | 94.0 ± 3.0 (a) | 60.1 ± 15.8 (b) |
| 1692 | Dihydro p-coumaryl alcohol | 10210-17-0 | 24.2 ± 2.2 (a,b) | 31.8 ± 1.8 (a,b) | 59.2 ± 28.2 (a) | 15.6 ± 3.6 (b) |
| 1764 | Vanillylacetone | 122-48-5 | 141.7 ± 31.8 | 145.4 ± 18.3 | 132.5 ± 11.0 | 95.3 ± 13.4 |
| 1778 | Vanillic acid | 121-34-6 | 166.8 ± 32.0 (a,b) | 198.4 ± 31.6 (a) | 164.5± 5.0 (a,b) | 108.5 ± 1.3 (b) |
| 1833 | 3-Vanillyl propanol | 3579-91-7 | 140.1 ± 160.6 | 550.8 ± 188.3 | 568.3 ± 100.7 | 317.0 ± 31.4 |
| 1861 | Dihydroferulic acid | 1135-23-5 | 9.4 ± 3.3 | 7.8 ± 3.0 | 14.9 ± 2.3 | 8.0 ± 2.7 |
| 1909 | Syringic acid | 530-57-4 | 26.4 ± 15.6 | 30.0 ± 17.5 | 5.9 ± 5.9 | 24.6 ± 12.8 |
| 1937 | Ferulic acid | 1135-24-6 | 56.4 ± 12.6 | 71.6 ± 1.9 | 79.8 ± 6.4 | 53.8 ± 10.9 |
| 1951 | p-Coumaric acid | 7400-08-0 | 40.1 ± 16.9 | 53.5 ± 8.6 | 55.8 ± 15.1 | 28.5 ± 5.2 |
| 2075 | Isoferulic acid | 537-73-5 | 95.6 ± 19.0 (a) | 167.1 ± 24.9 (b) | 169.8 ± 25.6 (b) | 96.2 ± 19.1 (a,b) |
Experimental Section
General
| Forest | Valdu-Niellu | Ospedale |
|---|---|---|
| Geographical coordinates | 42°17’03’’N 8°55’22’’E | 41°41’37’’N 9°12’36’’E |
| Elevation (m) | 1070 | 950 |
| Slope (%) | 5 to 10 | 5 to 10 |
| Substrate | Granitic | Granitic |
| Station type | Mesophylous | Mesophylous |
| Vegetation cover | 60% | 40% |
| Age stand (years) | 13 | 22 |
| Dbh (cm) | 13 | 13 |
| Total height (m) | 6.6 | 6.7 |
| Basal area (m²/ha) | 21.3 | 14.1 |
| Forest | Valdu-Niellu (VN) | Ospedale (OSP) | ||||
| Stations | VN1 | VN2 | OSP1 | OSP2 | OSP3 | OSP4 |
| Surface | 0.1 ha | 0.1 ha | 0.05 ha | 0.05 ha | 0.05 ha | 0.05 ha |
| Burning | No | April 24, 2006 | April 27, 2003 | April 27, 2003 April 4, 2006 | No | April 4, 2006 |
Total Phenolic Compounds
Simple Phenolic Compounds
Statistical Analysis
Acknowledgements
References
- Harborne, J.B. Secondary plant products. Encyclopaedia of Plant Physiology; Bell, E.A., Charlwood, B.V., Eds.; Springer-Verlag: Berlin-Heidelberg, 1980; vol. 8, pp. 329–402. [Google Scholar]
- Giertych, M.J.; Karolewski, P. Changes in phenolic compounds content in needles of Scots pine (Pinus sylvestris L.) seedlings following short term exposition to sulphur dioxide. Arbor. Korn. Rocz. 1993, 38, 43–51. [Google Scholar]
- Penuelas, J.; Estiarte, M.; Kimball, B.A.; Idso, S.B.; Pinter, P.J.; Wall, G.W.; Garcia, R.L.; Hansaker, D.J.; Lamortz, R.L.; Hendrix, D.L. Variety of responses of plant phenolic concentration to CO2 enrichment. J. Exp. Bot. 1996, 47, 1463–1467. [Google Scholar] [CrossRef]
- Loponen, J.; Lempa, K.; Ossipov, V.; Kozlov, M.V.; Girs, A.; Hangasmaa, K.; Haukioja, E.; Pihlaja, K. Patterns in content of phenolic compounds in leaves of mountain birches along a strong pollution gradient. Chemosphere 2001, 45, 291–301. [Google Scholar] [CrossRef]
- Ferrat, L.; Pergent-Martini, C.; Romeo, M. Assessment of the use of biomarkers in aquatic plants for the evaluation of environmental quality: application to seagrass. Aquat. Toxicol. 2003, 65, 187–204. [Google Scholar] [CrossRef]
- Pasqualini, V.; Robles, C.; Garzino, S.; Greff, S.; Bousquet-Melou, A.; Bonin, G. Phenolic compounds content in Pinus halepensis Mill. needles: a bioindicator of air pollution. Chemosphere 2003, 52, 239–248. [Google Scholar] [CrossRef]
- Robles, C.; Greff, S.; Pasqualini, V.; Garzino, S.; Bousquet-Melou, A.; Fernandez, C.; Korboulewski, N.; Bonin, G. Phenols and flavonoids in Pinus halepensis Mill. needles as bioindicators of air pollution. J. Environ. Qual. 2003, 32, 2265–2271. [Google Scholar] [CrossRef]
- Chalker-Scott, L.; Fuchigami, L.H. Low temperature stress physiology in crops; Li, P.H., Ed.; CRC Press: Florida, 1989; pp. 67–79. [Google Scholar]
- Alonso, M.; Rozados, M.J.; Vega, J.A.; Perez-Gorostiaga, P.; Cuinas, P.; Fonturbel, M.T.; Fernandez, C. Biochemical responses of Pinus pinaster trees to fire-induced trunk girdling and crown scorch: secondary metabolites and pigments as needle chemical indicators. J. Chem. Ecol. 2002, 28, 687–700. [Google Scholar] [CrossRef]
- Dela, G.; Etti, O.; Rinat, O.; Nissim-Levi, A.; Weiss, D.; Oren-Shamir, M. Changes in anthocyanidin concentration and composition in “Jaguar” rose flowers due to transient high-temperature conditions. Plant Sci. 2003, 164, 333–340. [Google Scholar] [CrossRef]
- Nacif de Abreu, I.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Bioch. 2005, 43, 241–248. [Google Scholar]
- Quézel, P.; Médail, F. Ecologie et biogéographie des forêts du bassin méditerranéen; Elsevier, Ed.; Lavoisier Publ.: Paris, 2003. [Google Scholar]
- Fernandes, P.M.; Rigolot, E. The fire ecology and management of maritime pine (Pinus pinaster Ait.). For. Ecol. Manag. 2007, 241, 1–13. [Google Scholar]
- Weber, M.G.; Taylor, S.W. The use of prescribed fire in the management of Canada's forested lands. For. Chron. 1992, 68, 324–334. [Google Scholar] [CrossRef]
- Graham, R.T.; McCaffrey, S.; Jain, T.B. Science basis for changing forest structure to modify wildfire behavior and severity. In Gen. Tech. Rep. RMRS-120; Rocky Mountain Research Station, USDA Forest Service, 2004. [Google Scholar]
- Gamisans, J.; Marzocchi, J.F. La Flore Endémique de la Corse; Edisud Ed.: Aix-en-Provence, France, 1996. [Google Scholar]
- Rezzi, S.; Bighelli, A.; Castola, V.; Casanova, J. Composition and chemical variability of the oleoresin of Pinus nigra ssp. laricio from corsicana. Ind. Crop. Prod. 2005, 21, 71–79. [Google Scholar]
- Cannac, M.; Ferrat, L.; Morandini, F.; Chiaramonti, N.; Santoni, P.A.; Pasqualini, V. Bioindicators for the short-term response of Pinus laricio needles to thermal pruning. In 4th International Wildland Fire Conference, Seville, Spain, 13–17 May 2007.
- Musika, R.M. Terpenes and phenolics in response to nitrogen fertilization: a test of the carbon/nutrient balance hypothesis. Chemoecology 1993, 4, 3–7. [Google Scholar] [CrossRef]
- Rama, R.S.; Pellisier, F.; Prasat, M.N.V. Plant Physiology; Prasat, M.N.V., Ed.; John Wiley and Sons: New York, 1997; pp. 253–303. [Google Scholar]
- Bryant, J.P.; Chapin, F.S.; Klein, D.R. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Coley, P.D.; Bryant, J.P.; Chapin, F.S., III. Resource availability and plant antiherbivore defense. Science 1985, 230, 895–899. [Google Scholar]
- Monleon, V.J.; Chromack, K.; Landsberg, J.D. Short- and long-term effects of prescribed underburning on nitrogen availability in ponderosa pine stands in central Oregon. Can. J. For. Res. 1997, 27, 369–378. [Google Scholar] [CrossRef]
- Rösler, J.; Krefel, F.; Amrhein, N.; Sohmid, I. Maize phenylalanina ammonia-lyase activity. Plant Physiol. 1997, 113, 175–179. [Google Scholar]
- Rivero, R.M.; Ruiz, J.M.; Garcia, P.C.; Lopez-Lefebre, L.R.; Sanchez, E.; Romero, L. Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Roitto, M.; Markkola, A.; Julkunen-Tiitto, R.; Sarjala, T.; Rautio, P.; Kuikka, K.; Tuomi, J. Defoliation-induced responses in peroxidases, phenolics, and polyamines in scots pine (Pinus sylvestris L.) needles. J. Chem. Ecol. 2003, 29, 1905–1918. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Cannac, M.C.; Pasqualini, V.; Greff, S.; Fernandez, C.; Ferrat, L. Characterization of Phenolic Compounds in Pinus laricio Needles and Their Responses to Prescribed Burnings. Molecules 2007, 12, 1614-1622. https://doi.org/10.3390/12081614
Cannac MC, Pasqualini V, Greff S, Fernandez C, Ferrat L. Characterization of Phenolic Compounds in Pinus laricio Needles and Their Responses to Prescribed Burnings. Molecules. 2007; 12(8):1614-1622. https://doi.org/10.3390/12081614
Chicago/Turabian StyleCannac, Magali Cannac, Vanina Pasqualini, Stéphane Greff, Catherine Fernandez, and Lila Ferrat. 2007. "Characterization of Phenolic Compounds in Pinus laricio Needles and Their Responses to Prescribed Burnings" Molecules 12, no. 8: 1614-1622. https://doi.org/10.3390/12081614




