Flavonoid Composition of Citrus Juices
Abstract
:Introduction
Citrus juice flavonoids
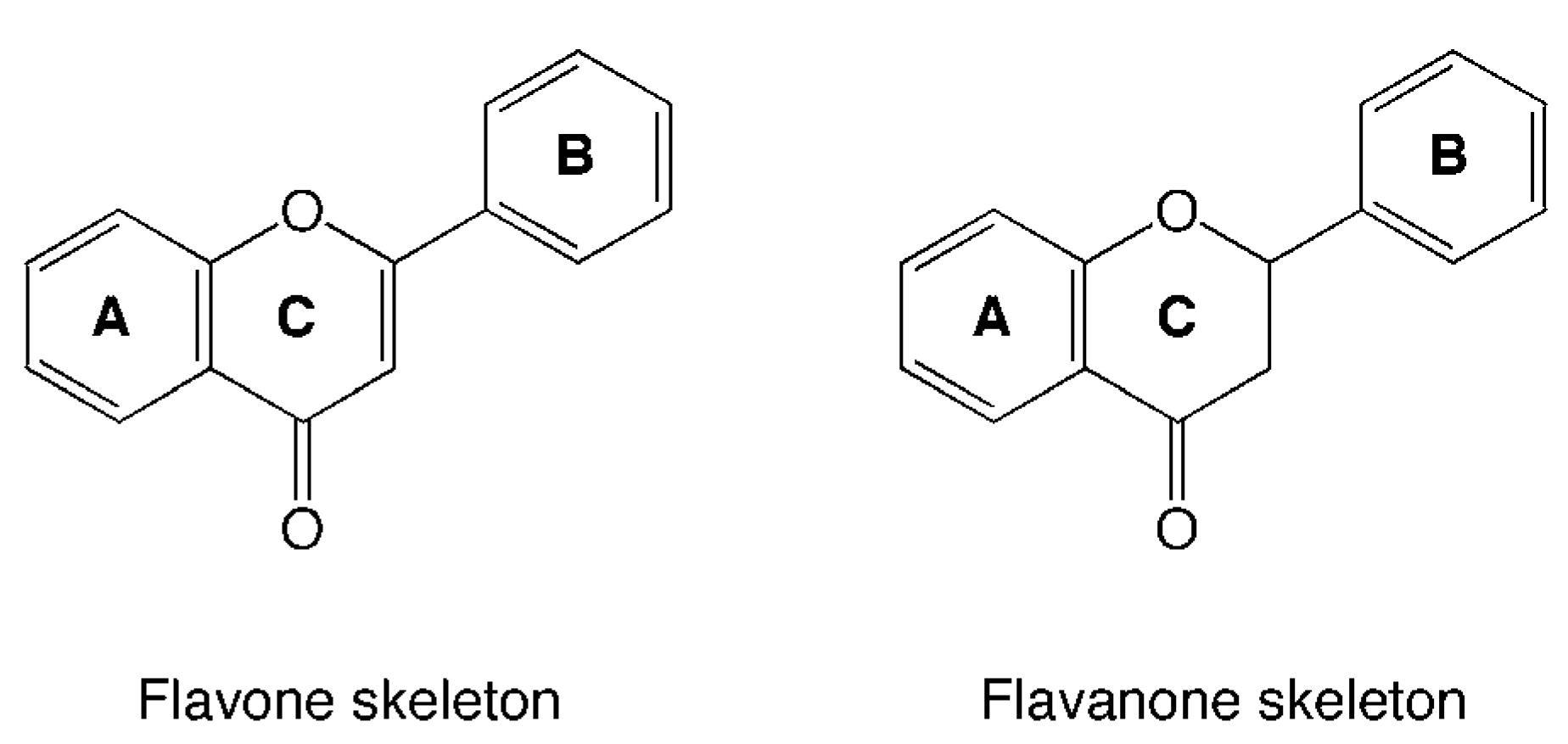

| Compound name | R1 | R2 | R3 | |
| 1 | Hesperetin | H | OH | OMe |
| 2 | Naringenin | H | H | OH |
| 3 | Taxifolin | OH | OH | OH |
| 4 | Isosakuranetin | H | H | OMe |
| 5 | Eriodictyol | H | OH | OH |

| Compound name | R1 | R2 | R3 | R4 | |
| 6 | Acacetin | H | H | H | OMe |
| 7 | Isoscutellarein | H | OH | H | OH |
| 8 | Luteolin | H | H | OH | OH |
| 9 | Kaempferol | OH | H | H | OH |
| 10 | Quercetin | OH | H | OH | OH |
| 11 | Apigenin | H | H | H | OH |
| 12 | Diosmetin | H | H | OH | OMe |
| 13 | Chrysoeriol | H | H | OMe | OH |

| Compound name | R1 | R2 | R3 | |
| 14 | Quercetogetin | OMe | H | OMe |
| 15 | 3,3’,4’,5,6,7,8-Heptamethoxyflavone | OMe | OMe | OMe |
| 16 | Natsudaidain | OH | OMe | OMe |
| 17 | Nobiletin | H | OMe | OMe |
| 18 | Sinensetin | H | H | OMe |
| 19 | Tangeretin | H | OMe | H |
| 20 | Tetramethylscutellarein | H | H | H |

| Compound name | R1 | R2 | R3 | |
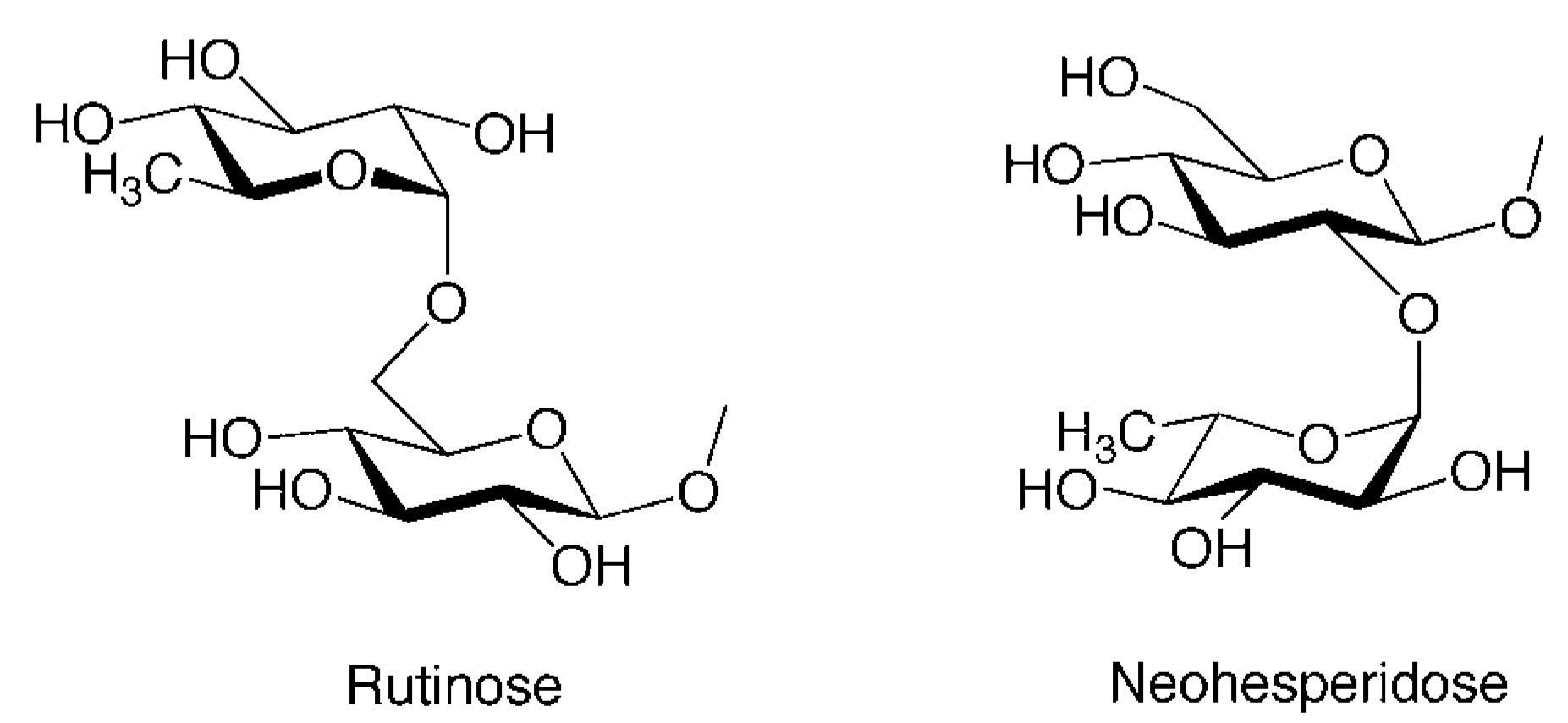
| 21 | Isosakuranetin 7-O-rutinoside (Didymin, Neoponcirin) | O-Rua | H | OMe |
| 22 | Eriodictyol 7-O-rutinoside (Eriocitrin) | O-Rua | OH | OH |
| 23 | Hesperetin 7-O-rutinoside (Hesperidin) | O-Rua | OH | OMe |
| 24 | Naringenin 7-O-neohesperidoside (Naringin) | O-Nhb | H | OH |
| 25 | Naringenin 7-O-rutinoside (Narirutin) | O-Rua | H | OH |
| 26 | Hesperetin 7-O-neohesperidoside (Neohesperidin) | O-Nhb | OH | OMe |
| 27 | Eriodictyol 7-O-neohesperidoside (Neoeriocitrin) | O-Nhb | OH | OH |
| 28 | Isosakuranetin 7-O-neohesperidoside (Poncirin) | O-Nhb | H | OMe |

| Compound name | R1 | R2 | R3 | R4 | R5 | R6 | |
| 29 | Luteolin 6,8-di-C-glucoside (Lucenin-2) | H | Glu | OH | Glu | OH | OH |
| 30 | Apigenin 6,8-di-C-glucoside (Vicenin-2) | H | Glu | OH | Glu | H | OH |
| 31 | Chrysoeriol 6,8-di-C-glucoside (Stellarin-2) | H | Glu | OH | Glu | OMe | OH |
| 32 | Diosmetin 6,8-di-C-glucoside (Lucenin-2 4´-methyl ether) | H | Glu | OH | Glu | OH | OMe |
| 33 | Apigenin 7-O-neohesperidoside-4´-glucoside (Rhoifolin 4´-glucoside) | H | H | O-Nhb | H | OH | O-Glu |
| 34 | Chrysoeriol 7-O-neohesperidoside- 4´-glucoside | H | H | O-Nhb | H | OMe | OH |
| 35 | Apigenin 6-C-glucoside (Isovitexin) | H | Glu | OH | H | H | OH |
| 36 | Luteolin 7-O-rutinoside | H | H | O-Rua | H | OH | OH |
| 37 | Chrysoeriol 8-C-glucoside (Scoparin) | H | H | OH | Glu | OMe | OH |
| 38 | Diosmetin 8-C-glucoside (Orientin 4´-methyl ether) | H | H | OH | Glu | OH | OMe |
| 39 | Quercetin 3-O-rutinoside (Rutin) | O-Rua | H | OH | H | OH | OH |
| 40 | Apigenin 7-O-neohesperidoside (Rhoifolin) | H | H | O-Nhb | H | OH | OH |
| 41 | Apigenin 7-O-rutinoside (Isorhoifolin) | H | H | O-Rua | H | OH | OH |
| 42 | Chrysoeriol 7-O-neohesperidoside | H | H | O-Nhb | H | OMe | OH |
| 43 | Diosmetin 7-O-rutinoside (Diosmin) | H | H | O-Rua | H | OH | OMe |
| 44 | Diosmetin 7-O-neohesperidoside (Neodiosmin) | H | H | O-Nhb | H | OH | OMe |
Analytical Methods
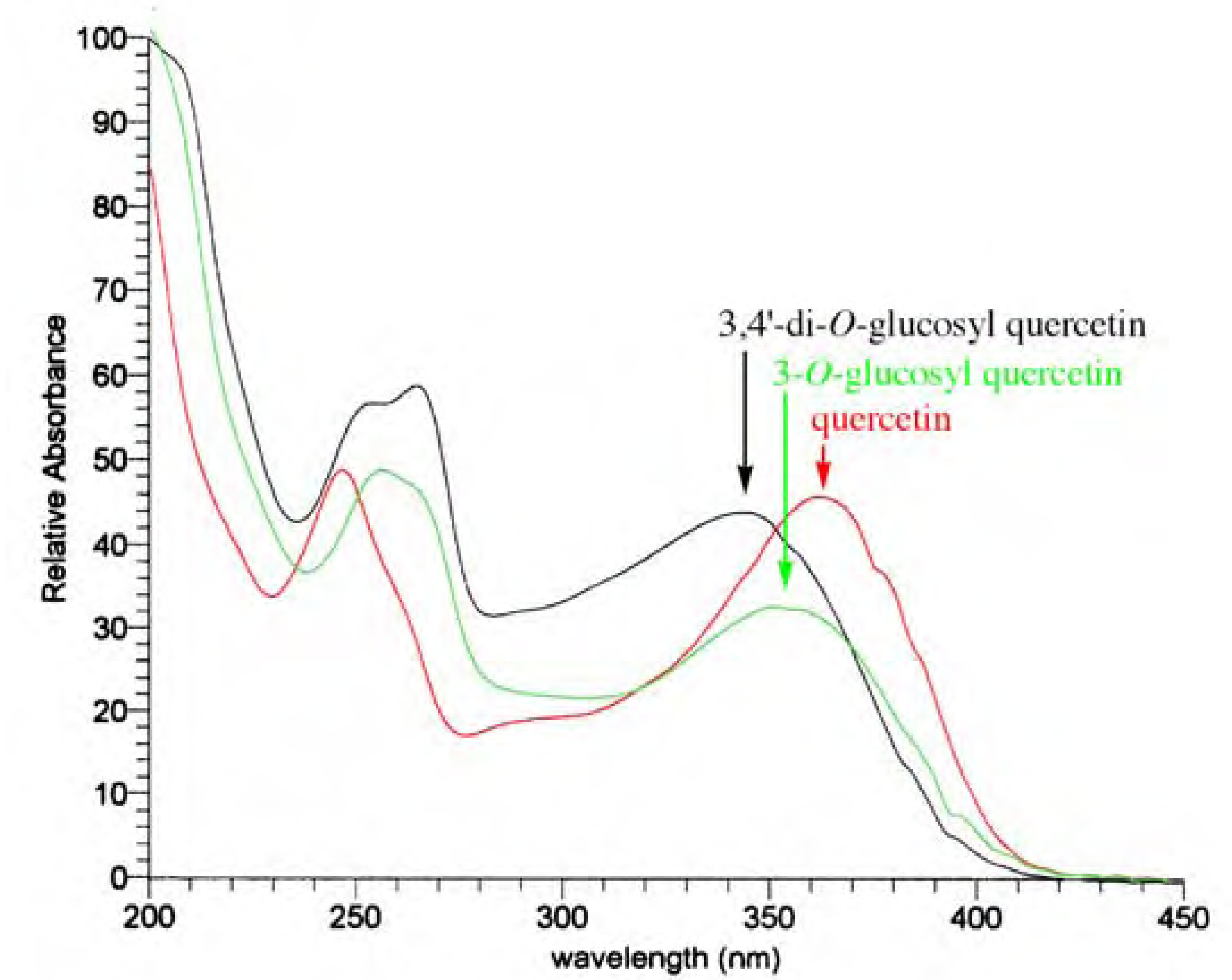
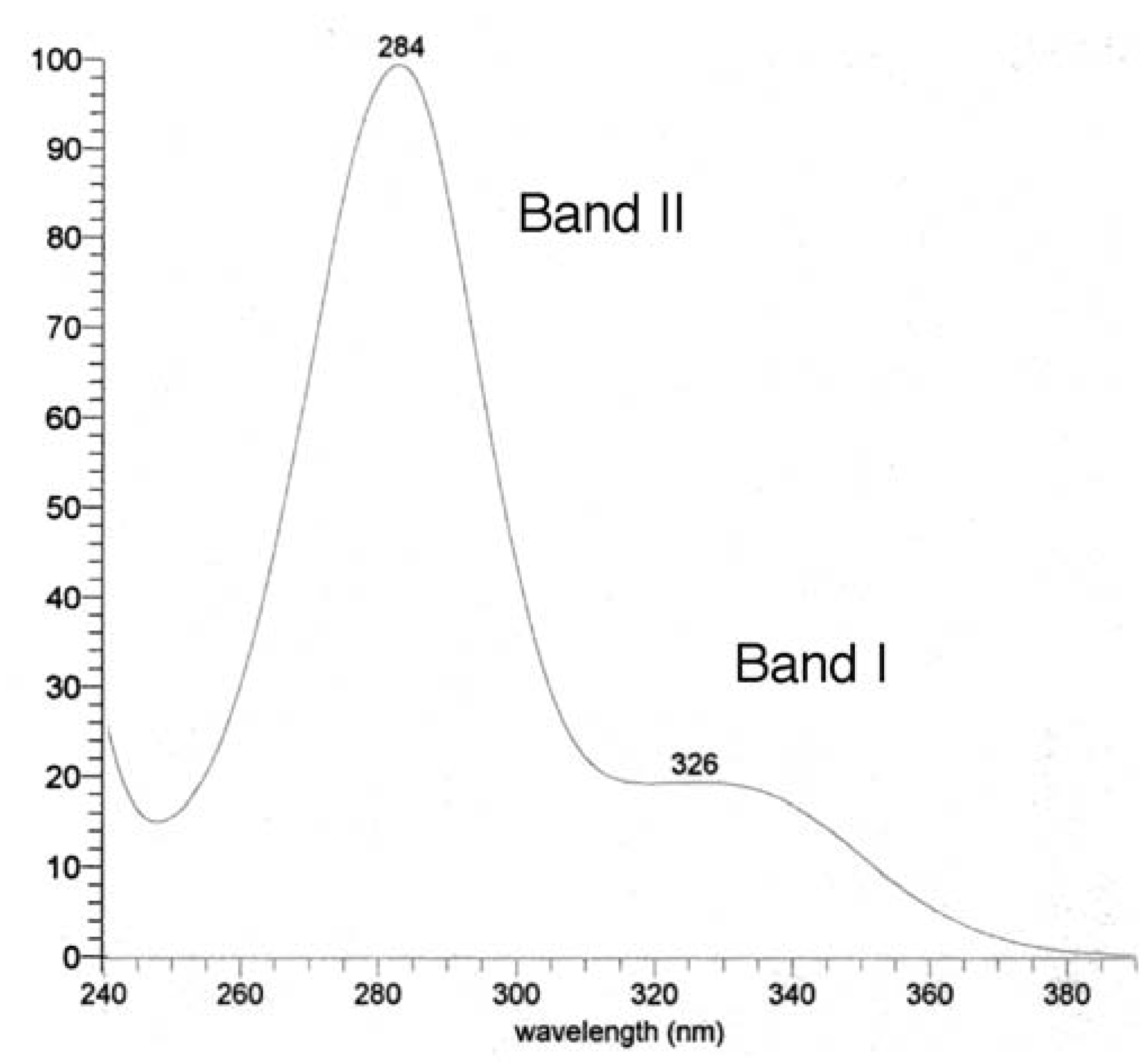
UV, MS, and NMR spectra of Citrus flavonoids
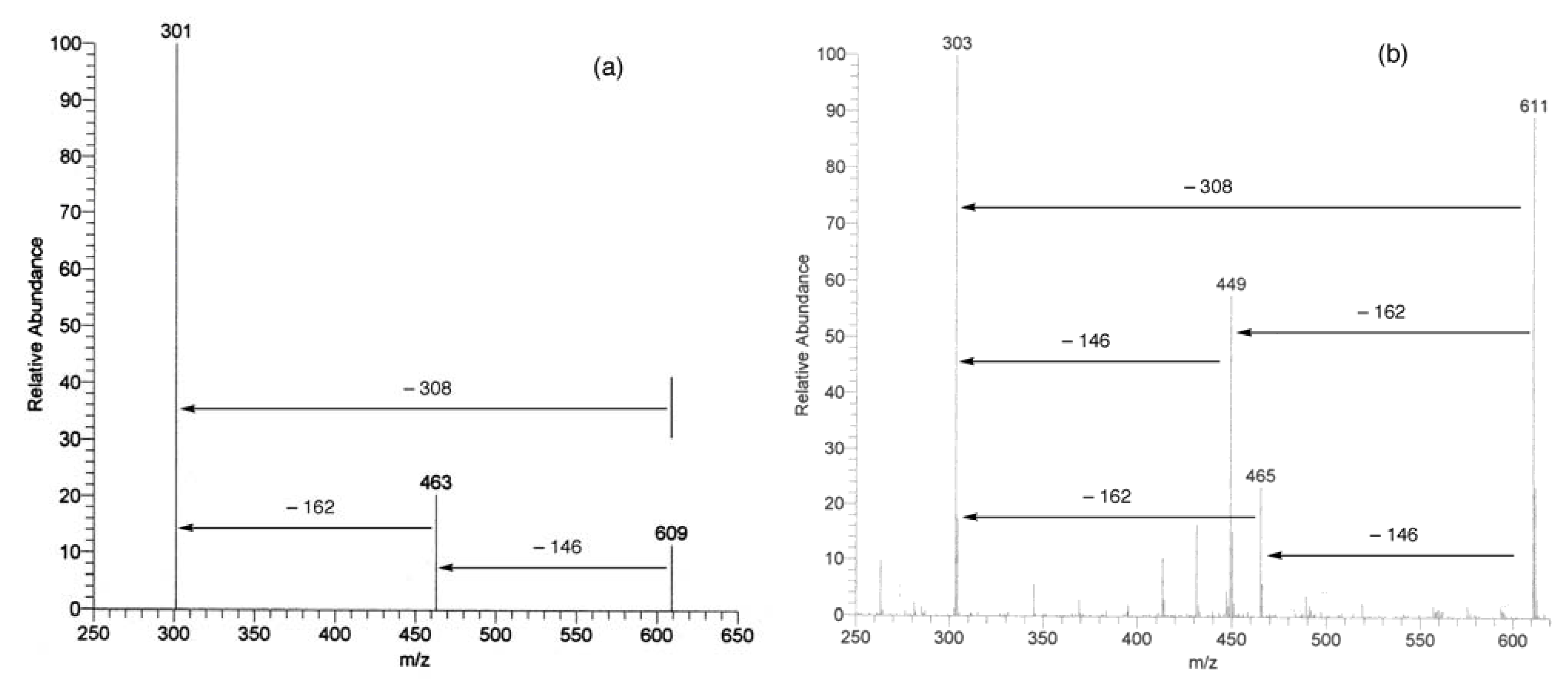
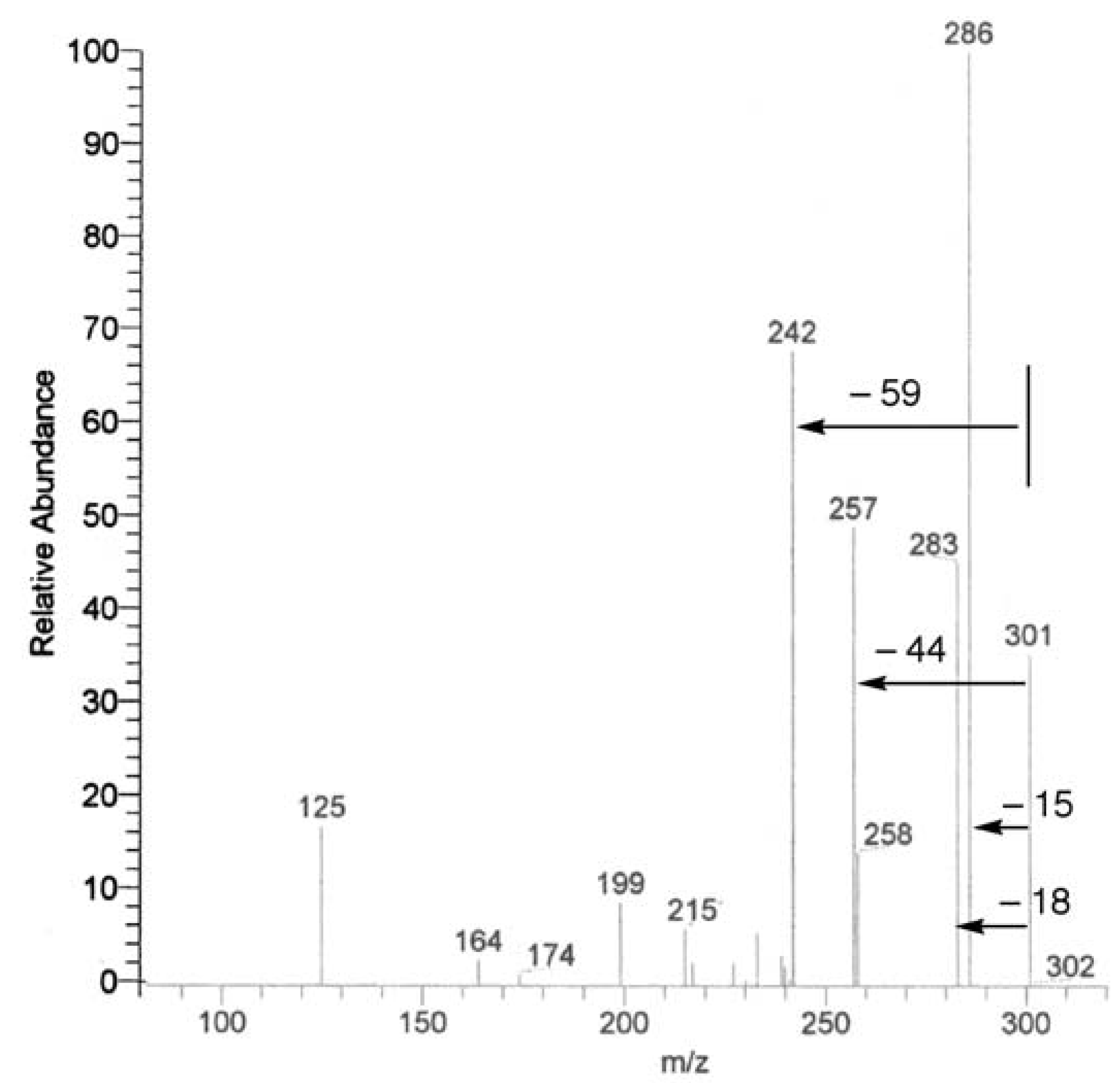
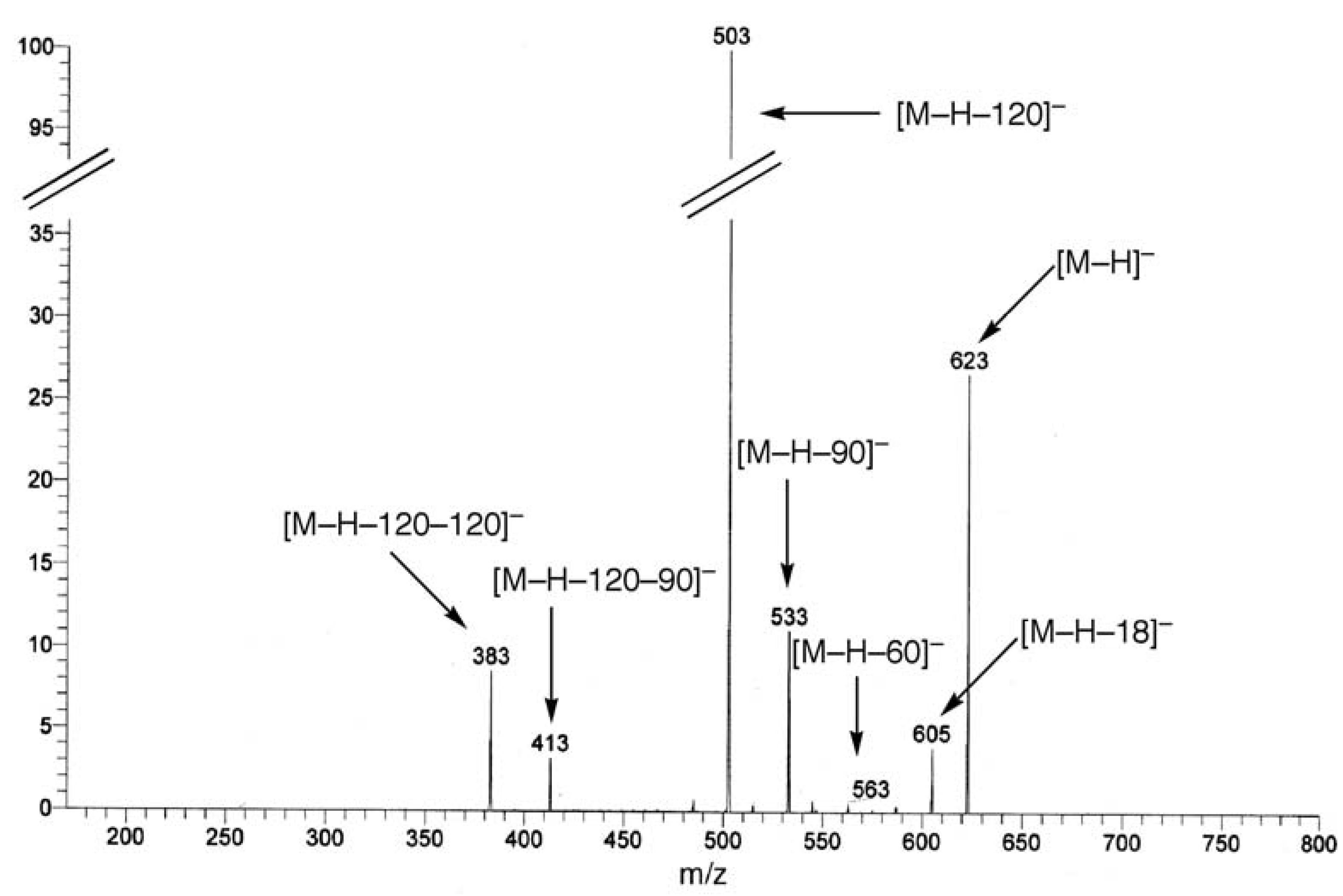
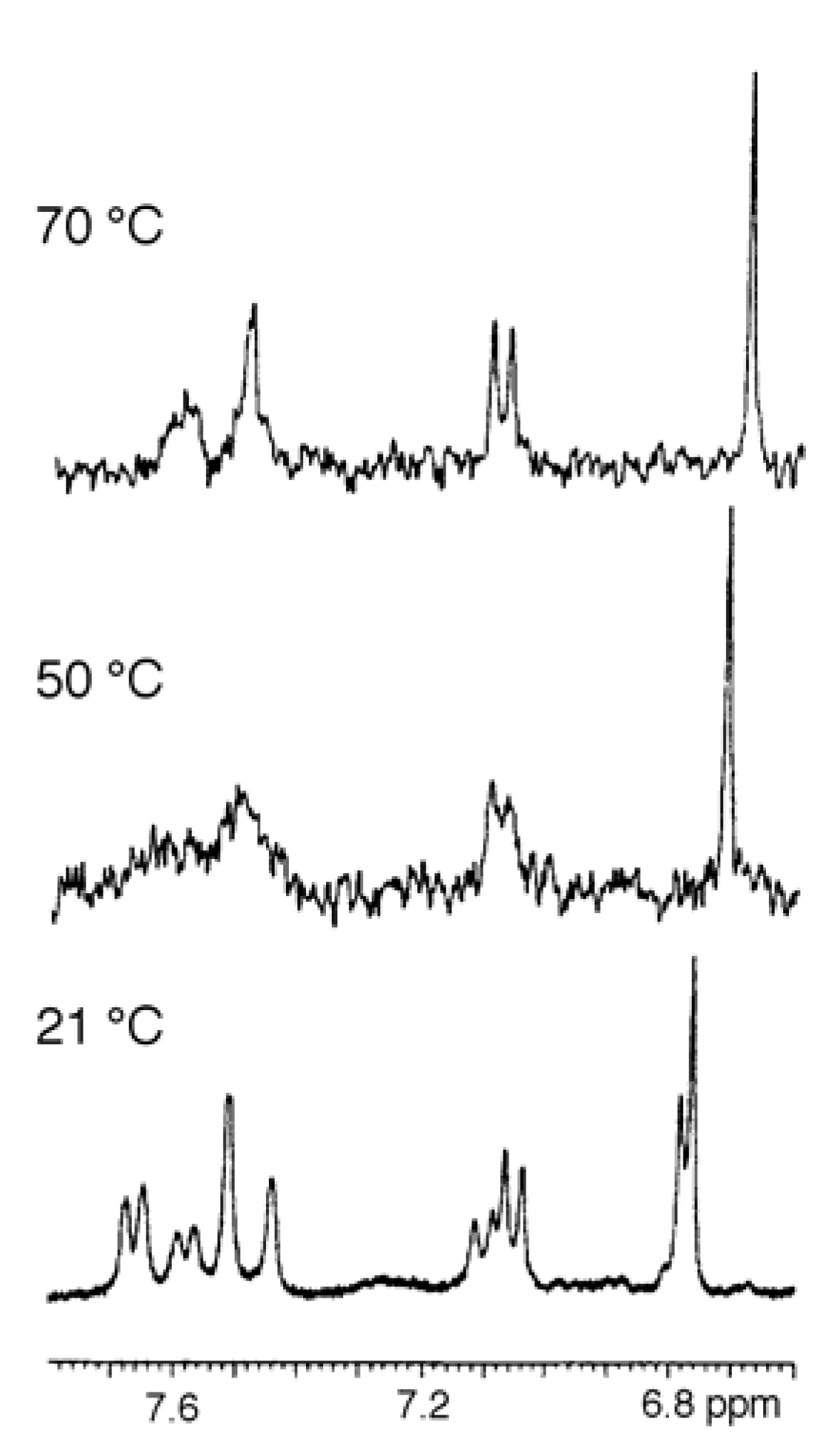
Sample preparation
Analytical approach to flavonoids determination
Gas chromatography (GC)
High Performance Liquid Chromatography (HPLC)
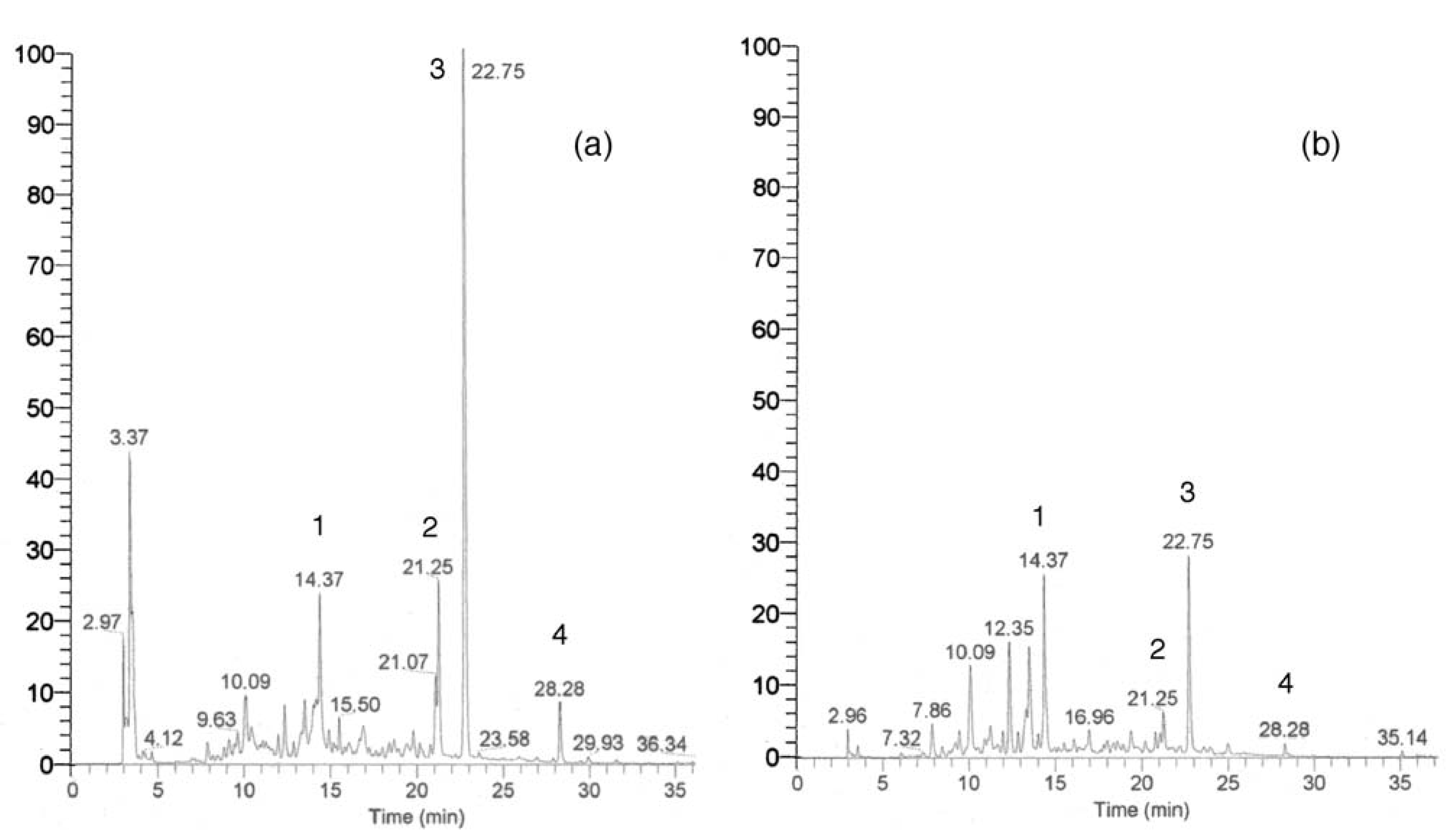
Other chromatographic and miscellaneous methods
Coupled methods
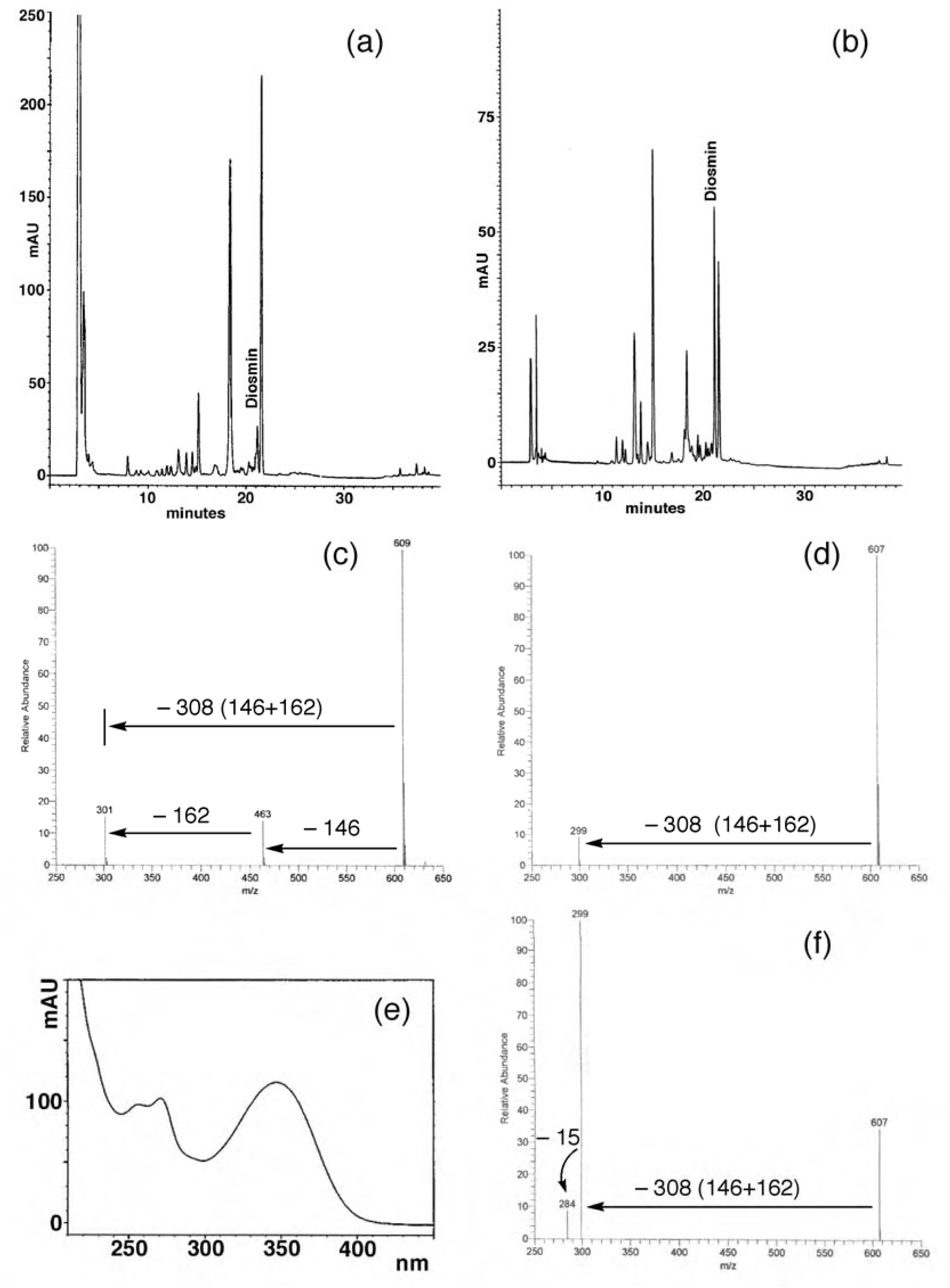
Flavonoid composition of juices
Orange
| Mean | SD | Median | MIN | MAX | N | Ref. | ||
| Flavanones | ||||||||
| Didymin | 21 | 1.89 | 0.92 | 1.60 | 0.80 | 3.10 | 7a | [71a] |
| Eriocitrin | 22 | 0.31 | 0.18 | 0.29 | 0.11 | 0.67 | 7a | [71a] |
| Hesperidin | 23 | 28.6 | 11.9 | 28.0 | 3.51 | 55.2 | 44b | [71] |
| Narirutin | 25 | 5.2 | 3.1 | 4.2 | 0.55 | 14.2 | 44b | [71] |
| Flavones | ||||||||
| Neoeriocitrin | 27 | 0.59 | – | – | – | – | 1c | [71d] |
| Poncirin | 28 | 1.04 | 0.78 | 1.04 | 0.49 | 1.59 | 2d | [71d] |
| 6,8-di-C-Glu-Apigenin | 30 | 5.72 | 2.02 | 5.00 | 4.15 | 8 | 3e | [38a,71e] |
| 6,8-di-C-Glu-Diosmetin | 32 | 0.35 | 0.14 | 0.35 | 0.25 | 0.45 | 2f | [38a] |
| Rhoifolin | 40 | 0.05 | – | – | – | – | 1c | [71d] |
| Isorhoifolin | 41 | 0.07 | – | – | – | – | 1c | [71d] |
| Diosmin | 43 | 0.09 | – | – | – | – | 1g | [71d] |
| Neodiosmin | 44 | 0.08 | – | – | – | – | 1g | [71d] |
| Polymethoxyflavones | ||||||||
| Heptamethoxyflavone | 15 | 0.08 | 0.06 | 0.08 | 0.04 | 0.12 | 2d | [71d,f] |
| Nobiletin | 17 | 0.33 | 0.19 | 0.33 | 0.19 | 0.46 | 2d | [71d,f] |
| Sinensetin | 18 | 0.37 | – | – | – | – | 1g | [71d,f] |
| Tangeretin | 19 | 0.04 | 0.04 | 0.04 | 0.01 | 0.07 | 2d | [71d,f] |
| Aglycones | ||||||||
| Taxifolin | 3 | 0.03 | – | – | – | – | 1c | [71d] |
| Acacetin | 6 | 0.03 | – | – | – | – | 1c | [71d] |
| Mean | SD | Median | MIN | MAX | N | Ref. | ||
| Flavanones | ||||||||
| Didymin | 21 | 1.89 | 0.56 | 1.78 | 1.14 | 3.53 | 32 | [30, 74b,d] |
| Hesperidin | 23 | 37.5 | 19.2 | 39.1 | 4.45 | 76.3 | 63 | [30, 47b, 49, 57b, 74b-f] |
| Naringin | 24 | 2.13 | 3.01 | 0.34 | 0.20 | 7.54 | 9 | [49, 74a,c,e] |
| Narirutin | 25 | 5.9 | 1.6 | 6.3 | 2.95 | 9.78 | 46 | [30, 47b, 49, 57b, 74b-d] |
| Neohesperidin | 26 | 0.95 | 0.32 | 1.10 | 0.53 | 1.29 | 5 | [74e] |
| Flavones | ||||||||
| 6,8-di-C-Glu-Apigenin | 30 | 4.16 | 1.37 | 3.95 | 2.78 | 8.00 | 13 | [71e] |
| Diosmin | 43 | 3.46 | 1.92 | 2.60 | 0.79 | 7.20 | 11 | [74c] |
| Polymethoxyflavones | ||||||||
| Quercetogetin | 14 | 0.04 | 0.01 | 0.04 | 0.03 | 0.05 | 4 | [30, 74d] |
| Heptamethoxyflavone | 15 | 0.05 | 0.03 | 0.05 | 0.03 | 0.07 | 2 | [30] |
| Nobiletin | 17 | 0.26 | 0.07 | 0.26 | 0.18 | 0.34 | 4 | [30, 74d] |
| Sinensetin | 18 | 0.24 | 0.09 | 0.22 | 0.17 | 0.36 | 4 | [30, 74d] |
| Tangeretin | 19 | 0.04 | 0.02 | 0.05 | 0.02 | 0.06 | 4 | [30, 74d] |
| Aglycones | ||||||||
| Naringenin | 2 | 0.8 | – | – | – | – | 1 | [74f] |
| Isoscutellarein | 7 | 0.05 | 0.04 | 0.05 | 0.02 | 0.08 | 2 | [30] |
| Mean | SD | Median | MIN | MAX | N | Ref. | ||
| Flavanones | ||||||||
| Didymin | 21 | 1.44 | 1.31 | 1.60 | 0.05 | 3.10 | 7a | [71a,d, 74d] |
| Eriocitrin | 22 | 0.31 | 0.18 | 0.31 | 0.09 | 0.59 | 5a | [71a,d, 74d] |
| Hesperidin | 23 | 24.3 | 18.2 | 28 | 0.81 | 45.8 | 7a | [71a,d, 74d] |
| Narirutin | 25 | 3.92 | 3.10 | 4.80 | 0.12 | 9.00 | 7a | [71a,d, 74d] |
| Neoeriocitrin | 27 | 0.05 | 0.01 | 0.05 | 0.04 | 0.06 | 2b | [71d] |
| Polymethoxyflavones | ||||||||
| Quercetogetin | 14 | 0,06 | – | – | – | – | 1c | [74d] |
| Heptamethoxyflavone | 15 | 0.07 | – | – | – | – | 1d | [74d] |
| Nobiletin | 17 | 0.23 | 0.10 | 0.20 | 0.15 | 0.35 | 3e | [71d, 74d] |
| Sinensetin | 18 | 1.05 | – | – | – | – | 1c | [74d] |
| Tangeretin | 19 | 0.26 | 0.23 | 0.19 | 0.07 | 0.52 | 3e | [71d, 74d] |
| Aglycones | ||||||||
| Acacetin | 6 | 0.02 | – | – | – | – | 1f | [71d] |
| Mean | SD | Median | MIN | MAX | N | Ref. | ||
| Flavanones | ||||||||
| Hesperidin | 23 | 39.9 | 29.4 | 34.9 | 5.21 | 86.1 | 8 | [71b,74c] |
| Naringin | 24 | 0.08 | 0.03 | 0.08 | 0.05 | 0.12 | 4 | [71b] |
| Narirutin | 25 | 4.64 | – | – | – | – | 1 | [71b] |
| Flavones | ||||||||
| 6,8-di-C-Glu-Apigenin | 30 | 0.5 | – | – | – | – | 1 | [38a] |
| 6,8-di-C-Glu-Diosmetin | 32 | 0.2 | – | – | – | – | 1 | [38a] |
| Diosmin | 43 | 1.25 | 0.51 | 1.26 | 0.67 | 2.12 | 7 | [74c] |
| Values | N | Ref. | ||
| Flavanones | ||||
| Hesperidin | 23 | 0.15 | 1 | [71b] |
| Narirutin | 25 | 1.97 | 1 | [71b] |
| Flavones | ||||
| 6,8-di-C-Glu-Apigenin | 30 | 2.5 | 1 | [38a] |
| 6,8-di-C-Glu-Diosmetin | 32 | 0.7 | 1 | [38a] |
| Values | N | Ref. | ||
| Flavanones | ||||
| Naringin | 24 | 1.97 | 1 | [71d] |
| Neohesperidin | 26 | 0.87 | 1 | [71d] |
| Neoeriocitrin | 27 | 0.77 | 1 | [71d] |
| Poncirin | 28 | 0.73 | 1 | [71d] |
| Flavones | ||||
| Diosmin | 43 | 0.15 | 1 | [71d] |
| Polymethoxyflavones | ||||
| Nobiletin | 17 | 0.2 | 1 | [71d] |
| Tangeretin | 19 | 0.08 | 1 | [71d] |
| Aglycones | ||||
| Kaempferol | 9 | 0.14 | 1 | [71d] |
Lemon
| Mean | SD | Median | MIN | MAX | N | Ref. | ||
| Flavanones | ||||||||
| Eriocitrin | 22 | 16.7 | 10.3 | 16.55 | 1.67 | 39.1 | 10a | [40, 71d, 78] |
| Hesperidin | 23 | 20.5 | 12.4 | 18.85 | 3.84 | 41 | 10a | [40, 71d, 78] |
| Flavones | ||||||||
| 6,8-di-C-Glu-Apigenin | 30 | 1.17 | 0.25 | 1.05 | 1 | 1.45 | 3b | [38a] |
| 6,8-di-C-Glu-Diosmetin | 32 | 4.95 | 0.88 | 5 | 4.05 | 5.8 | 3b | [38a] |
| 7-O-Rut-Luteolin | 36 | 3.93 | 2.14 | 3.5 | 1.5 | 6.5 | 6c | [78] |
| Diosmin | 43 | 3.12 | 1.66 | 3.65 | 0.51 | 5.1 | 10a | [40, 71d, 80] |
| Aglycones | ||||||||
| Luteolin | 8 | 0.08 | – | – | – | – | 1d | [71d] |
| Mean | SD | Median | MIN | MAX | N | Ref. | ||
| Flavanones | ||||||||
| Eriocitrin | 22 | 16.0 | – | – | – | – | 1 | [57b] |
| Hesperidin | 23 | 7.07 | 9.02 | 3.06 | 1.59 | 23.08 | 5 | [57b, 74c] |
| Naringin | 24 | 0.38 | – | – | – | – | 1 | [74c] |
| Neohesperidin | 26 | 1.45 | – | – | – | – | 1 | [74e] |
| Flavones | ||||||||
| Diosmin | 43 | 1.04 | 0.67 | 1.12 | 0.16 | 1.78 | 4 | [74c] |
Bergamot
| Mean | SD | Median | MIN | MAX | N | Ref. | ||
| Flavanones | ||||||||
| Eriocitrin | 22 | 0.29 | – | – | – | – | 1a | [71d] |
| Hesperidin | 23 | 1.77 | 0.35 | 1.77 | 1.52 | 2.02 | 2b | [71d] |
| Neoeriocitrin | 27 | 0.01 | – | – | – | – | 1c | [71d] |
| Flavones | ||||||||
| Diosmin | 43 | 0.08 | – | – | – | – | 1c | [71d] |
| Polymethoxyflavones | ||||||||
| Heptamethoxyflavone | 15 | 0.12 | – | – | – | – | 1c | [71d] |
| Natsudaidain | 16 | 0.04 | – | – | – | – | 1c | [71d] |
| Nobiletin | 17 | 0.52 | – | – | – | – | 1c | [71d] |
| Tangeretin | 19 | 0.18 | – | – | – | – | 1c | [71d] |
| Aglycones | ||||||||
| Taxifolin | 3 | 0.04 | – | – | – | – | 1c | [71d] |
| Luteolin | 8 | 0.61 | – | – | – | – | 1a | [71d] |
| Mean | SD | Median | MIN | MAX | N | Ref. | ||
| Flavanones | ||||||||
| Naringin | 24 | 2.23 | – | – | – | – | 1 | [71d] |
| Neohesperidin | 26 | 1.60 | – | – | – | – | 1 | [71d] |
| Neoeriocitrin | 27 | 1.38 | – | – | – | – | 1 | [71d] |
| Poncirin | 28 | 6.41 | – | – | – | – | 1 | [71d] |
| Flavones | ||||||||
| 6,8-di-C-Glu-Apigenin | 30 | 4.53 | 0.58 | 4.45 | 4.0 | 5.15 | 3a | [38a] |
| 6,8-di-C-Glu-Diosmetin | 32 | 3.95 | 0.71 | 4.05 | 3.20 | 4.60 | 3a | [38a] |
| Rhoifolin | 40 | 0.37 | – | – | – | – | 1 | [71d] |
| Diosmin | 43 | 0.39 | – | – | – | – | 1 | [71d] |
| Neodiosmin | 44 | 0.3 | – | – | – | – | 1 | [71d] |
| Mean | N | Ref. | ||
| Flavanones | ||||
| Eriocitrin | 22 | 1.45 | 1a | [66] |
| Naringin | 24 | 26.1 | 1a | [66] |
| Neohesperidin | 26 | 22.1 | 1a | [66] |
| Neoeriocitrin | 27 | 27.6 | 1a | [66] |
| Flavones | ||||
| 6,8-di-C-Glu-Luteolin | 29 | 0.69 | 1a | [66] |
| 6,8-di-C-Glu-Apigenin | 30 | 6.22 | 1a | [66] |
| 6,8-di-C-Glu-Chrysoeriol | 31 | 0.66 | 1a | [66] |
| 6,8-di-C-Glu-Diosmetin | 32 | 4.38 | 1a | [66] |
| 4'-O-Glu Rhoifolin- | 33 | 0.81 | 1a | [66] |
| 7-O-Neohesp-4'-Glu-Chrysoeriol | 34 | 1.24 | 1a | [66] |
| 6-C-Glu-Apigenin | 35 | 0.49 | 1a | [66] |
| 8-C-Glu-Chrysoeriol | 37 | 0.75 | 1a | [66] |
| 8-C-Glu-Diosmetin | 38 | 0.82 | 1a | [66] |
| Rhoifolin | 40 | 6.06 | 1a | [66] |
| 7-O-Neohesp-Chrysoeriol | 42 | 4.89 | 1a | [66] |
| Neodiosmin | 44 | 2.3 | 1a | [66] |
Grapefruit
| Mean | SD | Median | MIN | MAX | N | Ref. | ||
| Flavanones | ||||||||
| Didymin | 21 | 0.30 | 0.04 | 0.30 | 0.27 | 0.33 | 2a | [81d] |
| Eriocitrin | 22 | 0.41 | 0.19 | 0.41 | 0.27 | 0.54 | 2a | [81d] |
| Hesperidin | 23 | 0.93 | 0.58 | 0.87 | 0.25 | 1.79 | 6a | [57a, 71d, 81a,b,d] |
| Naringin | 24 | 23.0 | 12.8 | 21.9 | 4.5 | 60.2 | 19a | [57a, 71d, 81] |
| Narirutin | 25 | 7.60 | 5.80 | 7.70 | 2.50 | 17.0 | 5a | [71d, 81b,d] |
| Neohesperidin | 26 | 1.21 | 0.35 | 1.28 | 0.67 | 1.58 | 5a | [71d, 81b,d] |
| Neoeriocitrin | 27 | 0.32 | 0.02 | 0.32 | 0.30 | 0.33 | 2b | [71d] |
| Poncirin | 28 | 1.26 | 0.35 | 1.30 | 0.85 | 1.58 | 4a | [71d, 81d] |
| Flavones | ||||||||
| Rutin | 39 | 3.26 | – | – | – | – | 1 | [57a] |
| Rhoifolin | 40 | 0.28 | – | – | – | – | 1c | [71d] |
| Polymethoxyflavones | ||||||||
| Heptamethoxyflavone | 15 | 0.06 | 0.07 | 0.06 | 0.01 | 0.11 | 2b | [71d] |
| Nobiletin | 17 | 0.15 | 0.04 | 0.15 | 0.12 | 0.17 | 2b | [71d] |
| Tangeretin | 19 | 0.12 | – | – | – | – | 1c | [71d] |
| Aglycones | ||||||||
| Hesperetin | 1 | 0.74 | – | – | – | – | 1 | [57a] |
| Naringenin | 2 | 2.70 | 2.68 | 1.70 | 0.98 | 8.00 | 6 | [57a, 81c] |
| Taxifolin | 3 | 0.16 | – | – | – | – | 1d | [71d] |
| Quercetin | 10 | 0.19 | 0.03 | 0.19 | 0.17 | 0.21 | 2b | [71d] |
| Mean | SD | Median | MIN | MAX | N | Ref. | ||
| Flavanones | ||||||||
| Didymin | 21 | 0.8 | 0.5 | 0.8 | 0.0 | 1.7 | 11a | [74d, 84] |
| Hesperidin | 23 | 2.8 | 3.9 | 1.6 | 0.2 | 16.4 | 15a | [74c-e, 84] |
| Naringin | 24 | 43.5 | 23.3 | 38.8 | 4.8 | 119.7 | 102b | [47b, 57b, 60, 74b-e, 81a,c, 84] |
| Narirutin | 25 | 9.90 | 4.2 | 10.2 | 2.3 | 18.8 | 17a | [47b, 57b, 74b,d, 84] |
| Neohesperidin | 26 | 0.8 | 0.5 | 0.9 | 0.67 | 2.0 | 18a | [74b,d,e, 84] |
| Poncirin | 28 | 1.2 | 0.7 | 1.2 | 0.1 | 2.4 | 16a | [74b,d, 84] |
| Flavones | ||||||||
| Diosmin | 43 | 0.80 | 0.8 | 0.8 | 0.20 | 1.40 | 2.0 | [74c] |
| Aglycones | ||||||||
| Naringenin | 2 | 4.20 | 4.00 | 3.30 | 0.40 | 16.2 | 44a | [81c] |
| Quercetin | 10 | 0.6 | 0.2 | 0.6 | 0.2 | 0.9 | 7a | [74b, 84] |
Conclusions
References and Notes
- Harborne, J. B. The Flavonoids; Chapman & Hall, Ed.; CRC: Boca Raton, FL, 1999; p. 66. [Google Scholar] Grotewold, E. The Science of Flavonoids; Springer: New York, NY, 2006. [Google Scholar] Harborne, J. B.; Williams, C. A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Stafford, H. A. Flavonoid Metabolism; CRC: Boca Raton, FL, 1990; pp. 1–59. [Google Scholar]
- Buslig, B S.; Manthey, J. A. (Eds.) Flavonoids in cell function; Kluwer Academic/Plenum Publishers: New York, NY, 2002. Forkmann, G. Proc. 16th Int. Conf. Groupe Polyphenols Lisbon. 1992, vol. 16, 19–27. Herrmann, K. Chem. Mikrobiol. Technol. Lebensm. 1970, 12, 161. Cody, V.; Middleton, E.; Harborne, J. B.; Beretz, A. (Eds.) Plant Flavonoids in Biology and Medicine. II: Biochemical, Cellular and Medicinal Properties. Progress in Clinical and Biological Research Vol. 280; Alan R. Liss: New York, 1988.
- Sharma, D. K. Bioprospecting for drug, research and functional foods for the prevention of diseases – Role of flavonoids in drug development. J. Sci. Ind. Res. 2006, 65, 391–401. [Google Scholar] Cermak, R.; Wolffram, S. The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms. Curr. Drug Metab. 2006, 7, 729–744. [Google Scholar] [CrossRef] Wang, H. K. The therapeutic potential of flavonoids. Expert Opin. Investig. Drugs 2000, 9, 2103–2119. [Google Scholar] [CrossRef] Di Carlo, G.; Mascolo, N.; Izzo, A. A.; Capasso, F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef] Manach, C.; Donovan, J. L. Farmacokinetics and metabolism of dietary flavonoids in humans. Free Radical Res. 2004, 38, 771–785. [Google Scholar] Ortuno, A.; Gomez, P.; Baidez, A.; Frias, V.; Del Rio, J. A. Citrus sp: a source of flavonoids of pharmaceutical interest. Potential Health Benefits of Citrus (ACS Symp. Ser. 936) 2006, 175–185. [Google Scholar] Tapiero, H.; Tew, K. D.; Ba, G.N.; Mathe, G. Polyphenols: do they play a role in the prevention of human pathologies? Biomed. Pharmacother. 2002, 56, 200–207. [Google Scholar] [CrossRef]
- Valenzuela, A.; Sanhueza, J.; Nieto, S. Natural antioxidants in functional foods: from food safety to health benefits. Grasas Aceites 2003, 54, 295–303. [Google Scholar] Hooper, L.; Cassidy, A. A review of the health care potential of bioactive compounds. J. Sci. Food Agric. 2006, 86, 1805–1813. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] Zhang, H. Y.; Yang, D. P.; Tang, G. Y. Multipotent antioxidants: from screening to design. Drug Discov. Today 2006, 11, 749–754. [Google Scholar] Wang, H.; Cao, G.; Prior, R. L. Total antioxidant capacity of fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef] Cao, G.; Sofic, E.; Prior, R. L. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef] Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar] [CrossRef] Kaur, C.; Kapoor, H. C. Antioxidants in fruits and vegetables – the millennium’s health. Int. J. Food Sci. Tech. 2001, 36, 703–725. [Google Scholar] [CrossRef] Kaur, C.; Kapoor, H. C. Antioxidants in fruits and vegetables – the millennium’s health. Int. J. Food Sci. Tech. 2001, 36, 703–725. [Google Scholar] [CrossRef] Sánchez-Moreno, C.; Plaza, L.; De Ancos, B.; Cano, M. P. Quantitative bioactive compounds assessment and their relative contribution to the antioxidant capacity of commercial orange juices. J. Sci. Food Agric. 2003, 83, 430–439. [Google Scholar] [CrossRef] Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Kris-Etherton, P. M.; Lefevre, M.; Beecher, G. R.; Gross, M. D.; Keen, C. L.; Etherton, T. D. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Ann. Rev. Nutr. 2004, 24, 511–538. [Google Scholar] [CrossRef] Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: a review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar]
- Yochum, L; Kushi, L. H.; Meyer, K.; Folsom, A. R. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am. J. Epidemiol. 1999, 149, 943–949. [Google Scholar] [CrossRef] Knekt, P.; Jarvinen, R.; Reunanen, A.; Maatela, J. Flavonoid intake and coronary mortality in Finland: a cohort study. Brit. Med. J. 1996, 312, 478–481. [Google Scholar] [CrossRef] Hertog, M. G. L. Flavonoid intake and long-term risk of coronary heart-disease and cancer in the 7 countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef] Gross, M. Flavonoids and cardiovascular disease. Pharm. Biol. 2004, 42, 21–35. [Google Scholar] [CrossRef]
- Nichenametla, S. N.; Taruscio, T. G.; Barney, D. L.; Exon, J. H. A review of the effects and mechanism of polyphenolics in cancer. Crit. Rev. Food Sci. 2006, 46, 161–183. [Google Scholar] [CrossRef] Steinmetz, K. A.; Potter, J. D. Vegetables, fruit, and cancer prevention: a review. J. Am. Diet. Assoc. 1996, 96, 1027–1039. [Google Scholar] [CrossRef] Moon, Y. J.; Wang, X. D.; Morris, M. E. Effects on xenobiotic and carcinogen metabolism. Toxicol. in Vitro 2006, 20, 187–210. [Google Scholar] [CrossRef] Kris-Etherton, P. M.; Hecker, K. D.; Bonanome, A.; Coval, S. M.; Binkoski, A. E.; Hilpert, K. F.; Etherton, T. D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef] Le Marchand, L.; Murphy, S. P.; Hankin, J. H.; Wilkens, L. R.; Kolonel, L. N. Intake of flavonoids and lung cancer. J. Natl. Cancer Inst. 2000, 92, 154–160. [Google Scholar] [CrossRef]
- Asres, K.; Seyoum, A.; Veeresham, C.; Bucar, F.; Gibbons, S. Naturally derived anti-HIV agents. Phytother. Res. 2005, 19, 557–581. [Google Scholar] [CrossRef]
- Cushnie, T. P. T.; Lamb, A. J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agent. 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Kim, H. P.; Son, K. H.; Chang, H. W.; Kang, S. S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Marín, F. R.; Ortuño, A.; Del Río, J. A. Uses and properties of Citrus flavonoids. J. Agric. Food Chem. 1997, 45, 4505–4515. [Google Scholar] [CrossRef]
- Tijburg, L. B. M.; Mattern, T.; Folts, J. D.; Weisgerber, U. M.; Katan, M. B. Tea flavonoids and cardiovascular diseases: a review. Crit. Rev. Food Sci. 1997, 37, 771–785. [Google Scholar] [CrossRef]
- Borrelli, F.; Izzo, A. A. The plant kingdom as a source of anti-ulcer remedies. Phytother. Res. 2000, 14, 581–591. [Google Scholar] [CrossRef] Wightman, J. D. Red berries and their health benefits. Nutraceutical Beverages: Chemistry, Nutrition, and Health Effects (ACS Symp. Ser. 871) 2004, 123–132. [Google Scholar]
- Middleton, E.; Kandaswami, C. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992, 43, 1167–1179. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food. Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Burgess, J. R.; Andrade, J. E. Antioxidant effects of citrus flavonoid consumption. Potential health benefits of Citrus (ACS Symp. Ser. 936) 2006, 161–174. [Google Scholar] [CrossRef]
- Nijveldt, R. J.; van Nood, E.; van Hoorn, D. E. C.; Boelens, P. G.; van Norren, K.; van Leeuwen, P. A. M. Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar]
- Moufida, S.; Marzouk, B. Biochemical characterization of blood orange, sweet orange, lemon, bergamot and bitter orange. Phytochemistry 2003, 62, 1283–1289. [Google Scholar] [CrossRef]
- Hollman, P. C. H.; Arts, I. C. W. Flavonols, flavones and flavanols – nature, occurrence and dietary burden. J. Sci. Food Agr. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J. Flavonoids: Dietary occurrence and biochemical activity. Nutr Res. 1998, 18, 1995–2018. [Google Scholar] [CrossRef] Franke, A. A.; Cooney, R. V.; Henning, S. M.; Custer, L. J. Bioavailability and antioxidant effects of orange juice components in humans. J. Agric. Food. Chem. 2005, 53, 5170–5178. [Google Scholar] [CrossRef] Yao, L. H.; Jiang, Y. M.; Shi, J.; Tomas-Barberan, F. A.; Datta, N.; Singanusong, R.; Chen, S. S. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. Ross, J. A.; Kasum, C. M. Dietary flavonoids: bioavailability, metabolic effects, and safety. Ann. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Polydera, A. C.; Stoforos, N. G.; Taoukis, P. S. Effect of high hydrostatic pressure treatment on post processing antioxidant activity of fresh Navel orange juice. Food Chem. 2005, 91, 495–503. [Google Scholar] [CrossRef] Sánchez-Moreno, C.; Plaza, L.; Elez-Martínez, P.; De Ancos, B.; Martín-Belloso, O.; Cano, M. P. Impact of high pressure and pulsed electric fields on bioactive compounds and antioxidant activity of orange juice in comparison with traditional thermal processing. J. Agric. Food. Chem. 2005, 53, 4403–4409. [Google Scholar] [CrossRef] Del Caro, A.; Piga, A.; Vacca, V.; Agabbio, M. Changes of flavonoids, vitamin C and antioxidant capacity in minimally processed citrus segments and juices during storage. Food Chem. 2004, 84, 99–105. [Google Scholar] [CrossRef] Montijano, H.; Coll, M. D.; Borrego, F. Assessment of neohesperidine DC stability during pasteurization of juice-based drinks. Int. J. Food Sci. Technol. 1996, 31, 397–401. [Google Scholar]
- Schijlen, E. G. W.; de Vos, C. H. R.; van Tunen, A. J.; Bovy, A. G. Modification of flavonoid biosynthesis in crop plants. Phytochemistry. 2004, 65, 2631–2648. [Google Scholar] [CrossRef] Yonekura-Sakakibara, K.; Saito, K. Review: genetically modified plants for the promotion of human health. Biotechnol. Lett. 2006, 28, 1983–1991. [Google Scholar] [CrossRef] Andersen, O. M. Flavonoids: chemistry and biochemistry; CRC Press: Boca Raton, FL, 2005. [Google Scholar] Tucker, G. Nutritional enhancement of plants. Curr. Opin. Biotech. 2003, 14, 221–225. [Google Scholar] [CrossRef]
- Erlund, I. Review of the flavonoids quercetin, hesperetin and naringenin. Dietary sources, bioactivities, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef] Aherne, S. A.; O'Brien, N. M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef] Ting, S. V.; Roussef, R. L. Citrus Fruits and Their Products: Analysis and Technology; Marcel Dekker: New York, 1986. [Google Scholar]
- Harborne, J. B. (Ed.) Flavonoids: Advances in Research since 1986; Chapman and Hall: London, 1994.
- Mizrahi, S.; Berk, Z. Physio-chemical characteristics of orange juice cloud. J. Sci. Food Agric. 1970, 21, 250–253. [Google Scholar] [CrossRef] Amiot, M. J.; Tacchini, M.; Aubert, S.; Nicolas, J. J. Food Sci. 1992, 57, 958–962. [CrossRef]
- Horowitz, R. M.; Gentili, B. Phenolic glycosides of grapefruit: a relation between bitterness and structure. Arch. Biochem. Biophys. 1961, 92, 191–192. [Google Scholar] [CrossRef] Guadagni, D. G.; Maier, V. P.; Turnbaugh, J. G. Effect of some Citrus juice constituents on taste thresholds for limonin and naringin bitterness. J. Sci. Food Agric. 1973, 24, 1277–1288. [Google Scholar] [CrossRef] Horowitz, R. M. Taste effects of flavonoids. In Plant Flavonoids in Biology and Medicine, Biochemical, Pharmacological, and Structure-Activity; Cody, V., Middleton, E., Jr., Harborne, J., Eds.; Alan R. Liss: New York, 1986; pp. 163–175. [Google Scholar]
- Miyake, Y.; Yamamoto, K.; Morimitsu, Y.; Osawa, T. Isolation of C-glucosylflavone from lemon peel and antioxidative activity of flavonoid compounds in lemon fruit. J. Agric. Food Chem. 1997, 45, 4619–4623. [Google Scholar] [CrossRef]
- Leuzzi, U.; Caristi, C.; Panzera, V.; Licandro, G. Flavonoids in pigmented orange juice and second-pressure extracts. J. Agric. Food Chem. 2000, 48, 5501–5506. [Google Scholar] [CrossRef]
- Mabry, T. J.; Markham, K. R.; Thomas, M. B. The ultraviolet spectra of flavones and flavonols. In The systematic identification of flavonoids; Spinger-Verlag: Berlin, 1970. [Google Scholar]
- Bonaccorsi, P.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Flavonol glucoside profile of southern Italian red onion (Allium cepa L.). J. Agric. Food. Chem. 2005, 53, 2733–2740. [Google Scholar] [CrossRef]
- Ferreres, F.; Llorach, R.; Gil-Izquierdo, A. Characterization of the interglycosidic linkage in di-, tri-, tetra- and pentaglycosylated flavonoids and differentiation of positional isomers by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Mass. Spectrom. 2004, 39, 312–321. [Google Scholar] [CrossRef]
- Stobiecki, M. Application of mass spectrometry for identification and structural studies of flavonoid glycosides. Phytochemistry 2000, 54, 237–256. [Google Scholar] [CrossRef] Cuyckens, H.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass. Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Spectra in Figures 4 and 5 have been recorded in our laboratory to clarify the argument proposed.
- Ma, Y.-L.; Vedernikova, I.; Van den Heuvel, H.; Claeys, M. Internal glucose residue loss in protonated O-diglycosyl flavonoids upon low-energy collision-induced dissociation. J. Am. Soc. Mass. Spectrom. 2000, 11, 136–144. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass. Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] Cuyckens, F.; Rozenberg, R.; de Hoffmann, E.; Claeys, M. Structure characterization of flavonoid O-diglycosides by positive and negative nano-electrospray ionization ion trap mass spectrometry. J Mass Spectrom. 2001, 36, 1203–1210. [Google Scholar] [CrossRef]
- Caristi, C.; Bellocco, E.; Gargiulli, C.; Toscano, G.; Leuzzi, U. Flavone-di-C-glycosides in Citrus juices from southern Italy. Food. Chem. 2006, 95, 431–437. [Google Scholar] [CrossRef] Ferreres, F.; Silva, B. M.; Andrade, P. B.; Seabra, R. M.; Ferreira, M. A. Approach to the study of C-glycosyl flavones by ion trap HPLC-PAD-ESI/MS/MS: Application to seeds of quince (Cydonia oblonga). Phytochem. Anal. 2003, 14, 352–359. [Google Scholar] [CrossRef]
- Rayyan, S.; Fossen, T.; Nateland, H. S.; Andersen, O. M. Isolation and identification of flavonoids, including flavone rotamers, from the herbal drug 'Crataegi folium cum flore' (Hawthorn). Phytochem. Anal. 2005, 16, 334–341. [Google Scholar] [CrossRef]
- Caristi, C.; Bellocco, E.; Panzera, V.; Toscano, G.; Vadalà, R.; Leuzzi, U. Flavonoids detection by HPLC-DAD-MS-MS in lemon juices from sicilian cultivars. J. Agric. Food. Chem. 2003, 51, 3528–3534. [Google Scholar] [CrossRef]
- Ooghe, W. C.; Ooghe, S. J.; Detavernier, C. M.; Huyghebaert, A. Characterization of orange juice (Citrus sinensis) by polymethoxylated flavones. J. Agric. Food Chem. 1994, 42, 2191–2195. [Google Scholar] [CrossRef] de Pascual-Teresa, S.; Treutter, D.; Rivas-Gonzalo, J. C.; Santos-Buelga, C. Analysis of flavanols in beverages by high-performance liquid chromatography with chemical reaction detection. J. Agric. Food. Chem. 1998, 46, 4209–4213. [Google Scholar] [CrossRef] Carando, S.; Teissedre, P. L.; Pascual-Martinez, L.; Cabanis, J. C. Levels of flavan-3-ols in French wines. J. Agric. Food. Chem. 1999, 47, 4161–4166. [Google Scholar] [CrossRef] Marini, D.; Balestrieri, F. Multivariate analysis of flavanone glycosides in Citrus juices. Ital. J. Food Sci. 1995, 7, 255–264. [Google Scholar]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J. Pharmaceut. Biomed. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef] Tura, D.; Robards, K. Sample handling strategies for the determination of biophenols in food and plants. J. Chromatogr. A 2002, 975, 71–93. [Google Scholar] [CrossRef]
- Conde, E.; Cadahia, E.; Garcia-Vallejo, M. C. Low molecular weight polyphenols in leaves of Eucalyptus camaldulensis, E-globulus and E-rudis. Phytochem. Anal. 1997, 8, 186–193. [Google Scholar] [CrossRef]
- He, X. G.; Lian, L. Z.; Lin, L. Z.; Bernart, M. W. High-performance liquid chromatography electrospray mass spectrometry in phytochemical analysis of sour orange (Citrus aurantium L.). J. Chromatogr. A 1997, 791, 127–134. [Google Scholar] [CrossRef]
- Guyot, S.; Marnet, N.; Laraba, D.; Sanoner, P.; Drilleau, J. F. Reversed-phase HPLC following thiolysis for quantitative estimation and characterization of the four main classes of phenolic compounds in different tissue zones of a French cider apple variety (Malus domestica var. Kermerrien). J. Agric. Food. Chem. 1998, 46, 1698–1705. [Google Scholar] [CrossRef]
- Bonvehi, J. S.; Torrento, M. S.; Lorente, E. C. Evaluation of polyphenolic and flavonoid compounds in honeybee-collected pollen produced in Spain. J. Agric. Food. Chem. 2001, 49, 1848–1853. [Google Scholar] [CrossRef]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Nonomura-Nakano, M.; Nesumi, H.; Yoshida, T.; Sugiura, M.; Yano, M. Quantitative study of fruit flavonoids in Citrus hybrids of King (C. nobilis) and Mukaku Kishu (C. kinokuni). J. Agric. Food. Chem. 2001, 49, 3982–3986. [Google Scholar] [CrossRef] Bronner, W. E.; Beecher, G. R. Extraction and measurement of prominent flavonoids in orange and grapefruit concentrates. J. Chromatogr. A 1995, 705, 247–256. [Google Scholar] [CrossRef]
- Johnsson, P.; Peerlkamp, N.; Kamal-Eldin, A.; Andersson, R. E.; Andersson, R.; Lundgren, L. N.; Aman, P. Polymeric fractions containing phenol glucosides in flaxseed. Food. Chem. 2002, 76, 207–212. [Google Scholar] [CrossRef]
- Careri, M.; Elviri, L.; Mangia, A. Validation of a liquid chromatography ion spray mass spectrometry method for the analysis of flavanones, flavones and flavonols. Rapid Commun. Mass Spectrom. 1999, 13, 2399–2405. [Google Scholar] [CrossRef]
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A 2003, 1000, 657–691. [Google Scholar] [CrossRef]
- de Rijke, E.; Out, P.; Niessen, W. M. A.; Ariese, F.; Gooijer, C; Brinkman, U. A. T. Analytical separation and detection methods for flavonoids. J. Chromatogr. A 2006, 1112, 31–63. [Google Scholar] [CrossRef] Robards, K.; Antolovich, M. Analytical chemistry of fruit bioflavonoids - A review. Analyst 1997, 122, R11–R34. [Google Scholar] [CrossRef] Hasegawa, S.; Berhow, M. A.; Fong, C. H. Analysis of Bitter Principles in Citrus. In Modern Methods of Plant Analysis, Vol. 18, Fruit Analysis; Linskens, H.-F., Jackson, J.F., Eds.; Springer-Verlag: Berlin, 1996; pp. 59–80. [Google Scholar]
- Molyneux, R. J.; Mahoney, N.; Bayman, P.; Wong, R. Y.; Meyer, K.; Irelan, N. Eutypa dieback in grapevines: differential production of acetylenic phenol metabolites by strains of Eutypa lata. J. Agric. Food. Chem. 2002, 50, 1393–1399. [Google Scholar] [CrossRef] Gallardo-Williams, M. T.; Geiger, C. L.; Pidala, J. A.; Martin, D. F. Essential fatty acids and phenolic acids from extracts and leachates of southern cattail (Typha domingensis P.). Phytochemistry 2002, 59, 305–308. [Google Scholar] [CrossRef] Maciejewicz, W.; Daniewski, M.; Bal, K.; Markowski, W. GC-MS identification of the flavonoid aglycones isolated from propolis. Chromatographia 2001, 53, 343–346. [Google Scholar] [CrossRef] Bunzel, M.; Ralph, J.; Marita, J. M.; Hatfield, R. D.; Steinhart, H. Diferulates as structural components in soluble and insoluble cereal dietary fibre. J. Sci. Food Agr. 2001, 81, 653–660. [Google Scholar] [CrossRef] Tsikas, D. Affinity chromatography as a method for sample preparation in gas chromatography/mass spectrometry. J. Biochem. Bioph. Meth. 2001, 49, 705–731. [Google Scholar] [CrossRef]
- Martinez-Valverde, I.; Periago, M. J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agr. 2002, 82, 323–330. [Google Scholar] [CrossRef] Hvattum, E. Determination of phenolic compounds in rose hip (Rosa canina) using liquid chromatography coupled to electrospray ionisation tandem mass spectrometry and diode-array detection. Rapid Commun. Mass Spectrom. 2002, 16, 655–662. [Google Scholar] [CrossRef] Andreasen, M. F.; Christensen, L. P.; Meyer, A. S.; Hansen, A. Content of phenolic acids and ferulic acid dehydrodimers in 17 rye (Secale cereale L.) varieties. J. Agric. Food. Chem. 2000, 48, 2837–2842. [Google Scholar] [CrossRef] Martos, I.; Ferreres, F.; Yao, L. H.; D'Arcy, B.; Caffin, N.; Tomas-Barberan, F. A. Flavonoids in monospecific Eucalyptus honeys from Australia. J. Agric. Food. Chem. 2000, 48, 4744–4748. [Google Scholar] [CrossRef] Martos, I.; Ferreres, F.; Tomas-Barberan, F. A. Identification of flavonoid markers for the botanical origin of Eucalyptus honey. J. Agric. Food. Chem. 2000, 48, 1498–1502. [Google Scholar] [CrossRef] Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A.; Montedoro, G. High-performance liquid chromatography evaluation of phenols in olive fruit, virgin olive oil, vegetation waters, and pomace and 1D-and 2D-nuclear magnetic resonance characterization. J. Am. Oil Chem. Soc. 1999, 76, 873–882. [Google Scholar] [CrossRef] Escarpa, A.; Gonzalez, M. C. Fast separation of (poly)phenolic compounds from apples and pears by high-performance liquid chromatography with diode-array detection. J. Chromatogr. A 1999, 830, 301–309. [Google Scholar] [CrossRef] De Nino, A.; Mazzotti, F.; Morrone, S. P.; Perri, E.; Raffaelli, A.; Sindona, G. Characterization of cassanese olive cultivar through the identification of new trace components by ionspray tandem mass spectrometry. J. Mass. Spectrom. 1999, 34, 10–16. [Google Scholar] [CrossRef] Andrade, P. B.; Carvalho, A. R. F.; Seabra, R. M.; Ferreira, M. A. A previous study of phenolic profiles of quince, pear, and apple purees by HPLC diode array detection for the evaluation of quince puree genuineness. J. Agric. Food Chem. 1998, 46, 968–972. [Google Scholar] Mouly, P. P.; Arzouyan, C. R.; Gaydou, E. M.; Estienne, J. M. Differentiation of Citrus juices by factorial discriminant analysis using liquid chromatography of flavanone glycosides. J Agric. Food Chem. 1994, 42, 70–79. [Google Scholar] [CrossRef] Swatsitang, P.; Tucker, G.; Robards, K.; Jardine, D. Isolation and identification of phenolic compounds in Citrus sinensis. Anal. Chim. Acta 2000, 417, 231–240. [Google Scholar] Calabrò, M.L.; Galtieri, V.; Cutroneo, P.; Tommasini, S.; Ficarra, P.; Ficarra, R. Study of the extraction procedure by experimental design and validation of a LC method for determination of flavonoids in Citrus bergamia juice. J. Pharmaceut. Biomed. 2004, 35, 349–363. [Google Scholar] [CrossRef] Careri, M.; Elviri, L.; Mangia, A.; Musci, M. Spectrophotometric and coulometric detection in the highperformance liquid chromatography of flavonoids and optimization of sample treatment for the determination of quercetin in orange juice. J. Chromatogr. A 2000, 881, 449–460. [Google Scholar] [CrossRef] Mouly, P. P.; Gaydou, E. M.; Estienne, J. M. Column liquid chromatographic determination of flavanone glycosides in Citrus. J. Chromatogr. 1993, 634, 129–134. [Google Scholar] [CrossRef]
- Penazzi, G.; Caboni, M. F.; Zunin, P.; Evangelisti, F.; Tiscornia, E.; Toschi, T. G.; Lercker, G. Routine high-performance liquid chromatographic determination of free 7-ketocholesterol in some foods by two different analytical methods. J. Am. Oil Chem. Soc. 1995, 72, 1523–1527. [Google Scholar] [CrossRef]
- Merken, H. M.; Beecher, G. R. Measurement of food flavonoids by high-performance liquid chromatography: a review. J. Agric. Food. Chem. 2000, 48, 577–599. [Google Scholar] [CrossRef]
- Khokhar, S.; Magnusdottir, S. G. M. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J. Agric. Food. Chem. 2002, 50, 565–570. [Google Scholar] [CrossRef] Li, W. K.; Fong, H. H. S.; Singletary, K. W.; Fitzloff, J. F. Determination of catechins in commercial grape seed extract. J. Liq. Chromatogr. R. T. 2002, 25, 397–407. [Google Scholar] [CrossRef] Brenes, M.; Rejano, L.; Garcia, P.; Sanchez, A. H.; Garrido, A. Biochemical-changes in phenolic-compounds during spanish-style green olive processing. J. Agric. Food. Chem. 1995, 43, 2702–2706. [Google Scholar] [CrossRef]
- Wu, T.; Guan, Y.; Ye, J. Determination of flavonoids and ascorbic acid in grapefruit peel and juice by capillary electrophoresis with electrochemical detection. Food Chem. 2007, 100, 1573–1579. [Google Scholar] [CrossRef] Desiderio, C.; De Rossi, A.; Sinibaldi, M. Analysis of flavanone-7-O-glycosides in Citrus juices by short-end capillary electrochromatography. J. Chromatogr. A. 2005, 1081, 99–104. [Google Scholar] [CrossRef] Issaq, H. J. Capillary electrophoresis of natural products. Electrophoresis 1997, 18, 2438–2452. [Google Scholar] [CrossRef]
- Gel-Moreto, N.; Streich, R.; Galensa, R. Chiral separation of six diastereomeric flavanone-7-O-glycosides by capillary electrophoresis and analysis of lemon juice. J. Chromatogr. A. 2001, 925, 279–289. [Google Scholar] [CrossRef]
- Simó, C.; Ibañez, E.; Señoráns, F. J.; Barbas, C.; Reglero, G.; Cifuentes, A. Analysis of antioxidants from orange juice obtained by countercurrent supercritical fluid extraction, using micellar electrokinetic chromatography and reverse-phase liquid chromatography. J Agric. Food Chem. 2002, 50, 6648–6652. [Google Scholar] [CrossRef]
- Reichart, E.; Obendorf, D. Determination of naringin in grapefruit juice by catodic stripping differential pulse voltammetry at the hanging mercury drop electrode. Anal. Chim. Acta 1998, 360, 179–187. [Google Scholar] [CrossRef]
- Mullen, W.; Marks, S. C.; Crozier, A. Evaluation of phenolic compounds in commercial fruit juices and fruit drinks. J. Agric. Food. Chem. 2007, 55, 3148–3157. [Google Scholar] [CrossRef] Mattoli, L.; Cangi, F.; Maidecchi, A.; Ghiara, C.; Tubaro, M.; Traldi, P. A rapid liquid chromatography electrospray ionization mass spectrometry(n) method for evaluation of synephrine in Citrus aurantium L. samples. J. Agric. Food. Chem. 2005, 53, 9860–9866. [Google Scholar] [CrossRef] Sanchez-Rabaneda, F.; Jauregui, O.; Lamuela-Raventos, R. M.; Viladomat, F.; Bastida, J.; Codina, C. Qualitative analysis of phenolic compounds in apple pomace using liquid chromatography coupled to mass spectrometry in tandem mode. Rapid Commun. Mass Spectrom. 2004, 18, 553–563. [Google Scholar] [CrossRef] Hughes, R. J.; Croley, T. R.; Metcalfe, C. D.; March, R. E. A tandem mass spectrometric study of selected characteristic flavonoids. Int. J. Mass. Spectrom. 2001, 210, 371–385. [Google Scholar] Robards, K.; Li, X.; Antolovich, M.; Boyd, S. Characterisation of Citrus by chromatographic analysis of flavonoids. J. Sci. Food Agric. 1997, 75, 87–101. [Google Scholar] [CrossRef] Justesen, U.; Knuthsen, P.; Leth, T. Determination of plant phenols in Danish foodstuffs by HPLC-UV and LC-MS detection. Cancer Lett. 1997, 114, 165–167. [Google Scholar] [CrossRef]
- Singleton, J. A.; Stikeleather, L. F.; Sanford, J. H. LC-electrospray ionization and LC-FABMS study of flavonoid glycosides extracted from peanut meal. J. Am. Oil Chem. Soc. 2002, 79, 741–748. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Xu, Q.; Xue, X.-Y.; Zhang, F.-F.; Liang, X.-M. Identification of O-diglycosyl flavanones in Fructus aurantii by liquid chromatography with electrospray ionization and collision-induced dissociation mass spectrometry. J. Pharmaceut. Biomed. 2006, 42, 441–448. [Google Scholar] [CrossRef] Seeram, N. P.; Lee, R.; Scheuller, H. S.; Heber, D. Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food. Chem. 2006, 97, 1–11. [Google Scholar] [CrossRef] Dugo, P.; Lo Presti, M.; Oehman, M.; Fazio, A.; Dugo, G.; Mondello, L. Determination of flavonoids in Citrus juices by micro-HPLC-ESI/MS. J. Sep. Sci. 2005, 28, 1149–1156. [Google Scholar] [CrossRef] Tolonen, A.; Uusitalo, J. Fast screening method for the analysis of total flavonoid content in plants and foodstuffs by high-performance liquid chromatography/electrospray ionization time-of-flight mass spectrometry with polarity switching. Rapid Commun. Mass Spectrom. 2004, 18, 3113–3122. [Google Scholar] [CrossRef]
- Papp, I.; Apati, P.; Andrasek, V.; Blazovics, A.; Balazs, A.; Kursinszki, L.; Kite, G. C.; Houghton, P. J.; Kery, A. LC-MS analysis of antioxidant plant phenoloids. Chromatographia 2004, 60, S93–S100. [Google Scholar] Justesen, U.; Knuthsen, P. Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes. Food. Chem. 2001, 73, 245–250. [Google Scholar] [CrossRef]
- Han, J.; Ye, M.; Xu, M.; Sun, J. H.; Wang, B. R.; Guo, D. Characterization of flavonoids in the traditional Chinese herbal medicine-Huangqin by liquid chromatography coupled with electrospray ionization mass spectrometry. J. Chromatogr. B 2007, 848, 355–362. [Google Scholar] [CrossRef] De Lourdes Mata Bilbao, M.; Andrés-Lacueva, C.; Jáuregui, O.; Lamuela-Raventós, R. M. Determination of flavonoids in a Citrus fruit extract by LC–DAD and LC–MS. Food Chem. 2007, 101, 1742–1747. [Google Scholar] [CrossRef] Anagnostopoulou, M. A.; Kefalas, P.; Kokkalou, E.; Assimopoulou, A. N.; Papageorgiou, V. P. Analysis of antioxidant compounds in sweet orange peel by HPLC-diode array detection-electrospray ionization mass spectrometry. Biomed. Chromatogr. 2005, 19, 138–148. [Google Scholar] [CrossRef]
- Gattuso, G.; Caristi, C.; Gargiulli, C.; Bellocco, E.; Toscano, G.; Leuzzi, U. Flavonoid Glycosides in Bergamot Juice (Citrus bergamia Risso). J. Agric. Food Chem. 2006, 54, 3929–3935. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, F.; Ye, J. Quantitative and qualitative analysis of flavonoid markers in Fructus aurantii of different geographical origin by capillary electrophoresis with electrochemical detection. J. Chromatogr. B 2006, 830, 224–230. [Google Scholar] [CrossRef] Mattila, P.; Astola, J.; Kumpulainen, J. Determination of Flavonoids in Plant Material by HPLC with Diode-Array and Electro-Array Detections. J. Agric. Food Chem. 2000, 48, 5834–5841. [Google Scholar] [CrossRef] Gamache, P.; Ryan, E.; Acworth, N. Analysis of phenolic and flavonoid compounds in juice beverages using high-performance liquid chromatography with coulometric array detection. J. Chromatogr. 1993, 635, 143–150. [Google Scholar] [CrossRef] Roston, D. A.; Kissinger, P. T. Identification of phenolic constituents in commercial beverages by liquid chromatography with electrochemical detection. Anal. Chem. 1981, 53, 1695–1699. [Google Scholar] [CrossRef]
- Peterson, J. J.; Beecher, G. R.; Bhagwat, S. A.; Dwyer, J. T.; Gebhardt, S. E.; Haytowitz, D. B.; Holden, J. M. Flavanones in grapefruit, lemons, and limes: a compilation and review of the data from the analytical literature. J. Food Comp. Anal. 2006, 19, S74–S80. [Google Scholar] [CrossRef] Peterson, J. J.; Dwyer, J. T.; Beecher, G. R.; Bhagwat, S. A.; Gebhardt, S. E.; Haytowitz, D. B.; Holden, J. M. Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: a compilation and review of the data from the analytical literature. J. Food Comp. Anal. 2006, 19, S66–S73. [Google Scholar] [CrossRef]
- For some relevant and exhaustive examples, see: Nogata, Y.; Sagamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid compositon of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] Berhow, M.; Tisserat, B.; Kanes, K.; Vandercook, C. Survey of phenolic compounds produced in Citrus. USDA ARS Tech. Bull. 1998, 1856, 1–154, available also at http://www.ars.usda.gov/is/np/phenolics/title.htm. [Google Scholar] [CrossRef]
- Tanaka, T. Misunderstanding with regards citrus classification and nomenclature. Bull. Univ. Osaka Prefect., Ser. B 1969, 21, 139. [Google Scholar]
- Mouly, P. P.; Gaydou, E. M.; Arzouyan, C. R.; Estienne, J. M. Différenciation des jus de Citrus par analyses statistiques multivariées. Partie II. Cas des oranges et des mandarines. Analusis 1996, 24, 230–239. [Google Scholar] Dhuique-Mayer, C.; Caris-Veyrat, C.; Ollitrault, P.; Curk, F.; Amiot, M. Varietal and interspecific influence on micronutrient contents in Citrus from the Mediterranean area. J. Agric. Food Chem. 2005, 53, 2140–2145. [Google Scholar] [CrossRef] Pupin, A. M.; Dennis, M. J.; Toledo, M. C. F. Flavanone glycosides in Brazilian orange juice. Food Chem. 1998, 61, 275–280. [Google Scholar] [CrossRef] Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. HL-60 differentiating activity and flavonoid content of the readily extractable fraction prepared from Citrus juices. J. Agric. Food Chem. 1999, 47, 128–135. [Google Scholar] [CrossRef] Gil-Izquierdo, A.; Gil, M. I.; Ferreres, F.; Tomás-Barberán, F. A. In vitro availability of flavonoids and other phenolics in orange juice. J. Agric. Food Chem. 2001, 49, 1035–1041. [Google Scholar] Pupin, A. M.; Dennis, M. J.; Toledo, M. C. F. Polymethoxylated flavones in Brazilian orange juice. Food Chem. 1998, 63, 513–518. [Google Scholar] [CrossRef]
- On top of these, it should be mentioned that rutin (39) has also been found, but being cited only once, and reported in mg/100 g fresh weight [62a], it has been omitted from this discussion.
- Green, C. O.; Wheatley, A. O.; Osagie, A. U.; Morrison, E. Y. S. A.; Asemota, H. N. Determination of polymethoxylated flavones in peels of selected Jamaican and Mexican Citrus (Citrus spp.) cultivars by high-performance liquid chromatography. Biomed. Chromatogr. 2007, 21, 48–54. [Google Scholar] [CrossRef]
- Theodoridis, G.; Lasáková, M.; Skeríková, V.; Tegou, A.; Giantsiou, N.; Jandera, P. Molecular imprinting of natural flavonoid antioxidants: application in solid-phase extraction for the sample pretreatment of natural products prior to HPLC analysis. J. Sep. Sci. 2006, 29, 2310–2321. [Google Scholar] [CrossRef] Vanamala, J.; Reddivari, L.; Yoo, K. S.; Pike, L. M.; Patil, B. S. Variation in the content of bioactive flavonoids in different brands of orange and grapefruit juices. J. Food Comp. Anal. 2006, 19, 157–166. [Google Scholar] [CrossRef] Kanaze, F. I.; Gabrieli, C.; Kokkalou, E.; Georgarakis, M.; Niopas, I. Simultaneous reversed-phase high-performance liquid chromatographic method for the determination of diosmin, hesperidin, and naringin in different Citrus fruit juices and pharmaceutical formulations. J. Pharm. Biomed. Anal. 2003, 33, 243–249. [Google Scholar] [CrossRef] Mouly, P. P.; Gaydou, E. M.; Auffray, A. Simultaneous separation of flavanones glycosides and polymethoxylated flavones in Citrus juices using liquid chromatography. J. Chrom. A 1998, 800, 171–179. [Google Scholar] [CrossRef] Belajová, E.; Suhaj, M. Determination of phenolic constituents in Citrus juices: Method of high performance liquid chromatography. Food Chem. 2004, 86, 339–343. [Google Scholar] [CrossRef] Justesen, U.; Knuthsen, P.; Leth, T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A. 1998, 799, 101–110. [Google Scholar] [CrossRef]
- Ooghe, W. C.; Ooghe, S. J.; Detavernier, C. M.; Huyghebaert, A. Characterization of orange juice (Citrus sinensis) by flavanone glycosides. J. Agric. Food Chem. 1994, 42, 2183–2190. [Google Scholar] [CrossRef]
- Nogata, Y.; Ohta, H.; Yoza, K. I.; Berhow, M.; Hasegawa, S. High-performance liquid-chromatographic determination of naturally-occurring flavonoids in Citrus with a photodiode-array detector. J. Chrom. A 1994, 667, 59–66. [Google Scholar] [CrossRef]
- Ooghe, W. C.; Detavernier, C. M. Detection of the addition of Citrus reticulata and hybrids to Citrus sinensis by flavonoids. J Agric. Food Chem. 1997, 45, 1633–1637. [Google Scholar] [CrossRef]
- Marín, F. R.; Martinez, M.; Uribesalgo, T.; Castillo, S.; Frutos, M. J. Changes in nutraceutical composition of lemon juices according to different industrial extraction systems. Food Chem. 2002, 78, 319–324. [Google Scholar] [CrossRef]
- Quercetin has also been reported as a component of lemon juice by Hertog and coworkers: being the data presented not comparable to the data discussed in this review, it was omitted from Table 12. See. Hertog, M. G. L.; Hollman, P. C. H.; van de Putte, B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J. Agric. Food Chem. 1993, 41, 1242–1246. [Google Scholar] [CrossRef]
- Marín, F. R.; Martinez, M.; Uribesalgo, T.; Castillo, S.; Frutos, M. J. Changes in nutraceutical composition of lemon juices according to different industrial extraction systems. Food Chem. 2002, 78, 319–324. [Google Scholar] [CrossRef]
- Mertens-Talcott, S. U.; Zadezensky, I.; De Castro, W. V.; Derendorf, H.; Butterweck, V. Grapefruit-drug interactions: can interactions with drugs be avoided? J. Clin. Pharmacol. 2006, 46, 1390–1416. [Google Scholar] [CrossRef] De Castro, W. V.; Mertens-Talcott, S.; Rubner, A.; Butterweck, V.; Derendorf, H. Variation of flavonoids and furanocoumarins in grapefruit juices: a potential source of variability in grapefruit juice–drug interaction studies. J. Agric. Food Chem. 2006, 54, 249–255. [Google Scholar] Lee, H. S.; Kim, J. G. Effects of debittering on red grapefruit juice concentrate. Food Chem. 2003, 82, 177–180. [Google Scholar] [CrossRef] Ho, P. C.; Saville, D. J.; Coville, P. F.; Wanwimolruk, S. Content of CYP3A4 inhibitors, naringin, naringenin and bergapten in grapefruit and grapefruit juice products. Pharm. Acta Helv. 2000, 74, 379–385. [Google Scholar] [CrossRef] Mouly, P. P.; Arzouyan, C. R.; Gaydou, E. M.; Estienne, J. M. Chromatographie des flavanosides des jus de différentes variété de pamplenousses. Différenciation par analyses statistiques multidimensionnelles. Analusis 1995, 23, 336–341. [Google Scholar]
- Rao, A. V.; Rao, L. G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] Rao, A. V.; Ray, M. R.; Rao, L. G. Lycopene. Adv. Food Nutr. Res. 2006, 51, 99–164. [Google Scholar] [CrossRef]
- Trillini, B. Grapefruit: the last decade acquisitions. Fitoterapia 2000, 71, S29–S37. [Google Scholar] [CrossRef] Bailey, D. G.; Malcolm, J.; Arnold, O.; Spence, J. D. Grapefruit juice-drug interactions. Brit. J. Clin. Pharmacol. 1998, 46, 101–110. [Google Scholar] Ameer, B.; Weintraub, R. A. Drug interactions with grapefruit juice. Clin. Pharmacokinet. 1997, 33, 103–121. [Google Scholar] [CrossRef]
- Ross, S. A.; Ziska, D. S.; Zhao, K.; El Sohly, M. A. Variance of common flavonoids by brand of grapefruit juice. Fitoterapia 2000, 71, 154–161. [Google Scholar] [CrossRef]
- Singh, S. V.; Jain, R. K.; Gupta, A.; Dhatt, A. S. Debittering of Citrus juices - A review. J. Food Sci. Tech. Mys. 2003, 40, 247–253. [Google Scholar] Puri, M.; Marwaha, S. S.; Kothari, R. M; Kennedy, J. F. Biochemical basis of bitterness in Citrus fruit juices and biotech approaches for debittering. Crit. Rev. Biotechnol. 1996, 16, 145–155. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641-1673. https://doi.org/10.3390/12081641
Gattuso G, Barreca D, Gargiulli C, Leuzzi U, Caristi C. Flavonoid Composition of Citrus Juices. Molecules. 2007; 12(8):1641-1673. https://doi.org/10.3390/12081641
Chicago/Turabian StyleGattuso, Giuseppe, Davide Barreca, Claudia Gargiulli, Ugo Leuzzi, and Corrado Caristi. 2007. "Flavonoid Composition of Citrus Juices" Molecules 12, no. 8: 1641-1673. https://doi.org/10.3390/12081641




