Effect of Cooking Processes on the Contents of Two Bioactive Carotenoids in Solanum lycopersicum Tomatoes and Physalis ixocarpa and Physalis philadelphica Tomatillos
Abstract
:Introduction
Results and Discussion
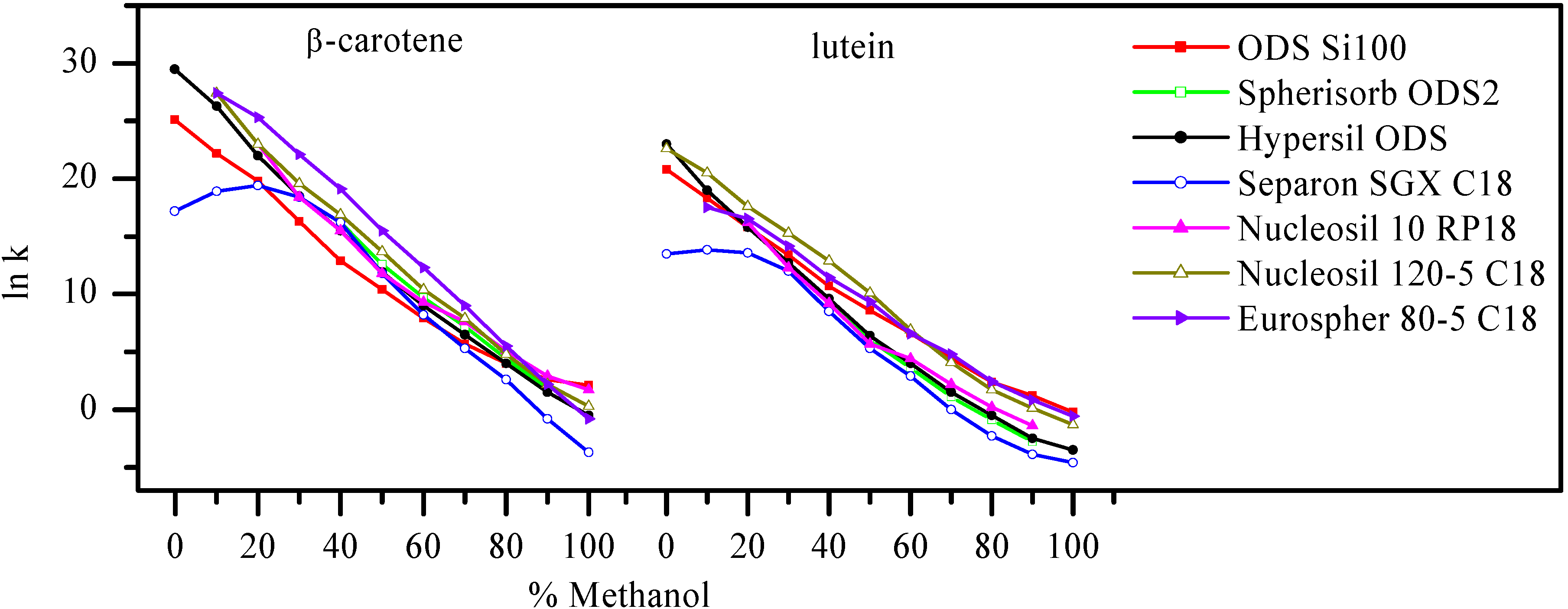
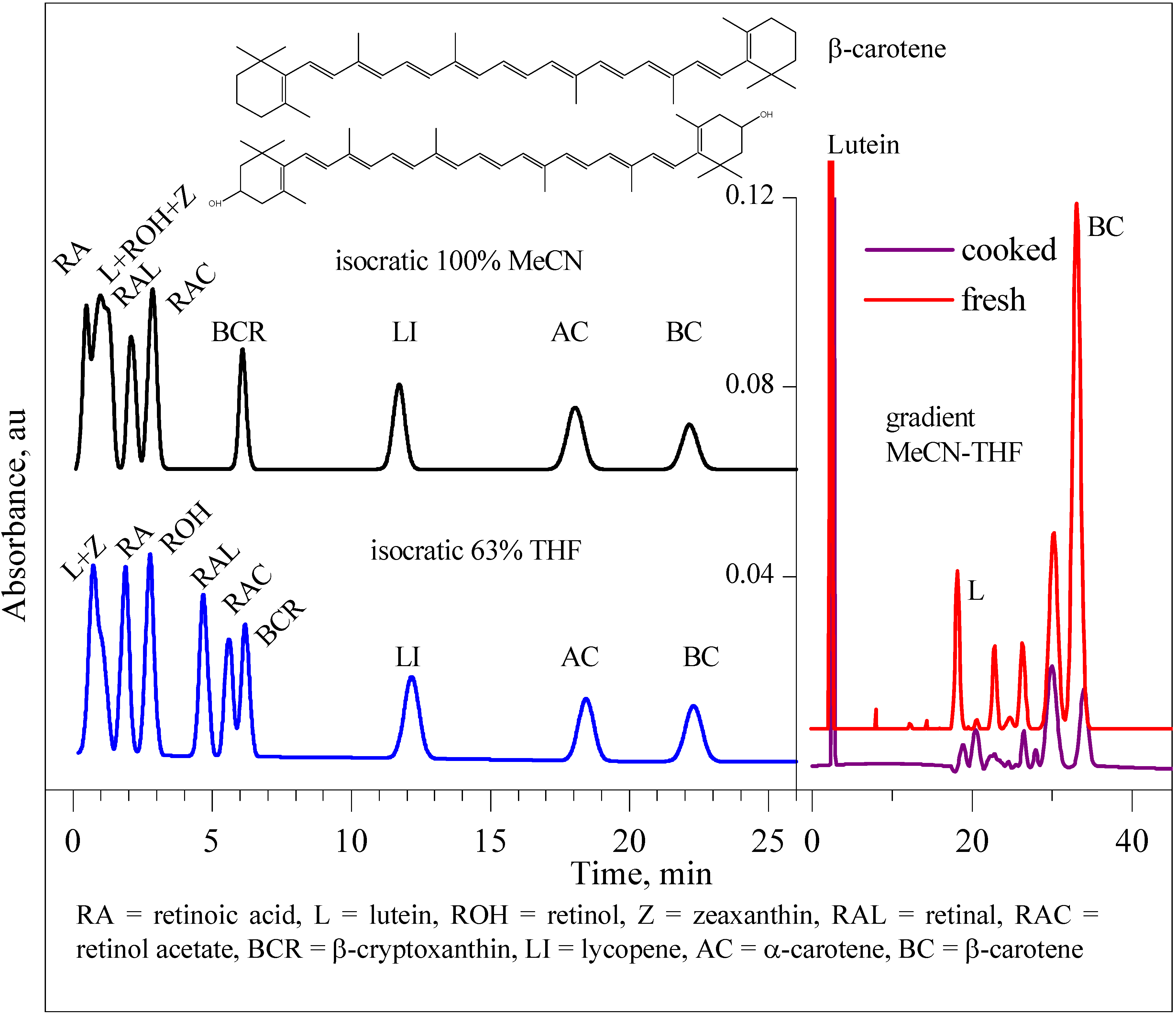
| Tomato/tomatillo | 1State | 2β-carotene, μg/100 mg | 2lutein, μg/100 mg |
|---|---|---|---|
| Guajillo tomato (Lycopersicum periforme Dun) | fresh | 403 ± 12 | 12 ± 2 |
| cooked | 292 ± 12 | 10 ± 2 | |
| Cherry tomato (Lycopersicum cerasiforme) | fresh | 254 ± 30 | 32 ± 3 |
| cooked | 26 ± 10 | 13 ± 1 | |
| Saladet tomato (Lycopersicum esculentum) | fresh | 6 ± 1 | 9 ± 1 |
| cooked | 1 ± 1 | 1 ± 1 | |
| Beef tomato (Lycopersicum esculentum Mill) | fresh | 6 ± 1 | 2 ± 1 |
| cooked | bdl | 2 ± 1 | |
| Tomatillo (Physalis philadelphica) | fresh | 21 ± 3 | 12 ± 1 |
| boiled | 21 ± 1 | 10 ± 1 | |
| cooked | 1 ± 1 | bdl | |
| microwaved | 10 ± 1 | 8 ± 1 | |
| Husk tomato (Physalis ixocarpa) | fresh | 2 ± 1 | 1 ± 1 |
| boiled | 2 ± 1 | bdl | |
| cooked | bdl | 1 ± 1 | |
| microwaved | bdl | bdl |
Conclusions
Experimental
General
Equipment
Sample preparation
Acknowledgements
References
- Aust, O.; Sies, H.; Stahl, W. Analysis of lipophilic antioxidants in human serum and tissues: tocopherols and antioxidants. J. Chromatogr. A. 2001, 936, 83–93. [Google Scholar] [CrossRef]
- Went, F.W.; LeRosen, A.L.; Zechmeister, L. Effect of external factors on tomato pigments as studied by chromatographic methods. Plant Physiol. 1942, 17, 91–100. [Google Scholar] Dharmapuri, S.; Rosati, C.; Pallara, P.; Aquilani, R.; Bouvier, F.; Camara, B.; Giuliano, G. Metabolic engineering of xanthophyll content in tomato fruits. FEBS Lett. 2002, 22, 30–4. [Google Scholar]
- Suvarnakuta, P.; Devahastin, S.; Mujumdar, A.S. Drying kinetics and β-carotene degradation in carrot undergoing different drying processes. J. Food Sci. 2005, 70, 520–526. [Google Scholar] [CrossRef]
- Shi, X.-M.; Chen, F. Stability of lutein under various storage conditions. Nahrung 1997, 41, 38–41. [Google Scholar] [CrossRef]
- Tai, C.-Y.; Chen, B.H. Analysis and stability of carotenoids in the flowers of Daylily (Hemerocallis disticha) as affected by various treatments. J. Agric. Food Chem. 2000, 48, 5962–5968. [Google Scholar] [CrossRef]
- Baranska, M.; Schütze, W.; Schulz, H. Determination of lycopene and β-carotene content in tomato fruits and related products. Comparison of FT-Raman, ATR-IR, and NIR spectroscopy. Anal. Chem. 2006, 78, 8456–8461. [Google Scholar] [CrossRef]
- Barua, A.B. Improved normal-phase and reversed-phase gradient high-performance liquid chromatography procedures for the analysis of retinoids and carotenoids in human serum, plant and animal tissues. J. Chromatogr. A 2001, 936, 71–82. [Google Scholar] Liu, H.L.; Kao, T.H.; Chen, B.H. Determination of carotenoids in the Chinese medical herb Jiao-Gu-Lan (Gynistemma Pentaphyllum MAKINO) by liquid chromatography. Chromatographia 2004, 60, 411–417. [Google Scholar]
- Vuong, L.T.; Dueker, S.R.; Murphy, S.P. Plasma β-carotene and retinol concentrations of children increase after a 30-d supplement with the fruit Momordica cochinchinensis (gac). Am J. Clin. Nutr. 2002, 75, 872–9. [Google Scholar]
- Pott, I.; Marx, M.; Neidhart, S.; Mühlbauer, W.; Carle, R. Quantitative determination of β-carotene stereoisomers in fresh, dried, and solar-dried mangoes (Mangifera indica L.). J. Agric. Food. Chem. 2003, 51, 4527–4531. [Google Scholar] [CrossRef]
- De La Cruz-García, C.; González-Castro, M.J.; Oruña-Concha, M.J.; López-Hernández, J.; Simal-Lozano, J.A.; Simal-Gándara, J. The effects of various culinary treatments on the pigment content of green beans (Phaseolus vulgaris, L.). Food Res. Int. 1997, 30, 787–791. [Google Scholar] [CrossRef]
- Ishida, B.K.; Chapman, M.H. A comparison of carotenoid content and total antioxidant activity in catsup from several commercial sources in the United States. J. Agric. Food Chem. 2004, 52, 8017–8020. [Google Scholar] [CrossRef]
- Bock, M.A.; Sanchez-Pilcher, J.; McKee, L.J.; Ortiz, M. Selected nutritional and quality analyses of tomatillos (Physalis ixocarpa). Plant Foods Hum. Nutr. 1995, 48, 127–133. [Google Scholar] [CrossRef]
- Kennelly, E.J.; Gerhäuser, C.; Song, L.L.; Graham, J.G.; Beecher, Ch.W.W.; Pezzuto, J.M.; Kinghorn, A.D. Induction of quinine reductase by whithanolides isolated from Physalis philadelphica (Tomatillos). J. Agric. Food Chem. 1997, 45, 3771–3777. [Google Scholar] [CrossRef]
- Choi, K.; Lee, G.; Han, Y.J.; Bunn, J.M. Tomato maturity evaluation using color image analysis. Trans. Am. Soc. Agric. Eng. 1995, 38, 171–176. [Google Scholar] [CrossRef]
- Gonzalez, E.; Montenegro, M.A.; Nazareno, M.A.; López de Mishima, B.A. Carotenoid composition and vitamin A value of an Argentinian squash (Cucurbita moschata). Arch. Latinoam. Nutr. 2001, 51, 395–399. [Google Scholar]
- Sample Availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Elizalde-González, M.P.; Hernández-Ogarcía, S.G. Effect of Cooking Processes on the Contents of Two Bioactive Carotenoids in Solanum lycopersicum Tomatoes and Physalis ixocarpa and Physalis philadelphica Tomatillos. Molecules 2007, 12, 1829-1835. https://doi.org/10.3390/12081829
Elizalde-González MP, Hernández-Ogarcía SG. Effect of Cooking Processes on the Contents of Two Bioactive Carotenoids in Solanum lycopersicum Tomatoes and Physalis ixocarpa and Physalis philadelphica Tomatillos. Molecules. 2007; 12(8):1829-1835. https://doi.org/10.3390/12081829
Chicago/Turabian StyleElizalde-González, María P., and Socorro G. Hernández-Ogarcía. 2007. "Effect of Cooking Processes on the Contents of Two Bioactive Carotenoids in Solanum lycopersicum Tomatoes and Physalis ixocarpa and Physalis philadelphica Tomatillos" Molecules 12, no. 8: 1829-1835. https://doi.org/10.3390/12081829




