Biological Activities of Hydrazone Derivatives
Abstract
:Contents:
| 1.Introduction | 1911 |
| 2. Biological activity | 1915 |
| 2.1. Anticonvulsant Activity | 1915 |
| 2.2. Antidepressant Activity | 1916 |
| 2.3. Analgesic, Antiinflammatory and Antiplatelet Activity | 1916 |
| 2.4. Antimalarial Activity | 1918 |
| 2.5. Antimicrobial Activity | 1919 |
| 2.6. Antimycobacterial Activity | 1922 |
| 2.7. Antitumoral Activity | 1929 |
| 2.8. Vasodilator Activity | 1933 |
| 2.9. Antiviral Activity | 1933 |
| 3.0. Schistosomiasis | 1933 |
| 3. Review articles related with hydrazones | 1934 |
Introduction





2. Biological activity
2.1. Anticonvulsant Activity



2.2. Antidepressant Activity

2.3. Analgesic, Antiinflammatory and Antiplatelet Activity






2.4. Antimalarial Activity



2.5. Antimicrobial Activity










2.6. Antimycobacterial Activity























2.7. Antitumoral Activity












2.8. Vasodilator Activity

3.0. Schistosomiasis
References and Notes
- Rollas, S.; Gülerman, N.; Erdeniz, H. Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines. Farmaco 2002, 57, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Küçükgüzel, Ş.G.; Rollas, S.; Küçükgüzel, İ; Kiraz, M. Synthesis and Antimycobacterial activity of some coupling products from 4-aminobenzoic acid hydrazones. Eur. J. Med. Chem. 1999, 34, 1093–1100. [Google Scholar]
- Küçükgüzel, Ş.G.; Oruç, E.E.; Rollas, S.; Şahin, F.; Özbek, A. Synthesis, Characterization and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur. J. Med. Chem. 2002, 37, 197–206. [Google Scholar]
- Kaymakçıoğlu, K.B.; Oruç, E.E.; Unsalan, S.; Kandemirli, F.; Shvets, N.; Rollas, S.; Anatholy, D. Synthesis and characterization of novel hydrazide-hydrazones and the study of their structure-antituberculosis activity. Eur. J. Med. Chem 2006, 41, 1253–1261. [Google Scholar]
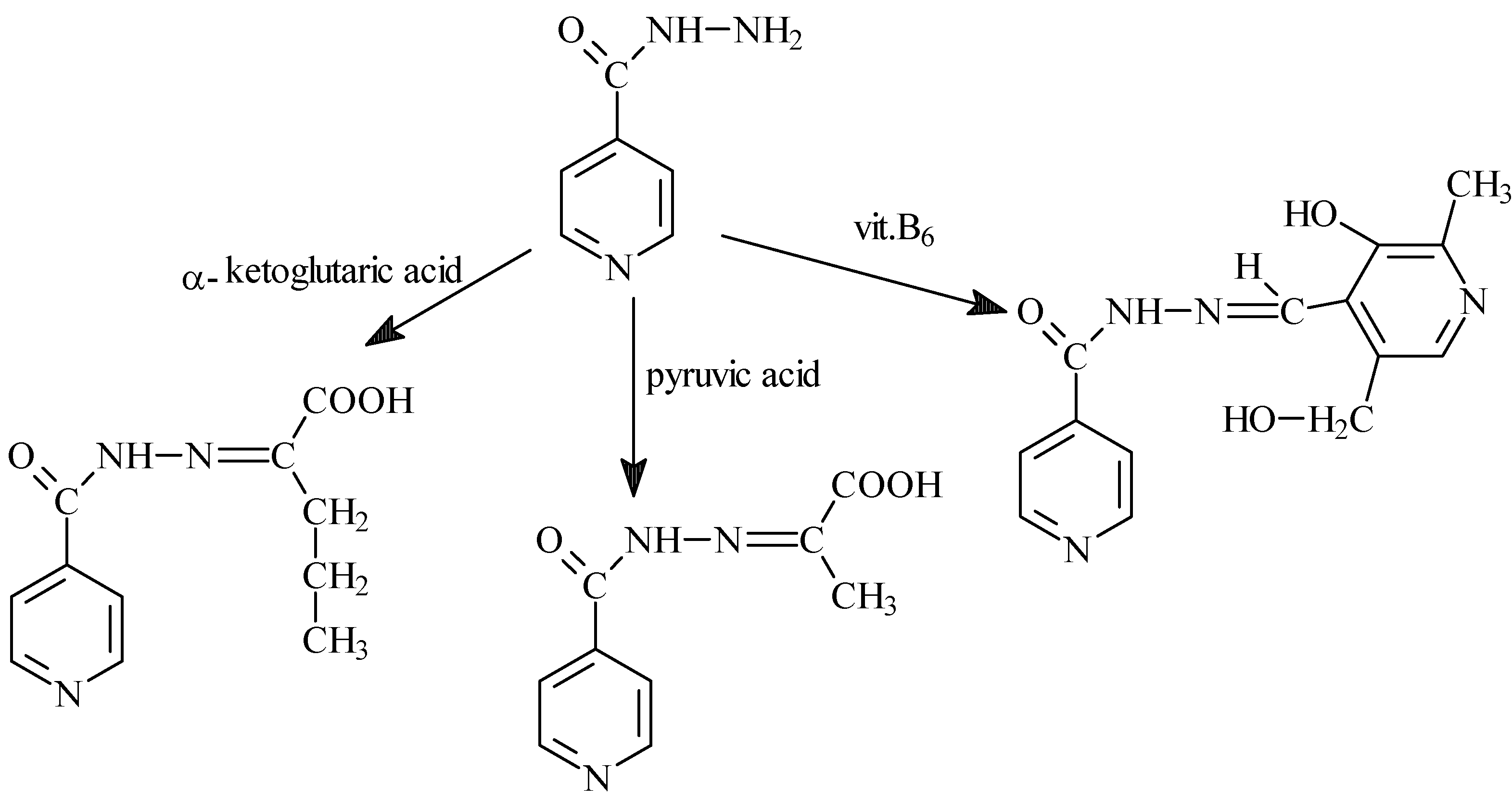
- Sah, P.P.T.; Peoples, S.A. Isonicotinyl hydrazones as antitubercular agents and derivatives for idendification of aldehydes and ketones. J. Am. Pharm. Assoc. 1954, 43, 513–524. [Google Scholar] [CrossRef]
- Bavin, E.M.; Drain, D.J.; Seiler, M.; Seymour, D.E. Some further studies on tuberculostatic compounds. J. Pharm. Pharmacol. 1954, 4, 844–855. [Google Scholar]
- Buu-Hoi, P.H.; Xuong, D.; Nam, H.; Binon, F.; Royer, R. Tuberculostatic hydrazides and their derivatives. J. Chem. Soc. 1953, 1358–1364. [Google Scholar]
- Amâl, H.; Ergenç, N. Some isonicoticoyl-hydrazones. İ.Ü. Fen Fakültesi Mecmuası. 1957, 22, 390–392. [Google Scholar]
- Singh, V.; Srivastava, V.K.; Palit, G.; Shanker, K. Coumarin congeners as antidepressants. Arzneim-Forsch. Drug. Res. 1992, 42, 993–996. [Google Scholar]
- Ergenç, N.; Günay, N.S. Synthesis and antidepressant evaluation of new 3-phenyl-5-sulfonamidoindole derivatives. Eur. J. Med. Chem. 1998, 33, 143–148. [Google Scholar]
- Durgun, B.B.; Çapan, G.; Ergenç, N.; Rollas, S. Synthesis, characterisation and biological evaluation of new benylidenebenzohydrazides and 2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazoles. Pharmazie 1993, 48, 942–943. [Google Scholar]
- Doğan, H.N.; Duran, A.; Rollas, S.; Şener, G.; Armutak, Y.; Keyer-Uysal, M. Synthesis and structure elucidation of some new hydrazones and oxadiazolines : anticonlsant activitites of 2-(3-acetyloxy-2-naphtyl)-4-acetyl-5-substituted-1,3,4-oxadiazolines. Med. Sci. Res. 1998, 26, 755–758. [Google Scholar]
- Kalsi, R.; Shrimali, M.; Bhalla, T.N.; Barthwal, J.P. Synthesis and anti-inflammatory activity of indolyl azetidinones. Indian J. Pharm. Sci. 2006, 41, 353–359. [Google Scholar]
- Küçükgüzel, Ş.G.; Kocatepe, A.; De Clercq, E.; Şahin, F.; Güllüce, M. Synthesis and biological activity of 4-thiazolidinones, thiosemicarbazides derived from diflunisal hydrazide. Eur. J. Med. Chem. 2006, 41, 353–359. [Google Scholar]
- Kömürcü, Ş.G.; Rollas, S.; Ülgen, M.; Gorrod, J.W.; Çevikbaş, A. Evaluation of Some Arylhydrazones of p-Aminobenzoic acid hydrazide as Antimicrobial Agents and Their in-vitro Hepatic Microsomal Metabolism. Boll. Chim. Farm. 1995, 134, 375–379. [Google Scholar]
- Ülgen, M.; Durgun, B.B.; Rollas, S.; Gorrod, J.W. The in vitro hepatic microsomal metabolism of benzoic acid benzylidenehydrazide. Drug Metab. Interact. 1997, 13, 285–294. [Google Scholar]
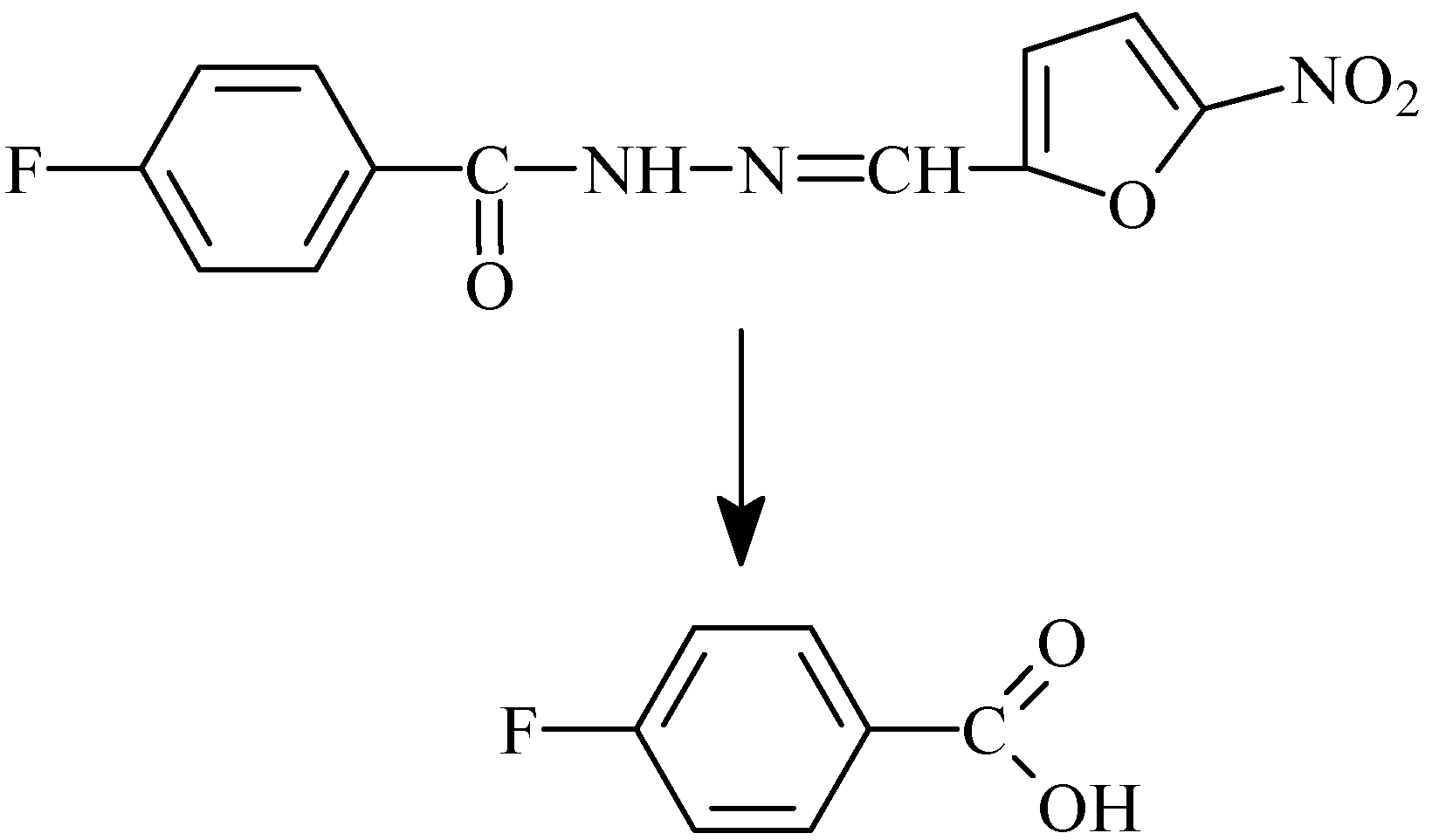
- Gülerman, N.N.; Oruç, E.E.; Kartal, F.; Rollas, S. In vivo metabolism of 4-fluorobenzoic acid [(5-nitro-2-furanyl)methylene] hydrazide in rats. Eur. J .Drug Metab. Pharmacokinet. 2000, 25, 103–108. [Google Scholar]
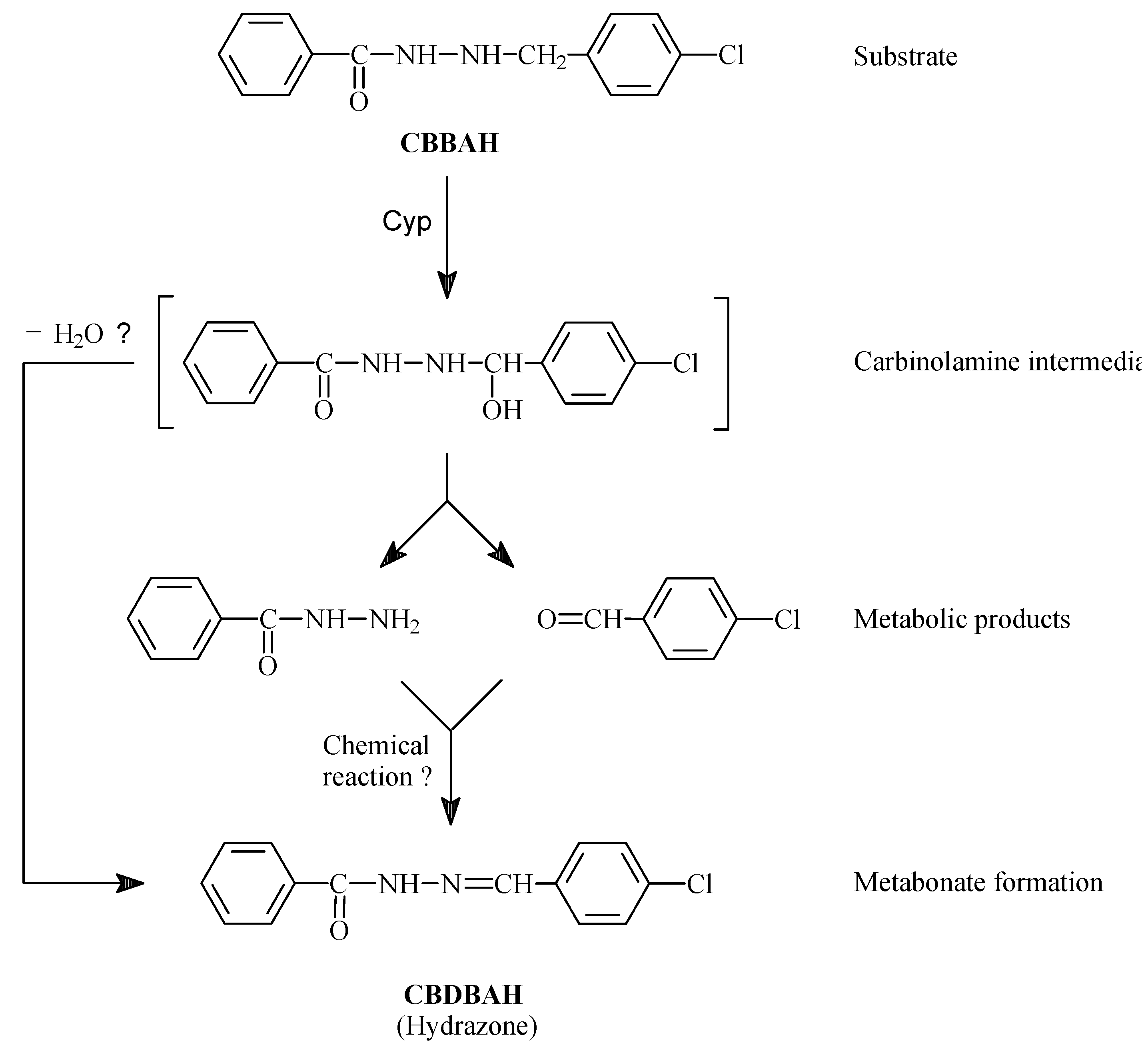
- Küçükgüzel, Ş.G.; Küçükgüzel, İ.; Ülgen, M. Metabolic and Chemical Studies on N-(4-chlorobenzyl)-N′-benzoylhydrazine. Farmaco 2000, 55, 624–630. [Google Scholar]
- Dimmock, J.R.; Vashishtha, S.C.; Stables, J.P. Anticonvulsant properties of various acetylhydrazones, oxamoylhydrazones and semicarbazones derived from aromatic and unsaturated carbonyl compounds. Eur. J. Med. Chem. 2000, 35, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Çakır, B.; Dağ, Ö.; Yıldırım, E.; Erol, K.; Şahin, M.F. Synthesis and anticonvulsant activity of some hydrazones of 2-[(3H)-oxobenzoxazolin-3-yl-aceto]hydrazide. J. Fac. Pharm. Gazi. 2001, 18, 99–106. [Google Scholar]
- Ragavendran, J.; Sriram, D.; Patel, S.; Reddy, I.; Bharathwajan, N.; Stables, J.; Yogeeswari, P. Design and synthesis of anticonvulsants from a combined phthalimide-GABA-anilide and hydrazone pharmacophore. Eur. J. Med. Chem. 2007, 42, 146–151. [Google Scholar]
- Todeschini, A.R.; Miranda, A.L.; Silva, C.M.; Parrini, S.C.; Barreiro, E.J. Synthesis and evaluation of analgesic, antiinflammatory and antiplatelet properties of new 2-pyridylarylhydrazone derivatives. Eur. J. Med. Chem. 1998, 33, 189–199. [Google Scholar] [CrossRef]
- Lima, P.C.; Lima, L.M.; Silva, K.C.; Leda, P.H.; Miranda, A.L.P.; Fraga, C.A.M; Barreiro, E.J. Synthesis and analgesic activity of novel N-acylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000, 35, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Fraga, A.G.M.; Rodrigues, C.R.; Miranda, A.L.P.; Barreiro, E.J.; Fraga, C.A.M. Synthesis and pharmacological evaluation of novel heterocyclic acylhydrazone derivatives, designed as PAF antagonists. Eur. J. Pharm. Sci. 2000, 11, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.; Costa, L.M.M.; Brito, F.C.F.; Miranda, A.L.P.; Barreiro, E.J.; Fraga, C.A.M. New class of potent antinociceptive and antiplatelet 10H-phenothiazine-1-acylhydrazone derivatives. Bioorg. Med. Chem 2004, 12, 3149–3158. [Google Scholar]
- Salgın-Gökşen, U.; Gökhan-Kelekçi, N.; Göktaş, Ö.; Köysal, Y.; Kılıç, E.; Işık, Ş.; Aktay, G.; Özalp, M. 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg. Med. Chem. 2007, 15, 5738–5751. [Google Scholar]
- Duarte, C.D.; Tributino, J.L.M.; Lacerda, D.I.; Martins, M.V.; Alexandre-Moreira, M.S.; Dutra, F.; Bechara, E.J.H.; De-Paula, F.S.; Goulart, M.O.F.; Ferreira, J.; Calixto, J.B.; Nunes, M.P.; Bertho, A.L.; Miranda, A.L.P.; Barreiro, E.J.; Fraga, C.A.M. Synthesis, pharmacological evaluation and electrochemical studies of novel 6-nitro-3,4-methylenedioxyphenyl-N-acylhydrazone derivatives : Discovery of LASSBio-881, a new ligand of cannabinoid receptors. Bioorg. Med. Chem. 2007, 15, 2421–2433. [Google Scholar] [CrossRef] [PubMed]
- Walcourt, A.; Loyevsky, M.; Lovejoy, D.B.; Gordeuk, V.R.; Richardson, D.R. Novel aroylhydrazone and thiosemicarbazone iron chelators with anti-malarial activity against chloroquine-resistant and -sensitive parasites. Int. J. Biochem. Cell Biol. 2004, 36, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Gemma, S.; Kukreja, G.; Fattorusso, C.; Persico, M.; Romano, M.; Altarelli, M.; Savini, L.; Campiani, G.; Fattorusso, E.; Basilico, N. Synthesis of N1-arylidene-N2-quinolyl- and N2-acrydinylhydrazones as potent antimalarial agents active against CQ-resistant P. falciparum strains. Bioorg. Med. Chem. Lett. 2006, 16, 5384–5388. [Google Scholar] [CrossRef]
- Bernardino, A.; Gomes, A.; Charret, K.; Freitas, A.; Machado, G.; Canto-Cavalheiro, M.; Leon, L.; Amaral, V. Synthesis and leishmanicidal activities of 1-(4-X-phenyl)-N′-[(4-Y-phenyl)methylene]-1H-pyrazole-4-carbohydrazides. Eur. J. Med. Chem. 2006, 41, 80–87. [Google Scholar]
- Küçükgüzel, Ş.G.; Rollas, S.; Erdeniz, H.; Kiraz, M. Synthesis, Characterization and Antimicrobial Evaluation of Ethyl 2-Arylhydrazono-3-oxobutyrates. Eur. J. Med. Chem. 1999, 34, 153–160. [Google Scholar]
- Tavares, L.C.; Chiste, J.J.; Santos, M.G.B.; Penna, T.C.V. Synthesis and biological activity of nifuroxazide and analogs. II. Boll. Chim. Farm. 1999, 138, 432–436. [Google Scholar]
- Ulusoy, N.; Çapan, G.; Ötük, G.; Kiraz, M. Synthesis and antimicrobial activity of new 6- phenylimidazo[2,1-b]thiazole derivatives. Boll. Chim. Farm. 2000, 139, 167–172. [Google Scholar]
- Turan-Zitouni, G.; Blache, Y.; Güven, K. Synthesis and antimicrobial activity of some imidazo-[1,2-a]pyridine-2-carboxylic acid arylidenehydrazide derivatives. Boll. Chim. Farm. 2001, 140, 397–400. [Google Scholar]
- Vicini, P.; Zani, F.; Cozzini, P.; Doytchinova, I. Hydrazones of 1,2-benzisothiazole hydrazides: synthesis, antimicrobial activity and QSAR investigations. Eur. J. Med. Chem. 2002, 37, 553–564. [Google Scholar]
- Küçükgüzel, Ş.G.; Mazi, A.; Şahin, F.; Öztürk, S.; Stables, J. P. Synthesis and biological activities of diflunisal hydrazide-hydrazones. Eur. J. Med. Chem. 2003, 38, 1005–1009. [Google Scholar]
- Loncle, C.; Brunel, J.; Vidal, N.; Dherbomez, M.; Letourneux, Y. Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur. J. Med. Chem. 2004, 39, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Masunari, A.; Tavares, L.C. A new class of nifuroxazide analogues : Synthesis of 5-nitrophene derivatives with antimicrobial activity against multidrug-resistant Staphylococcus aureus. Bioorg. Med. Chem. 2007, 15, 4229–4236. [Google Scholar] [CrossRef] [PubMed]
- www.TAACF.org.
- Bukowski, L.; Janowiec, M. 1-Methyl-1H-2-imidazo[4,5-b]pyridinecarboxylic acid and some of derivatives with suspected antituberculotic activity. Pharmazie 1996, 51, 27–30. [Google Scholar]
- Cocco, M.T.; Congiu, C.; Onnis, V.; Pusceddo, M.C.; Schivo, M.L.; De Logu, A. Synthesis and antimycobacterial activity of some isonicotinoylhydrazones. Eur. J. Med. Chem. 1999, 34, 1071–1076. [Google Scholar] [CrossRef]
- Bukowski, L.; Janowiec, M.; Zwolska-Kwiek, Z.; Andrzejczyk, Z. Synthesis and some reactions of 2- acetylimidazo[4,5-b]pyridine. Antituberculotic activity of the obtained compounds. Pharmazie 1999, 54, 651–654. [Google Scholar]
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Vio, L.; Banfi, E. Synthesis and antimycobacterial activity of [5-(pyridin-2-yl)-1,3,4-thiadiazole-2-ylthio]acetic acid arylidene-hydrazide derivatives. Farmaco 2001, 56, 587–592. [Google Scholar]
- Ulusoy, N.; Gürsoy, A.; Ötük, G.; Kiraz, M. Synthesis and antimicrobial activity of some 1,2,4-triazole-3-mercaptoacetic acid derivatives. Farmaco 2001, 56, 947–952. [Google Scholar]
- Savini, L.; Chiasserini, L.; Gaeta, A.; Pellerano, C. Synthesis and Anti-tubercular Evaluation of Quinolylhydrazones. Bioorg. Med. Chem. 2002, 10, 2193–2198. [Google Scholar]
- Rando, D.; Sato, D.N.; Siqueira, L.; Malvezzi, A.; Leite, C.Q.F.; Amaral, A.T.; Ferreira, E.I.; Tavares, L.C. Potential tuberculostatic agents. Topliss application on benzoic acid [(5-Nitro-thiophene-2-yl)methylene] hydrazide series. Bioorg. Med. Chem. 2002, 10, 557–560. [Google Scholar]
- Kaymakçıoğlu, K.B.; Rollas, S. Synthesis, characterization and evaluation of antituberculosis activity of some hydrazones. Farmaco 2002, 57, 595–599. [Google Scholar]
- Küçükgüzel, Ş.G.; Rollas, S. Synthesis, Characterization of Novel Coupling Products and 4-Arylhydrazono-2-pyrazoline-5-ones as Potential Antimycobacterial Agents. Farmaco 2002, 57, 583–587. [Google Scholar]
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Vio, L.; Banfi, E.; Scialino, G. Synthesis and antimycobacterial activity of (3,4-diaryl-3H-thiazole-2-ylidene)hydrazide derivatives. Farmaco 2003, 58, 631–637. [Google Scholar]
- Sriram, D.; Yogeeswari, P.; Madhu, K. Synthesis and in vitro and in vivo antimycobacterial activity of isonicotinoyl hydrazones. Bioorg. Med. Chem. Lett. 2005, 15, 4502–4505. [Google Scholar] [CrossRef] [PubMed]
- Maccari, R.; Ottana, R.; Vigorita, M.G. In vitro advanced antimycobacterial screening of isoniazid-related hydrazones, hydrazides and cyanoboranes: Part 14. Bioorg. Med. Chem. Lett. 2005, 15, 2509–2513. [Google Scholar] [CrossRef] [PubMed]
- Shindikar, A.V.; Viswanathan, C.L. Novel fluoroquinolones : design, synthesis, and in vivo activity in mice against Mycobacterium tuberculosis H37 Rv. Bioorg. Med. ChemLett. 2005, 15, 1803–1806. [Google Scholar]
- Sinha, N.; Jain, S.; Tilekar, A.; Upadhayaya, R.S.; Kishore, N.; Jana, G.H.; Arora, S.K. Synthesis of isonicotinic acid N′-Arylidene-N-[2-oxo-2-(4-aryl-piperazin-1-yl)ethyl]hydrazides as antituberculosis agents. Bioorg. Med. Chem Lett. 2005, 15, 1573–1576. [Google Scholar]
- Sriram, D.; Yogeeswari, P.; Madhu, K. Synthesis and in vitro antitubercular activity of some 1-[(4-sub)phenyl]-3-(4-{1-[(pyridine-4-carbonyl)hydrazono]ethyl} phenyl)thiourea. Bioorg. Med. Chem. 2006, 14, 876–878. [Google Scholar] [CrossRef]
- Nayyar, A.; Malde, A.; Coutinho, E.; Jain, R. Synthesis, anti-tuberculosis activity, and 3D-QSAR study of ring-substituted-2/4-quinolinecarbaldehyde derivatives. Bioorg. Med. Chem. 2006, 14, 7302–7310. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Yogeeswari, P.; Devakaram, R.V. Synthesis, in vitro and in vivo antimycobacterial activities of diclofenac acid hydrazones and amides. Bioorg. Med. Chem. 2006, 14, 3113–3118. [Google Scholar] [CrossRef] [PubMed]
- Bijev, A. New heterocyclic hydrazones in the search for antitubercular agents: Synthesis and in vitro evaluations. Lett.Drug Des. Discov. 2006, 3, 506–512. [Google Scholar]
- Shiradkar, M.R.; Murahari, K.K.; Gangadasu, H.R.; Suresh, T.; Kalyan, C.C.; Panchal, D.; Kaur, R.; Burange, P.; Ghogare, J.; Mokale, V.; Raut, M. Synthesis of new S-derivatives of clubbed triazolyl thiazole as anti-Mycobacterium tuberculosis agents. Bioorg. Med. Chem. 2007, 15, 3997–4008. [Google Scholar] [CrossRef] [PubMed]
- Imramovský, A.; Polanc, S.; Vinšová, J.; Kočevar, M.; Jampílek, J.; Rečková, Z.; Kaustová, J. A new modification of anti-tubercular active molecules. Bioorg. Med. Chem. 2007, 15, 2551–2559. [Google Scholar]
- Pandey, J.; Pal, R.; Dwivedi, A.; Hajela, K. Synthesis of some new diaryl and triaryl hydrazone derivatives as possible estrogen receptor modulators. Arzneimittelforschung. 2002, 52, 39–44. [Google Scholar] [PubMed]
- Abadi, A.H.; Eissa, A.A.H.; Hassan, G.S. Synthesis of novel 1,3,4-trisubstituted pyrazole derivatives and their evaluation as antitumor and antiangiogenic agents. Chem. Pharm. Bull. 2003, 51, 838–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terzioğlu, N.; Gürsoy, A. Synthesis and anticancer evaluation of some new hydrazone derivatives of 2,6-dimethylimidazo[2,1-b]-[1,3,4]thiadiazole-5-carbohydrazide. Eur. J. Med. Chem. 2003, 38, 781–786. [Google Scholar]
- Gürsoy, A.; Karali, N. Synthesis and primary cytotoxicity evaluation of 3-[[(3-phenyl-4(3H)-quinazolinone-2-yl)mercaptoacetyl]hydrazono]-1H-2-indolinones. Eur. J. Med. Chem. 2003, 38, 633–643. [Google Scholar]
- Savini, L.; Chiasserini, L.; Travagli, V.; Pellerano, C.; Novellino, E.; Cosentino, S.; Pisano, M.B. New α-heterocyclichydrazones : evaluation of anticancer, anti-HIV and antimicrobial activity. Eur.J.Med.Chem. 2004, 39, 113–122. [Google Scholar]
- Zhang, H.; Drewe, J.; Tseng, B.; Kasibhatla, S.; Cai, S.X. Discovery and SAR of indole-2-carboxylic acid benzylidenehydrazides as a new series of potent apoptosis inducers using a cell-based HTS assay. Bioorg. Med. Chem. 2004, 12, 3649–3655. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, N.; Karaoglu, S.; Demirbas, A.; Sancak, K. Synthesis and antimicrobial activities of some new 1-(5-phenylamino-[1,3,4]thiadiazol-2-yl)methyl-5-oxo-[1,2,4]triazole and 1-(4-phenyl-5-thioxo-[1,2,4]triazol-3-yl)methyl-5-oxo-[1,2,4]triazole derivatives. Eur. J. Med. Chem. 2004, 39, 793–804. [Google Scholar]
- Cocco, M. T.; Congiu, C.; Lilliu, V.; Onnis, V. Synthesis and in vitro antitumoral activity of new hydrazinopyrimidine-5-carbonitrile derivatives. Bioorg. Med. Chem. 2005, 14, 366–372. [Google Scholar]
- Vicini, P.; Incerti, M.; Doytchinova, I.; La Colla, P.; Busonera, B.; Loddo, R. Synthesis and antiproliferative activity of benzo[d]isothiazole hydrazones. Eur. J.Med. Chem. 2006, 41, 624–632. [Google Scholar]
- Jin, L.; Chen, J.; Song, B.; Chen, Z.; Yang, S.; Li, Q.; Hu, D.; Xu, R. Synthesis, structure, and bioactivity of N′-substitutedbenzylidene-3,4,5-trimethoxybenzohydrazide and 3-acetyl-2-substituted phenyl-5-(3,4,5-trimethoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 5036–5040. [Google Scholar]
- Gürsoy, E.; Güzeldemirci-Ulusoy, N. Synthesis and primary cytotoxicity evaluation of new imidazo[2,1-b]thiazole derivatives. Eur. J. Med. Chem. 2007, 42, 320–326. [Google Scholar]
- El-Hawash, S.A.M.; Abdel Wahab, A.E.; El-Dewellawy, M.A. Cyanoacetic acid hydrazones of 3-(and 4-) acetylpyridine and some derived ring systems as potential antitumor and anti-HCV agents. Arch. Pharm. Chem. Life Sci. 2006, 339, 14–23. [Google Scholar]
- Silva, A.G.; Zapata-Suto, G.; Kummerle, A.E.; Fraga, C.A.M.; Barreiro, E.J.; Sudo, R.T. Synthesis and vasodilatory activity of new N-acylhydrazone derivatives, designed as LASSBio-294 analogues. Bioorg. Med. Chem. 2005, 13, 3431–3437. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, M.T.; El-Sayed, W.A.; El-Ashry, E.H. Synthesis and antriviral evaluation of some sugar arylglycinoylhydrazones and their oxadiazoline derivatives. Arch. Pharm. Chem. Life Sci. 2006, 339, 656–663. [Google Scholar]
- Friedman, J.F.; Mital, P.; Kanzaria, H.K.; Olds, G.R.; Kurtis, J.D. Schistosomiasis and pregnancy. Trends Parasitol. 2007, 23, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.M.; Ali, H.M.; Homeida, M.M.; Bennett, J.L. Efficacy of a new Hoffmann-La Roche compound (RO-15-5458) against Schistosoma mansoni (Gezira strain, Sudan) in vervet monkeys (Cercopithecus aethiops). Trop. Med. Parasitol. 1989, 40, 335–336. [Google Scholar] [PubMed]
- Pereira, L.H.; Coelho, P.M.; Costa, J.O.; de Mello, R.T. Activity of 9-acridanone-hydrazone drugs detected at the prepostural phase, in the experimental schistosomiasis mansoni. Mem. Inst. Oswaldo Cruz. 1995, 90, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, A.; Jain, R. Recent advances in new structural classes of anti-tuberculosis agents. Curr. Med. Chem. 2005, 12, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Scior, T.; Garcés-Eisele, S.J. Isoniazid is not a lead compound for its pyridyl ring derivatives, isonicotinoyl amides, hydrazides and hydrazones: A critical review. Curr. Med. Chem. 2006, 13, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Janin, Y. Antituberculosis drugs: Ten years of research. Bioorg. Med. Chem. 2007, 15, 2479–2513. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Rollas, S.; Küçükgüzel, S.G. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910-1939. https://doi.org/10.3390/12081910
Rollas S, Küçükgüzel SG. Biological Activities of Hydrazone Derivatives. Molecules. 2007; 12(8):1910-1939. https://doi.org/10.3390/12081910
Chicago/Turabian StyleRollas, Sevim, and S. Güniz Küçükgüzel. 2007. "Biological Activities of Hydrazone Derivatives" Molecules 12, no. 8: 1910-1939. https://doi.org/10.3390/12081910



