Supramolecular Chirality in Crystalline Assemblies of Bile Acids and Their Derivatives; Three-Axial, Tilt, Helical, and Bundle Chirality
Abstract
:Table of Contents
| 1. | Introduction | 1974 |
| 2. | Diverse Structures of Inclusion Crystals of Bile Acids and Their Derivatives | 1976 |
| 3. | Hierarchical Assemblies of Bile Acids and Their Derivatives | 1979 |
| 4. | Supramolecular Chirality in Crystalline Molecular Assemblies | 1982 |
| 5. | Supramolecular Chirality of Crystalline Assemblies of Bile Acids and Their Derivatives | 1988 |
| 6. | Conclusion and Perspectives | 1991 |
1. Introduction
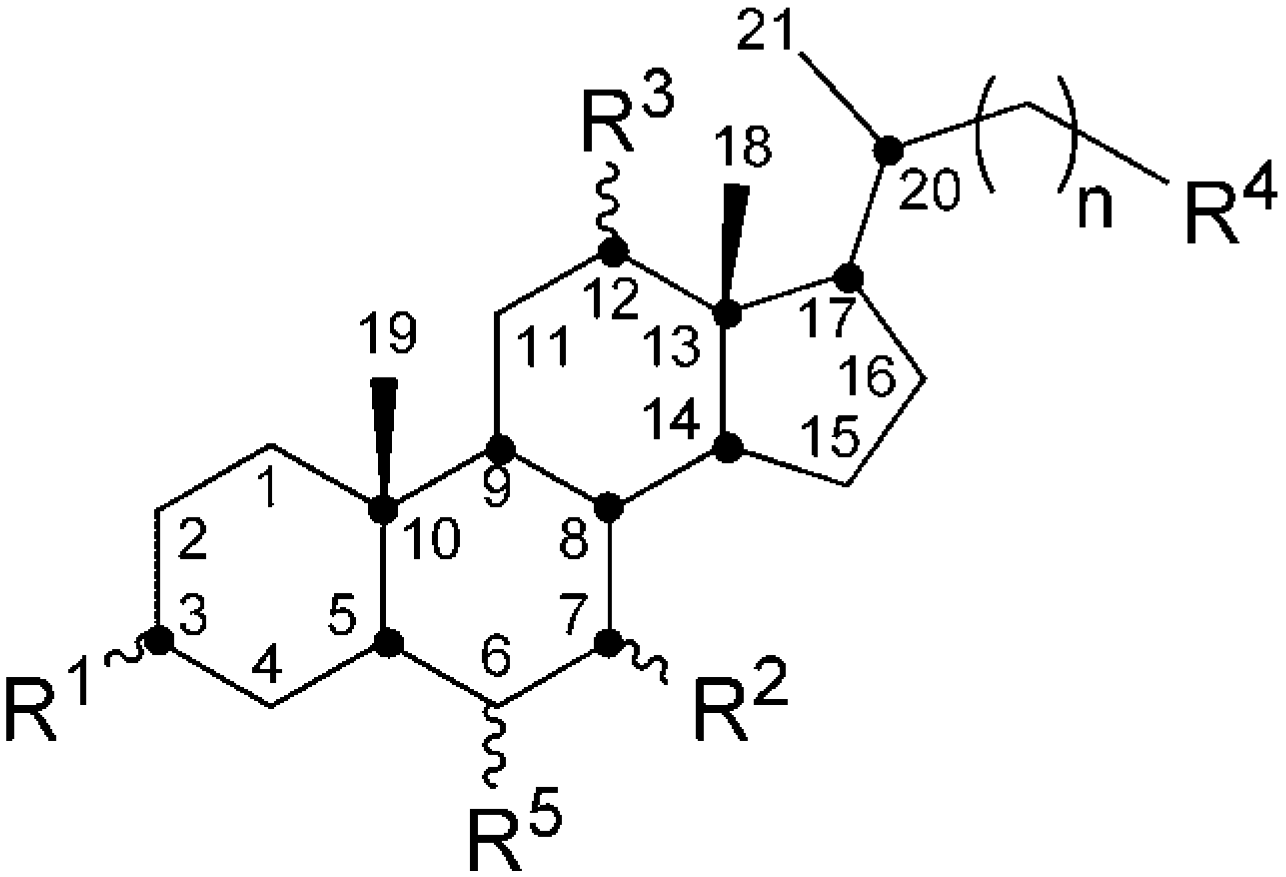
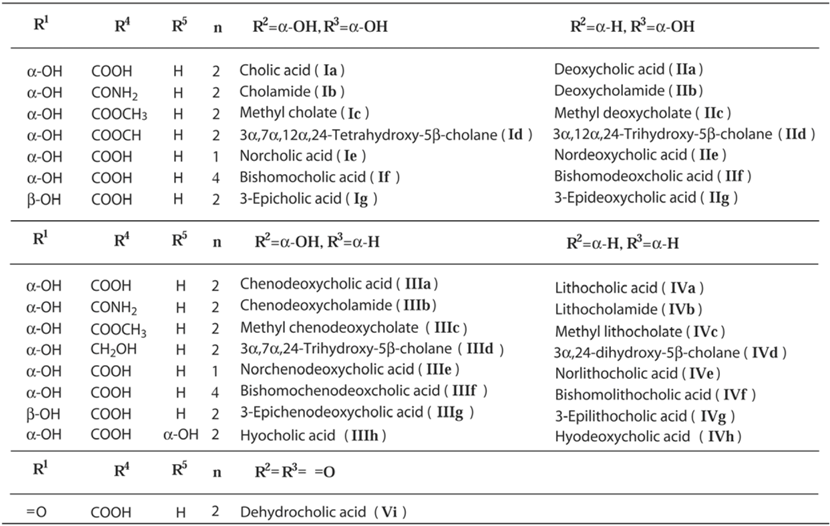
2. Diverse Structures of Inclusion Crystals of Bile Acids and Their Derivatives
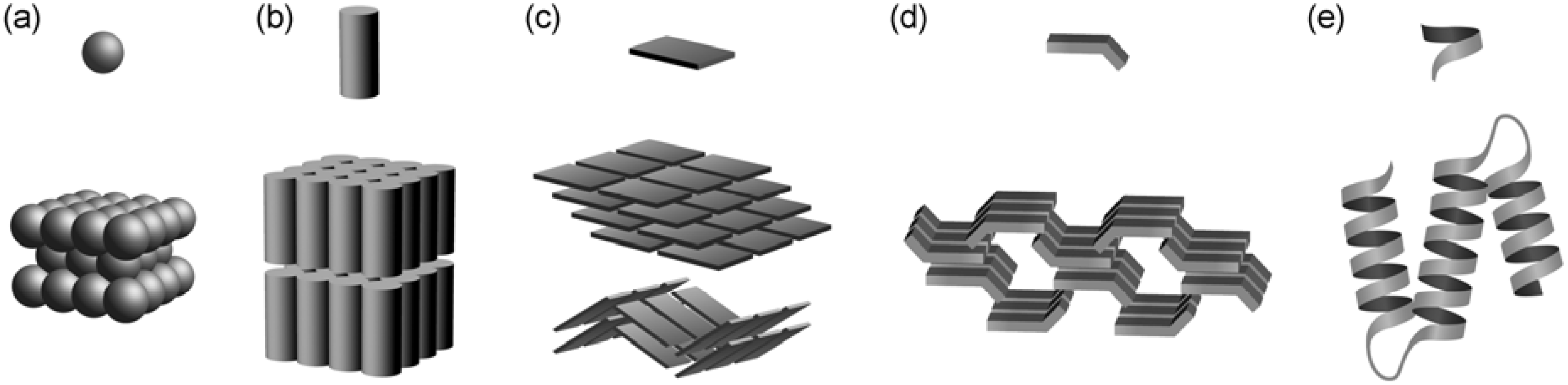
2.1. Basic Patterns of Assembly Modes
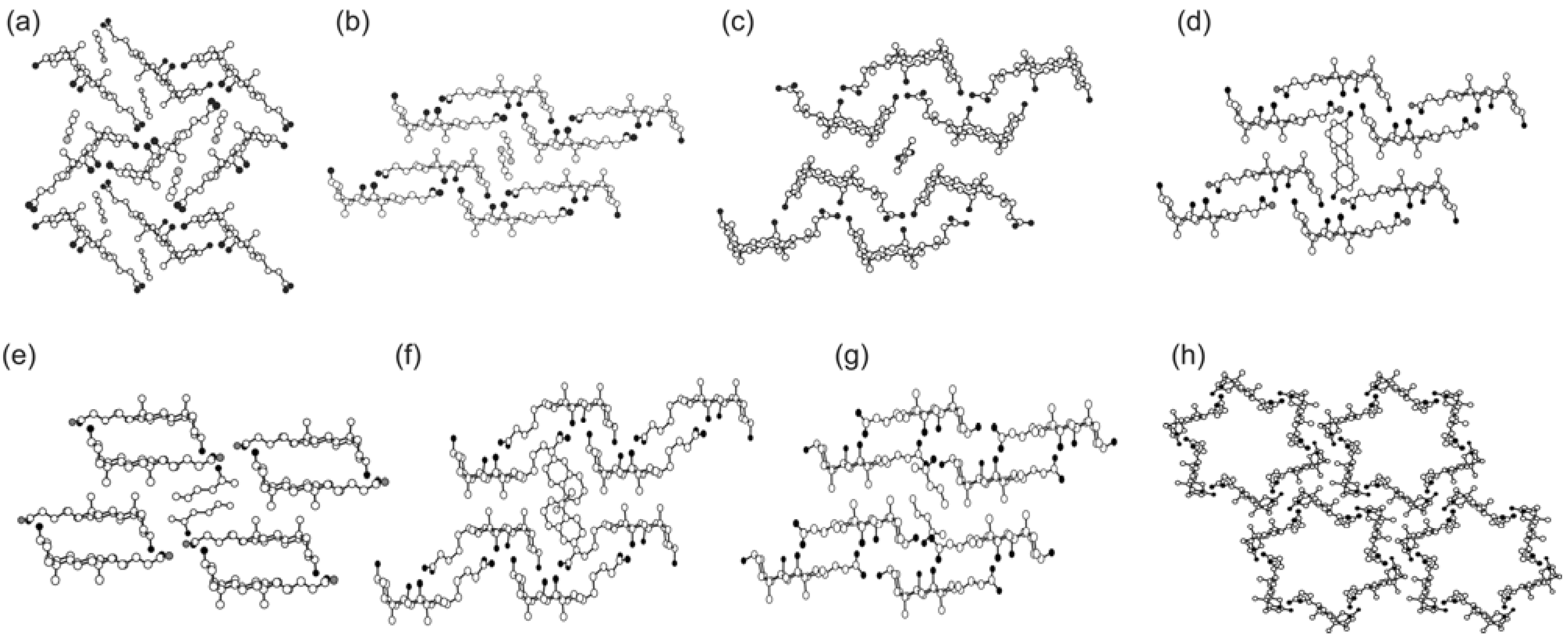
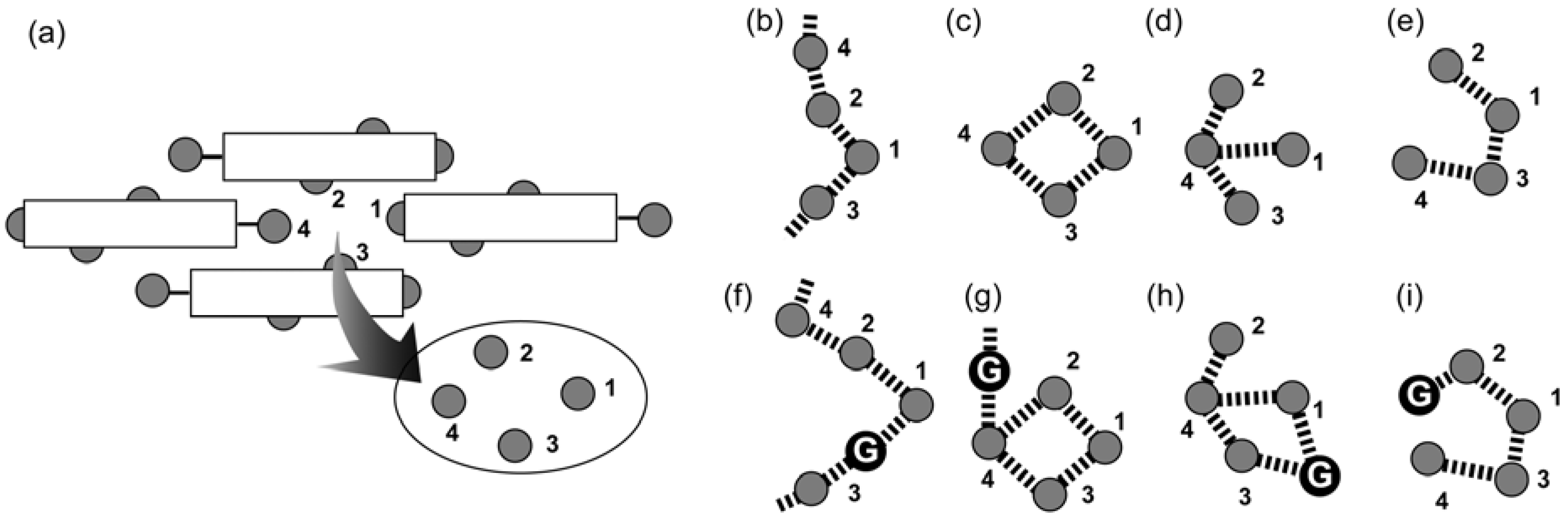
2.2. Carboxylic Acid Hosts
2.3. Amide, Ester, and Alcohol Hosts
2.4. Hosts with Different Side-Chain Length and Reversed Hydroxyl Groups
2.5. Other Hosts
3. Hierarchical Assemblies of Bile Acids and Their Derivatives
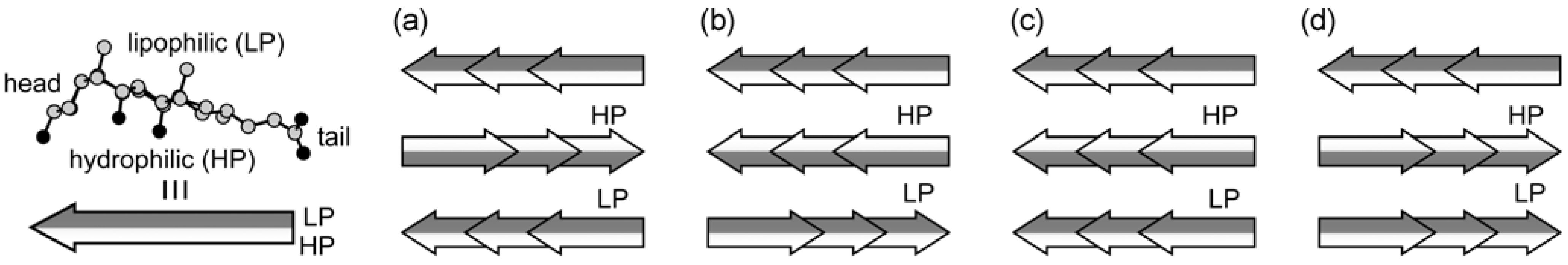
3.1. Intermediate among Various Molecules and Assemblies
3.2. Hierarchical Interpretation of the Steroidal Assemblies
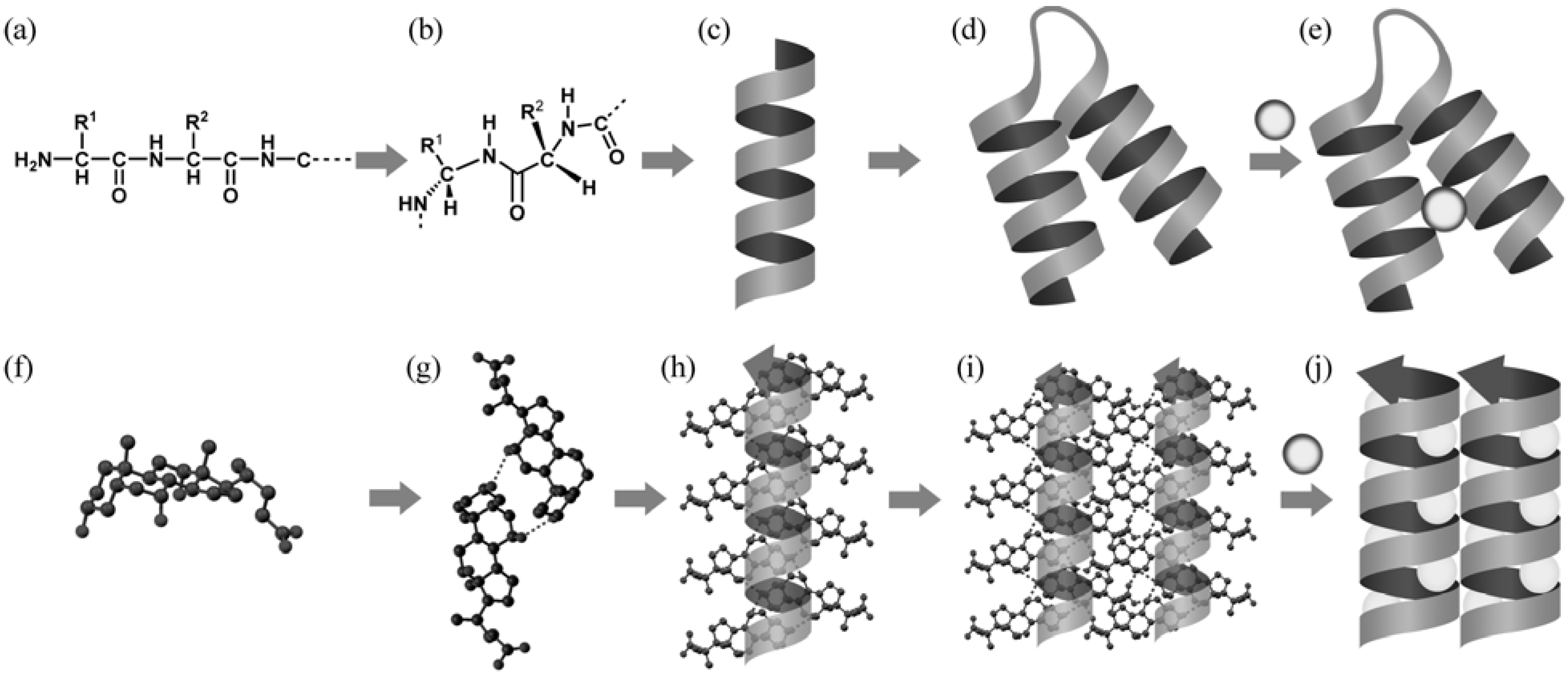
3.3. Molecular Information and Expression of Chiral Chains
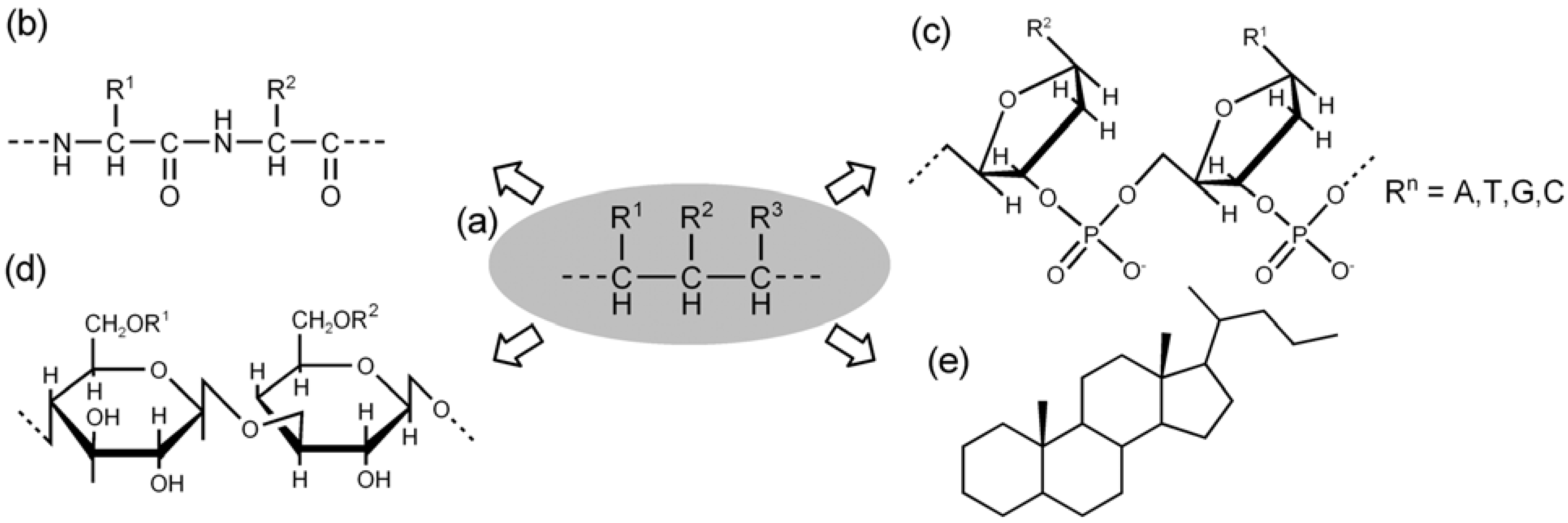
4. Supramolecular Chirality in Crystalline Molecular Assemblies
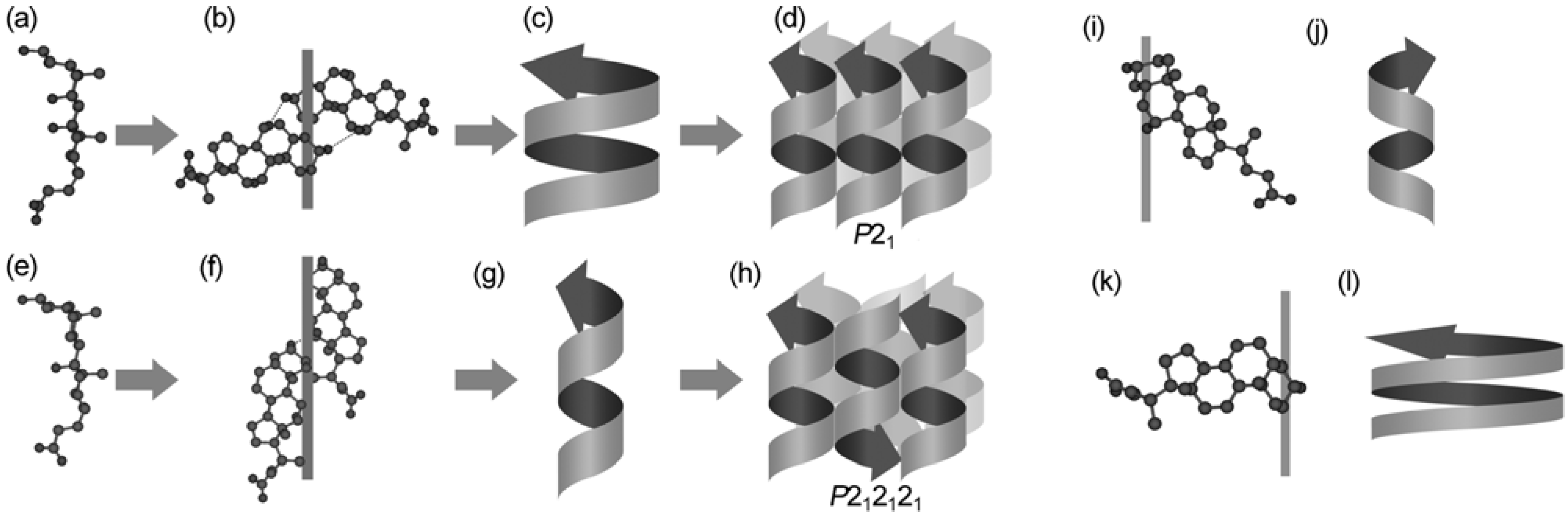
4.1. Chirality of the Hierarchical Assemblies, Especially 21 Helical Assemblies
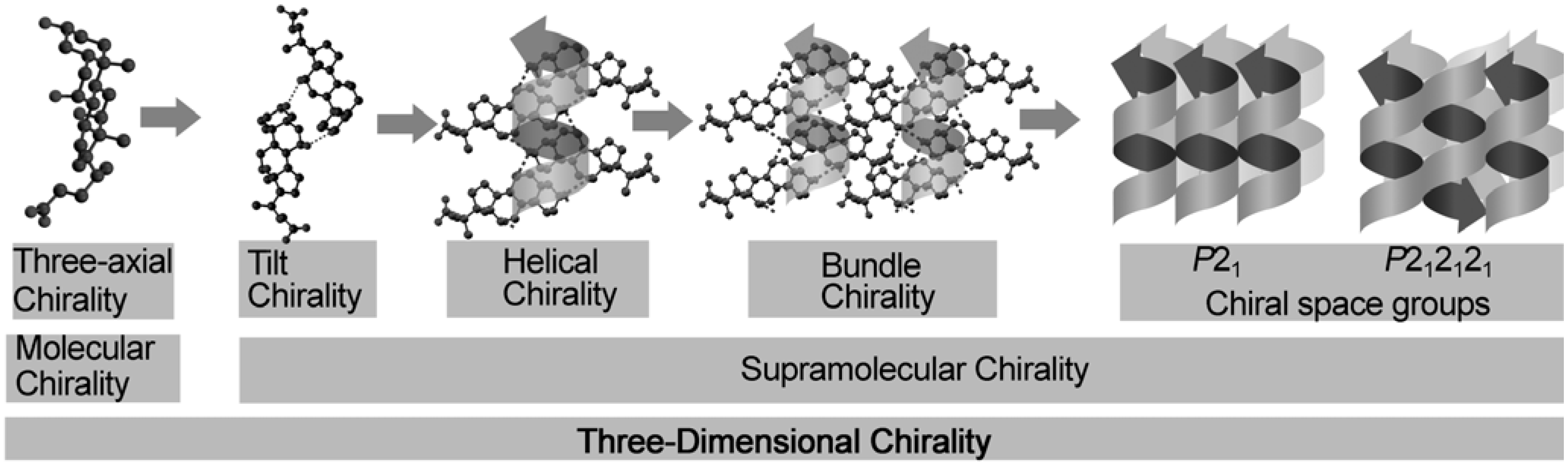
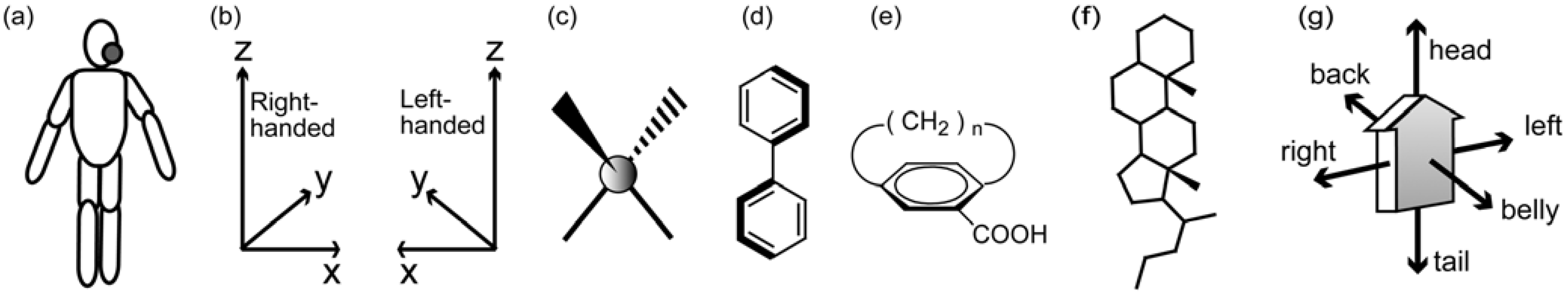
4.2. Three-axial Chirality of Molecules
4.3. Tilt Chirality of Bimolecular Assemblies Based on Three-Axial Chirality
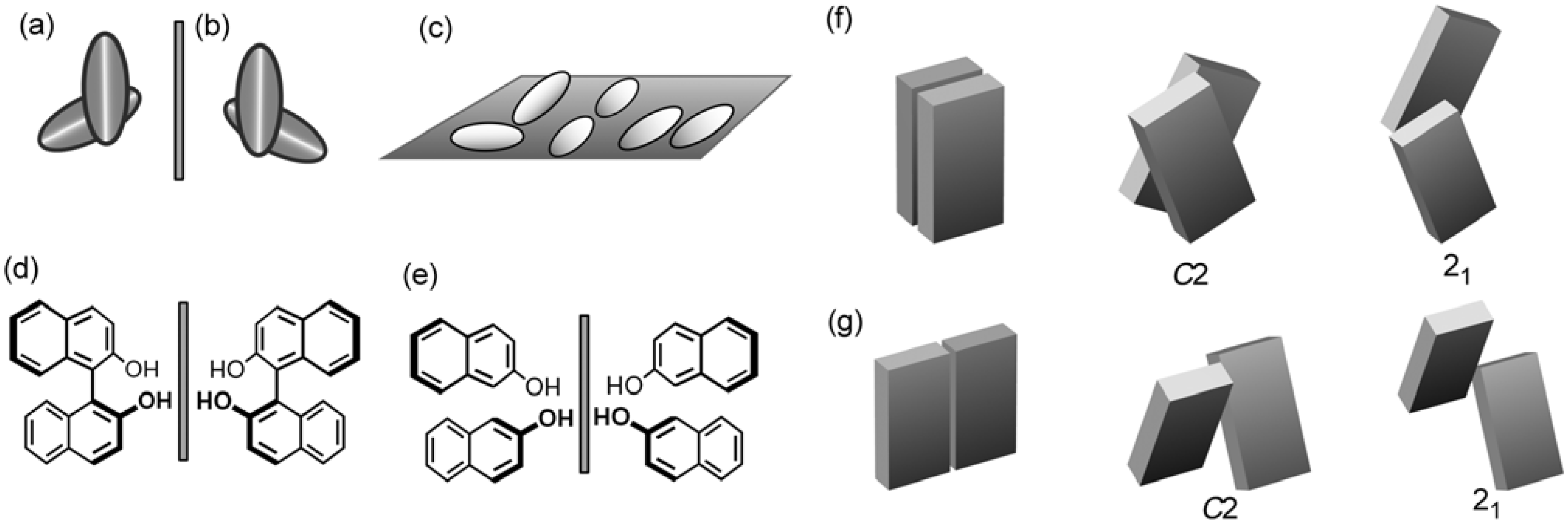

4.4. Helical Chirality of 21 Helical Assemblies Based on Three-axial and Tilt Chirality
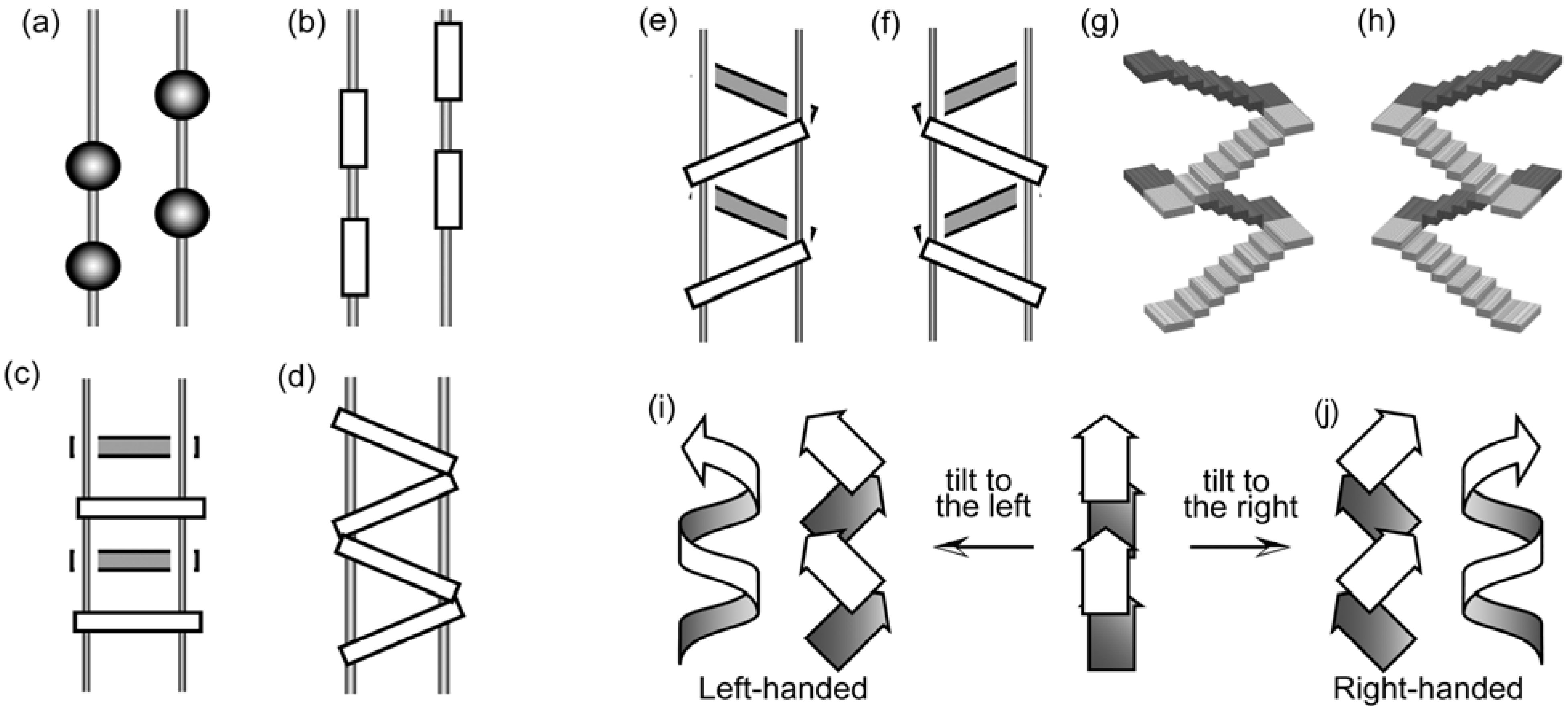
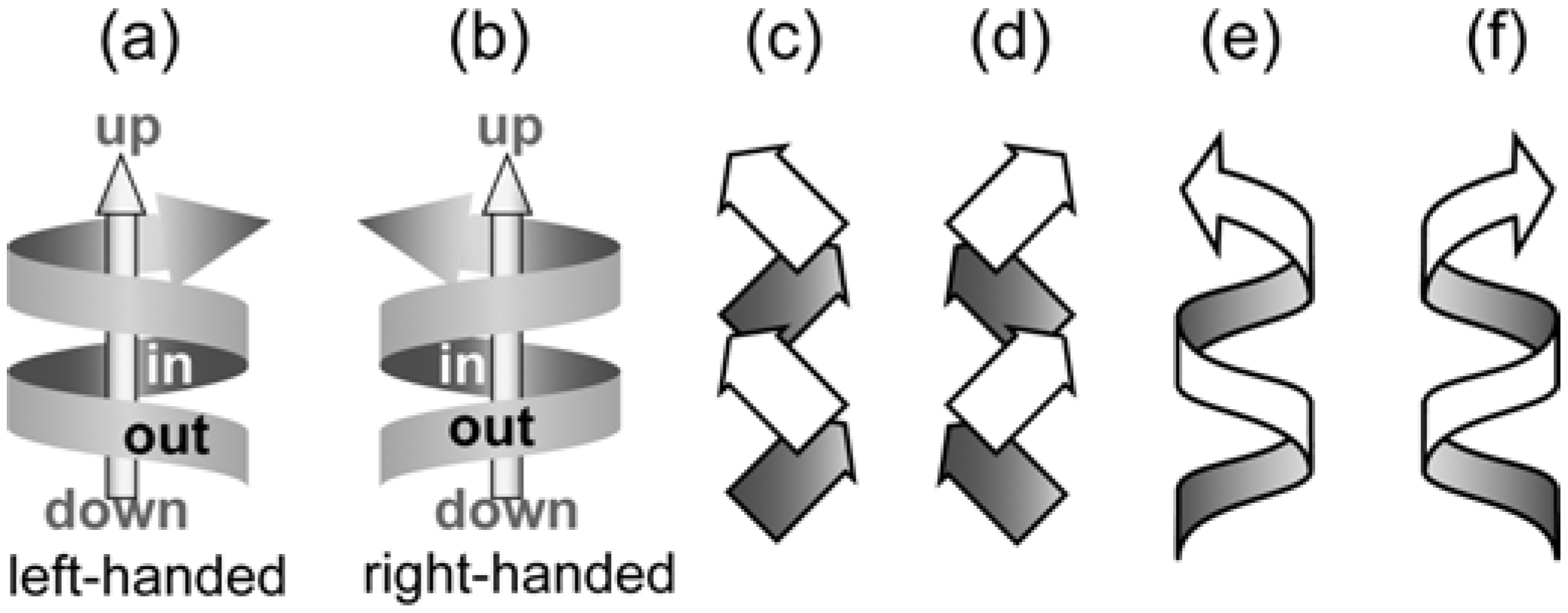
4.5. Bundle Chirality Based on Three-axial, Tilt, and Helical Chirality
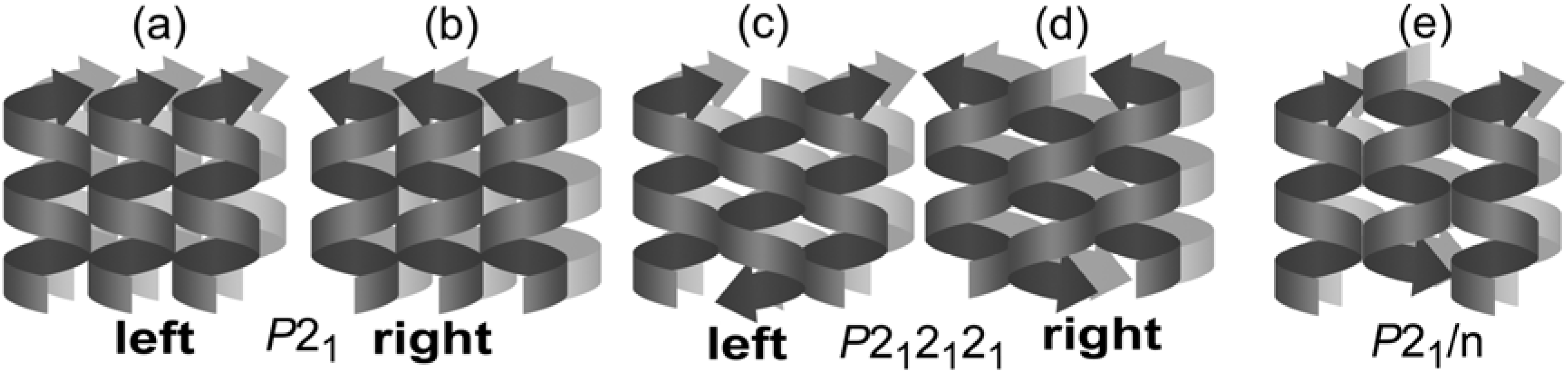
5. Supramolecular Chirality of Crystalline Assemblies of Bile Acids and Their Derivatives
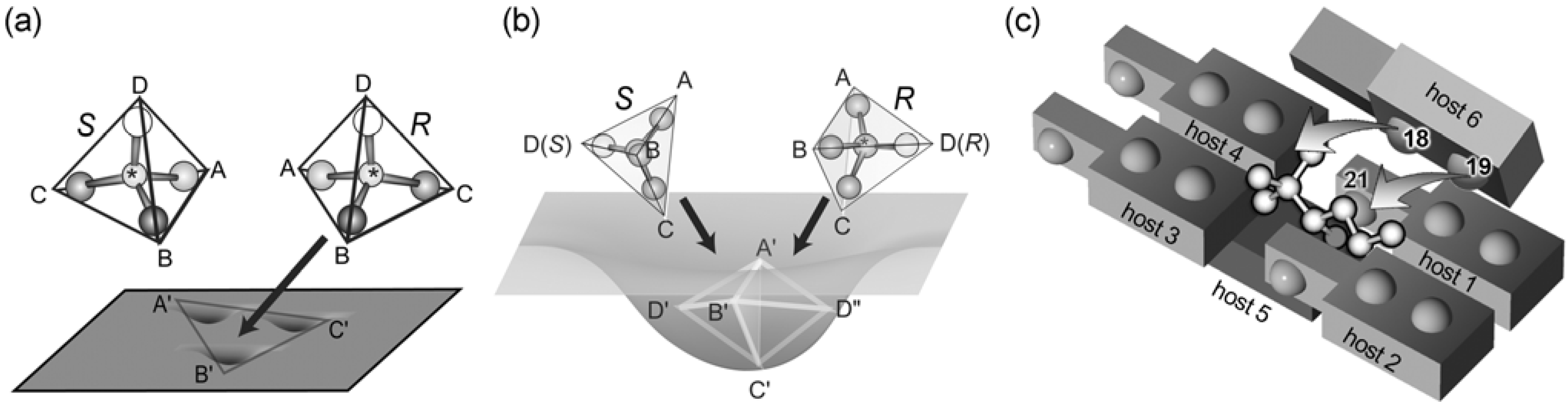
5.1. Steroidal Molecules with Three-Axial Chirality
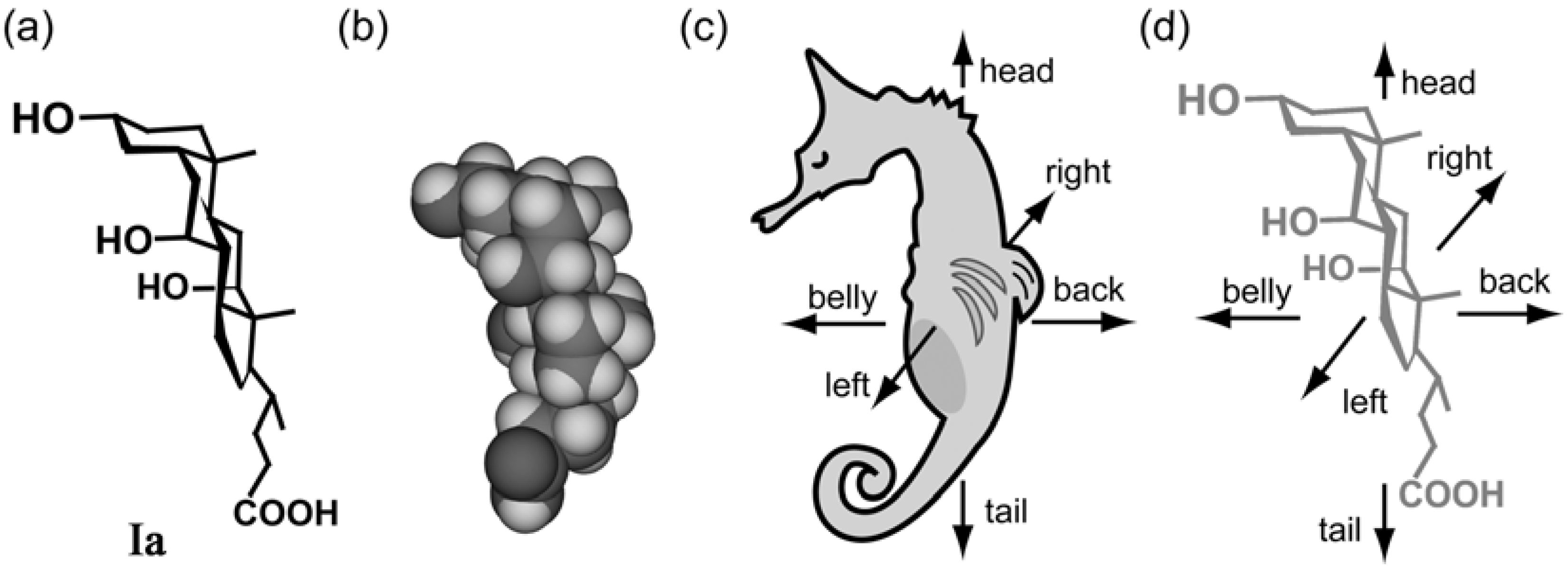
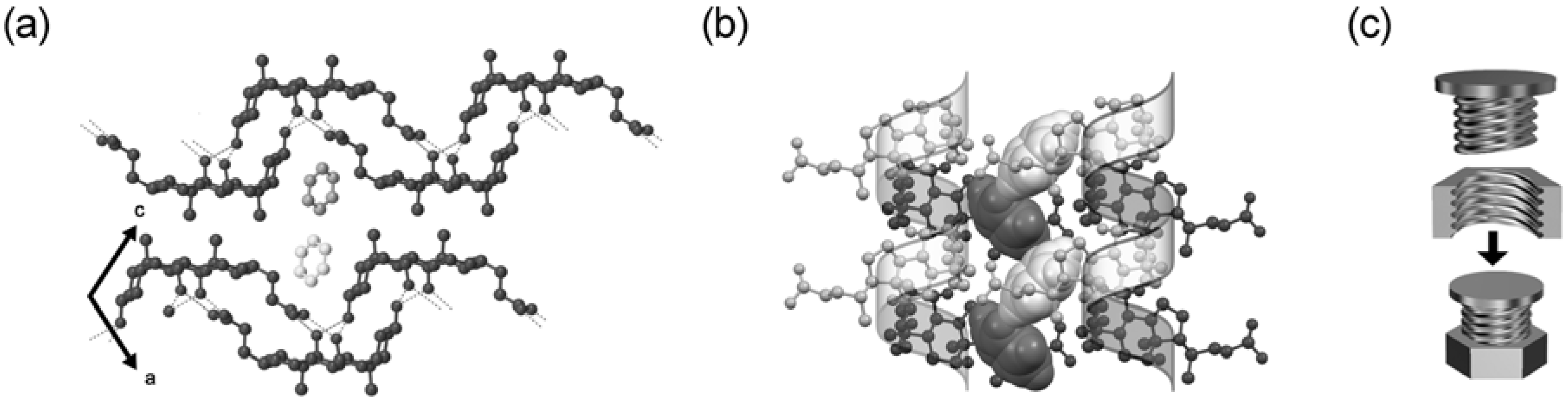
5.2. Steroidal Assemblies with Three-axial, Tilt, Helical, and Bundle Chirality
5.3. Complementary Chirality in Host-Guest Complexes
5.4. Chirality Recognition Based on Supramolecular Chirality
6. Conclusions and Perspectives
References and Notes
- Miyata, M.; Sada, K. Deoxycholic Acid and Related Hosts. In Solid-State Supramolecular Chemistry, Comprehensive Supramolecular Chemistry; MacNicol, D. D., Toda, F., Bishop, R., Eds.; Pergamon: Oxford, 1996; Vol. 6, pp. 147–176. [Google Scholar]
- Miyata, M.; Sada, K.; Yoswathananont, N. Deoxycholic, Cholic, and Apocholic Acids. In Encyclopedia of Supramolecular Chemistry; Atwood, J. L., Steed, J. W., Eds.; Marcel Dekker: New York, 2004; Vol. 1, pp. 441–451. [Google Scholar]
- Miyata, M.; Yoswathananont, N.; Nakano, K.; Sada, K. Separation of Isomers and Enantiomers by Bile Acid Derivatives. In Separation and Reaction in Organic Supramolecular Chemistry, Perspectives in Supramolecuar Chemistry; Toda, F., Bishop, R., Eds.; John Wiley & Sons: Chichester, U,K., 2004; Vol. 8, pp. 87–122. [Google Scholar]
- Miyata, M.; Tohnai, N.; Hisaki, I. Crystalline Host-Guest Assemblies of Steroidal and Related Molecules: Diversity, Hierarchy, and Supramolecular Chirality. Acc. Chem. Res. 2007, 40, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.D.; Kemula, W.; Powell, H.M.; Smith, N.O. Inclusion Compounds – Past, Present, and Future. J. Inclus. Phenom. 1983, 1, 3–44. [Google Scholar]
- Atwood, J.L.; Davies, J.E.D.; MacNicol, D.D. (Eds.) Inclusion Compounds; Academic Press: London, 1984; Vol. 1–3.
- Lehn, J.-M. Supramolecular Chemistry: Concepts and Perspectives; VCH: Weinheim, 1995. [Google Scholar]
- Desiraju, G.R. (Ed.) The Crystal as a Supramolecular Entity, Perspectives in Supramolecuar Chemistry”; John Wiley & Sons: Chichester, U.K., 2004; Vol. 2.
- Sobotka, H. The Chemistry of the Bile Acids and Related Substances. Chem. Rev. 1934, 15, 311–375. [Google Scholar] [CrossRef]
- Fieser, L.F.; Fieser, M. Steroids; Reinhold: New York, 1959; pp. 56–61. [Google Scholar]
- Herndon, W.C. The Structure of Choleic Acids. J. Chem. Educ. 1967, 44, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Craven, B.M.; DeTitta, G.T. Crystal Structure Determination of the 1:1 Complex of Deoxycholic Acid and Acetic Acid. J. Chem. Soc., Chem. Commun. 1972, 530–531. [Google Scholar]
- Giglio, E. Inclusion Compounds of Deoxycholic Acid. In Inclusion Compounds; Atwood, J.L., Davies, J.E.D., MacNicol, D. D., Eds.; Academic Press: London, 1984; Vol. 2, pp. 207–229. [Google Scholar]
- Johnson, P.L.; Schaefer, J.P. The Crystal and Molecular Structure of an Addition Compound of Cholic Acid and Ethanol. Acta Cryst. 1972, B28, 3083–3088. [Google Scholar] [CrossRef]
- Miyata, M.; Shibakami, M.; Goonewardena, W.; Takemoto, K. Inclusion Compounds of Cholic Acid with a Variety of Organic Substances. Chem. Lett. 1987, 605–608. [Google Scholar] [CrossRef]
- Miki, K.; Masui, A.; Kasai, N.; Miyata, M.; Shibakami, M.; Takemoto, K. New Channel-Type Inclusion Compound of Steroidal Bile Acid. Structure of a 1:1 Complex between Cholic Acid and Acetophenone. J. Am. Chem. Soc. 1988, 110, 6594–6596. [Google Scholar]
- Miyata, M.; Shibakami, M.; Chirachanchai, S.; Takemoto, K.; Kasai, N.; Miki, K. Guest-Responsive Structural Changes in Cholic Acid Intercalation Crystals. Nature 1990, 343, 446–447. [Google Scholar] [CrossRef]
- Jones, E.L.; Nassimbeni, L.R. Crystal and Molecular Structures of the Inclusion Compounds of Cholic Acid with Methanol, Ethanol and 1-Propanol. Acta Cryst. 1990, B46, 399–405. [Google Scholar] [CrossRef]
- Miki, K.; Kasai, N.; Shibakami, M.; Chirachanchai, S.; Takemoto, K.; Miyata, M. Crystal Structure of Cholic Acid with No Guest Molecules. Acta Cryst. 1990, C46, 2442–2445. [Google Scholar]
- Miyata, M.; Sada, K.; Hirayama, K.; Yasuda, Y.; Miki, K. Guest-Dependent Conformations of Side Chains in Cholic Acid Inclusion Compounds. Supramol. Chem. 1993, 2, 283–288. [Google Scholar] [CrossRef]
- Nakano, K.; Sada, K.; Miyata, M. Inclusion Compounds of Cholic Acid with Various Hydrocarbons and the Crystal Structure of a 1:1 Complex of Cholic Acid and Benzene. Chem. Lett. 1994, 137–140. [Google Scholar] [CrossRef]
- Shibakami, M.; Sekiya, A. Crystal Structures of Cholic Acid-Aniline and -3-Fluoroaniline Inclusion Compound; Fluorine Atom Effect on Channel and Hydrogen Bonding Pattern. J. Chem. Soc. Chem. Commun. 1994, 429–430. [Google Scholar] [CrossRef]
- Caira, M.R.; Nassimbeni, L. R.; Scott, J. L. Inclusion Compounds of Cholic Acid with Aliphatic Esters. J. Chem. Soc. Perkin Trans. 2 1994, 623–628. [Google Scholar]
- Nakano, K.; Sada, K.; Kurozumi, Y.; Miyata, M. Importance of Packing Coefficients of Host Cavities in the Isomerization of Open Host Frameworks: Guest-Size-Dependent Isomerization in Cholic Acid Inclusion Crystals with Monosubstituted Benzenes. Chem. Eur. J. 2001, 7, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Yoswathananont, N.; Sada, K.; Miyata, M. Inclusion Compounds of Cholic Acid with Large Aliphatic Alcohols. Mol. Cryst. Liq. Cryst. 2002, 389, 47–51. [Google Scholar] [CrossRef]
- Lessinger, L. Cholic Acid Monohydrate, C24H40O5·H2O. Cryst. Struc. Commun. 1982, 11, 1787–1792. [Google Scholar]
- Lessinger, L.; Low, B.W. Crystal Structure and Hydrogen-Bonding System of Cholic Acid Hemihydrate, C24H40O5·1/2H2O. J. Cryst. Spectrosc. Res. 1993, 23, 85–99. [Google Scholar] [CrossRef]
- Caira, M.R.; Nassimbeni, L.R.; Scott, J.L. Crystal Structure and Multiphase Decomposition of a Novel Cholic Acid Inclusion Compound with Mixed Guests. J. Chem. Soc. Perkin Trans. 2. 1994, 1403–1405. [Google Scholar] [CrossRef]
- Miki, K.; Kasai, N.; Tsutsumi, H.; Miyata, M.; Takemoto, K. Structure of a 2:1 Complex between Deoxycholic Acid and Ferrocene. J. Chem. Soc. Chem. Commun. 1987, 545–546. [Google Scholar] [CrossRef]
- Nakano, K.; Sada, K.; Miyata, M. Guest-Participating Reversion of Molecular Arrangements in Asymmetric Multibilayers of Cholic Acid Inclusion Crystals. J. Chem. Soc. Chem. Commun. 1995, 953–954. [Google Scholar] [CrossRef]
- Yoswathananont, N.; Chirachanchai, S.; Tashiro, K.; Nakano, K.; Sada, K.; Miyata, M. A Novel Host Framework of Cholic Acid Inclusion Crystals by Slide and Flip of the Layers. Cryst. Eng. Comm. 2001, 3, 74–77. [Google Scholar] [CrossRef]
- Nakano, K.; Sada, K.; Aburaya, K.; Nakagawa, K.; Yoswathananont, N.; Tohnai, N.; Miyata, M. Guest-Induced Inversion of an Asymmetric Host Layer in Inclusion Crystals of Cholic Acid. Cryst. Eng. Comm. 2006, 8, 461–467. [Google Scholar] [CrossRef]
- Lindley, P.F.; Mahmound, M.M.; Watson, F.E.; Jones, W.A. The Structure of Chenodeoxycholic Acid, C24H40O4. Acta Cryst. 1980, B36, 1893–1897. [Google Scholar] [CrossRef]
- Rizkallah, P.J.; Harding, M.M.; Lindley, P.F.; Aigner, A.; Bauer, A. Structure of a Low-Temperature Polymoph of Chenodeoxycholic Acid, C24H40O4, Determined with Synchrotron Radiation. Acta Cryst. 1990, B46, 262–266. [Google Scholar] [CrossRef]
- Sluis, P.; Schouten, A.; Kanters, J.A. Structure of Chenodeoxycholic Acid in Chenodeoxycholic Acid Ethyl Acetate Solvate. Acta Cryst. 1990, C46, 2165–2168. [Google Scholar]
- Chikada, M.; Sada, K.; Miyata, M. Intercalation and Polymerization in Chenodeoxycholic Acid Channels with Retention of a Crystalline State. Polym. J. 1999, 31, 1061–1064. [Google Scholar] [CrossRef]
- Arora, S.K.; Germain, G.; Declercq, J.P. The Crystal and Molecular Structure of Lithocholic Acid. Acta Cryst. 1976, B32, 415–419. [Google Scholar] [CrossRef]
- Sada, K.; Kondo, T.; Miyata, M.; Tamada, T.; Miki, K. A Novel Molecular Channel with a Hydrogen Bond ’Hook’; Inclusion Phenomena of Cholamide and the Crystal Structure of a 1:1 Complex of Cholanamide and 1,4-dioxane. J. Chem. Soc. Chem. Commun. 1993, 753–755. [Google Scholar] [CrossRef]
- Sada, K.; Kondo, T.; Miyata, M.; Miki, K. Inclusion Crystals of Cholic Acid and Cholanamide with Alcohols: Importance of Hydrogen Bond “Double Hooks”. Chem. Mater. 1994, 6, 1103–1105. [Google Scholar] [CrossRef]
- Sada, K.; Matsuura, Y.; Miyata, M. Hydrogen Bond ’Triple Hooks’ in an Inclusion Crystal of Cholanamide with Aniline. Structural Difference of Inclusion Crystals of Cholic Acid and Cholanamide with Same Guest. Mol. Cryst. Liq. Cryst. 1996, 276, 121–127. [Google Scholar]
- Sada, K.; Kondo, T.; Ushioda, M.; Matsuura, Y.; Nakano, K.; Miyata, M.; Miki, K. Functionalization of Inclusion Cavities of Bile Acid Hosts. Channel-Type Inclusion Compounds of Cholamide. Bull. Chem. Soc. Jpn. 1998, 71, 1931–1937. [Google Scholar]
- Yoswathananont, N.; Sada, K.; Nakano, K.; Aburaya, K.; Shigesato, M.; Hishikawa, Y.; Tani, K.; Tohnai, N.; Miyata, M. The Effect of a Host-guest Hydrogen Bond on the Inclusion of Alcoholic Guests in the Host Cavities of Cholamide. Eur. J. Org. Chem. 2005, 5330–5338. [Google Scholar] [CrossRef]
- Sada, K.; Kondo, T.; Miyata, M. Channel-Type Inclusion Crystal with Hydrogen Bond ’Hooks’ of Deoxycholanamide. Supramol. Chem. 1995, 5, 189–191. [Google Scholar] [CrossRef]
- Hishikawa, Y.; Aoki, Y.; Sada, K.; Miyata, M. Selective Inclusion Phenomena in Lithocholamide Crystal Lattices; Design of Bilayered Assemblies through Ladder-Type Hydrogen Bonding Network. Chem. Lett. 1998, 1289–1290. [Google Scholar] [CrossRef]
- Miyata, M.; Goonewardena, W.; Shibakami, M.; Takemoto, K.; Masui, A.; Miki, K.; Kasai, N. Inclusion Compounds of Methyl Esters of Cholic and Deoxycholic Acids; Structure of a 1:1 Complex between Methyl Cholate and Methanol. J. Chem. Soc., Chem. Commun. 1987, 1140–1141. [Google Scholar]
- Miki, K.; Masui, A.; Kasai, N.; Goonewardena, W.; Shibakami, M.; Takemoto, K.; Miyata, M. Structures of 1:1 Addition Compounds of Methyl Cholate with Methanol and with 2-Propanol. Acta Cryst. 1992, C48, 503–507. [Google Scholar]
- Goonewardena, W.; Miyata, M.; Takemoto, K. One-Dimensional Inclusion Polymerization of Diene and Vinyl Monomers by Using Methyl Cholate as a Host. Polym. J. 1993, 25, 731–740. [Google Scholar] [CrossRef]
- Miki, K.; Masui, A.; Kasai, N.; Miyata, M.; Goonewardena, W.; Shibakami, M.; Takemoto, K. Structure of a 2:1 Addition Compound of Methyl Deoxycholate with Methanol. Acta Cryst. 1989, C45, 79–83. [Google Scholar]
- Sada, K.; Kondo, T.; Yasuda, Y.; Miyata, M.; Miki, K. Interpretation of Variable Inclusion Abilities of Cholic Acid and Its Derivatives on the Basis of the Crystal Structure of 5β-Petromyzonol. Chem. Lett. 1994, 727–730. [Google Scholar] [CrossRef]
- Sada, K.; Matsuo, A.; Miyata, M. Novel Molecular Arrangement in Asymmetric Bilayered Crystals of the Inclusion Compounds of 3α, 12α, 24-Trihydroxy-5β-cholane. Chem. Lett. 1995, 877–878. [Google Scholar] [CrossRef]
- Sugahara, M.; Hirose, J.; Sada, K.; Miyata, M. Inclusion Abilities of Bile Acids with Different Side Chain Length. Mol. Cryst. Liq. Cryst. 2001, 356, 155–162. [Google Scholar] [CrossRef]
- Sada, K.; Sugahara, M.; Nakahata, Y.; Yasuda, Y.; Nishio, A.; Miyata, M. First Columnar Monolayer Structure of Bile Acids Inclusion Crystal. Inclusion Compounds of 23-Nordeoxycholic Acid. Chem. Lett. 1998, 31–32. [Google Scholar]
- Sugahara, M.; Sada, K.; Miyata, M. A Robust Structural Motif in Inclusion Crystals of Norbile Acids. Chem. Commun. 1999, 293–294. [Google Scholar] [CrossRef]
- Kato, K.; Sugahara, M.; Tohnai, N.; Sada, K.; Miyata, M. Drastic Increase in the Flexibility of Open Host Framework of a Steroidal Host Compound upon Shortening Its Spacer. Eur. J. Org. Chem. 2004, 981–994. [Google Scholar] [CrossRef]
- Sada, K.; Sugahara, M.; Kato, K.; Miyata, M. Controlled Expansion of a Molecular Cavity in a Steroid Host Compound. J. Am. Chem. Soc. 2001, 123, 4386–4392. [Google Scholar]
- Miyata, M.; Sada, K.; Miyake, Y. Combinatorial Chemistry of Inclusion Compounds By Using Steroids. Mol. Cryst. Liq. Cryst. 1998, 313, 173–178. [Google Scholar] [CrossRef]
- Miyake, Y.; Hirose, J.; Hasegawa, Y.; Sada, K.; Miyata, M. Novel Channel-Type Inclusion Compounds of 3-Epiursodeoxycholic Acid. Chem. Commun. 1998, 111–112. [Google Scholar] [CrossRef]
- Popovitz-Biro, R.; Tang, C.P.; Chang, H.C.; Lahav, M.; Leiserowitz, L. Solid-State Photochemistry of Guest Aliphatic Ketones Inside the Channels of Host Deoxycholic and Apocholic Acids. J. Am. Chem. Soc. 1985, 107, 4043–4058. [Google Scholar]
- Miyake, Y.; Sada, K.; Miyata, M. Inclusion Compounds of Hyocholic Acid with Specific Alcohols. Supramol. Chem. 1998, 10, 107–109. [Google Scholar] [CrossRef]
- Miyake, Y.; Sada, K.; Miyata, M. Inclusion Compounds of Hyodeoxycholic Acid and the Crystal Structure of a 1:1 Complex between the Acid and Pyridine. Chem. Lett. 1997, 1263–1264. [Google Scholar] [CrossRef]
- Sada, K.; Maeda, T.; Miyata, M. Chiral Discrimination of 1-Phenylethylamine by Diastereomeric Salt Formation with Bile Acids. Crystal Structures of Cholic Acid Salts with (R)- and (S)-1-Phenylethylamine. 1996, 837–838. [Google Scholar]
- Sada, K.; Shiomi, N.; Miyata, M. Nanocavities with Fine Adjustment in Channel-Type Inclusion Crystals of Alkylammonium Deoxycholates. Control of Molecular Cavities by Partial Filling of Molecular Channels. J. Am. Chem. Soc. 1998, 120, 10543–10544. [Google Scholar]
- Sada, K.; Hishikawa, Y.; Kondo, T.; Miyata, M. Specific Inclusion of Water by N-Methyldeoxycholanamide Revealed by the Crystal Structure of the Hemihydrate. Chem. Lett. 1994, 2113–2116. [Google Scholar] [CrossRef]
- Hishikawa, Y.; Watanabe, R.; Sada, K.; Miyata, M. Molecular Arrangements in Chiral Sheets of N-Alkylcholamides Bilayered Crystals. Chirality 1998, 10, 600–618. [Google Scholar] [CrossRef]
- Hishikawa, Y.; Sada, K.; Miyata, M. Extremely Specific Recognition of Ethylene Glycol in Three-leaved Building Blocks of N-Methyldeoxycholamide. Supramol. Chem. 1999, 11, 101–108. [Google Scholar] [CrossRef]
- Sada, K.; Kitamura, T.; Miyata, M. Inclusion Phenomena of Glycine-Conjugated Bile Acids and the Crystal Structure of 1:1 Complex of Glycodeoxycholic Acid and Tetrahydrofuran. J. Chem. Soc. Chem. Commun. 1994, 905–906. [Google Scholar] [CrossRef]
- Nakano, K.; Sada, K.; Miyata, M. Novel Additive Effect of Inclusion Crystals on Polymorphs – Cholic Acid Crystals Having Different Hydrogen-Bonded Networks with the Same Organic Guest. Chem. Commun. 1996, 989–990. [Google Scholar] [CrossRef]
- Nakano, K.; Tani, K.; Sada, K.; Miyata, M. Molecular Architecture of Steroidal Acids and Their Derivatives: Selective Acquisition of Polymorphic Inclusion Crystal of Cholic Acid. Progr. Colloid Polym. Sci. 1997, 106, 249–251. [Google Scholar] [CrossRef]
- Yoswathananont, N.; Kita, H.; Tohnai, N.; Sada, K.; Miyata, M. A Novel Three-Component Pseudo-Polymorphism in the Cholamide Inclusion Crystals Promoted by the Combination of Organic Guest and Water. Chem. Lett. 2002, 1234–1235. [Google Scholar] [CrossRef]
- Caira, M.R.; Nassimbeni, L.R.; Scott, J.R. Selective Inclusion by Cholic Acid. J. Chem. Soc. Chem. Commun. 1993, 612–614. [Google Scholar] [CrossRef]
- Nakano, K.; Mochizuki, E.; Yasui, N.; Morioka, K.; Yamauchi, Y.; Kanehisa, N.; Kai, Y.; Yoswathananont, N.; Sada, K.; Miyata, M. Mechanism of Selective and Unselective Enclathration by a Host Compound Possessing Open, Flexible Host Frameworks. Eur. J. Org. Chem 2003, 2428–2436. [Google Scholar]
- Nakano, K.; Katsuta, M.; Sada, K.; Miyata, M. Conformational Polymorphism in Inclusion Crystals of Cholic Acid with Ethyl Acetate. Cryst. Eng. Comm. 2001, 3, 44–45. [Google Scholar] [CrossRef]
- Miyata, M.; Sada, K.; Hori, S.; Miki, K. Intercalation Phenomena and Polymorphism of Cholic Acid Crystals. Mol. Cryst. Liq. Cryst. 1992, 219, 71–74. [Google Scholar] [CrossRef]
- Nakano, K.; Sada, K.; Miyata, M. Reversible Layer Sliding in Cholic Acid Crystals. Mol. Cryst. Liq. Cryst. 1996, 276, 129–132. [Google Scholar] [CrossRef]
- Nakano, K.; Sada, K.; Nakagawa, K.; Aburaya, K.; Yoswathananont, N.; Tohnai, N.; Miyata, M. Organic Intercalation Material: Reversible Change in Interlayer Distances by Guest Release and Insertion in Sandwich-Type Inclusion Crystals of Cholic Acid. Chem. Eur. J. 2005, 11, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Shibakami, M.; Tamura, M.; Sekiya, A. Topological and Nontopological Rearrangement in Crystal Lattice of Cholic Acid Inclusion Complex Induced by Guest Exchange. J. Am. Chem. Soc. 1995, 117, 4499–4505. [Google Scholar] [CrossRef]
- Scott, J.L. Solid-Vapour Reactions of Cholic Acid and Methyl Cholate with Acetonitrile: Structures and Reaction Kinetics. J. Chem. Soc. Perkin Trans. 2. 1995, 495–502. [Google Scholar] [CrossRef]
- Takemoto, K.; Miyata, M. Polymerization of Vinyl and Diene Monomers in Canal Complexes. J. Macromol. Sci. -Rev. Macromol. Chem. 1980, C18, 83–107. [Google Scholar]
- Miyata, M. Polymerization in Constrained Media. In Polymerization in Organized Media; Paleos, C. M., Ed.; Gordon and Breach Science Publishers: New York, 1992; pp. 327–367. [Google Scholar]
- Takemoto, K.; Miyata, M. Inclusion Polymerization in Steroidal Canal Complexes. In Advances in Supramolecular Chemistry; Gokel, G. W., Ed.; JAI Press Inc.: Greenwich, CT, 1993; Vol.3, pp. 37–63. [Google Scholar]
- Miyata, M. Inclusion Polymerization. In Special Topics Related to Supramolecular Chemistry, Comprehensive Supramolecular Chemistry; Reinhoudt, N.D., Ed.; Pergamon Press: Oxford, 1996; Vol.10, pp. 557–582. [Google Scholar]
- Miyata, M.; Sada, K. Inclusion Polymerization – Low-Dimensional and Space-Dependent Polymerization. In Soft Chemistry leading to Novel Materials; Agarwala, R. P., Ed.; Scitec Publications: Switzerland, 2001; pp. 99–123. [Google Scholar]
- Miyata, M. Inclusion Polymerization. In Encyclopedia of Supramolecular Chemistry; Atwood, J. L., Steed, J. W., Eds.; Marcel Dekker: New York, 2004; Vol. 1, pp. 705–711. [Google Scholar]
- Yoswathananont, N.; Sada, K.; Miyata, M.; Akita, S.; Nakano, K. Dependence of Selective Enclathration on Types of Cholic Acid Crystals. Org. Biomol. Chem. 2003, 1, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Akita, S.; Yoswathananont, N.; Sada, K.; Miyata, M. Relationship between Separation Behavior and Structural Flexibility in Inclusion Crystal of Cholic Acid. J. Incl. Phenom. Macrocyclic Chem. 2004, 48, 181–184. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: The Design of Organic Solids; Elsevier: New York, 1989. [Google Scholar]
- Gavezzotti, A. Are Crystal Structures Predictable? Acc. Chem. Res. 1994, 27, 309–314. [Google Scholar] [CrossRef]
- Dunitz, J.D. Are Crystal Structures Predictable? Chem. Commun. 2003, 545–548. [Google Scholar]
- Kato, K.; Inoue, K.; Tohnai, N.; Miyata, M. Helical Tape Assemblies in Inclusion Crystals of Bile Acids and Their Derivatives. J. Incl. Phenom. Macrocyclic Chem. 2004, 48, 61–67. [Google Scholar] [CrossRef]
- Kato, K.; Sugahara, M.; Tohnai, N.; Sada, K.; Miyata, M. Systematic Structural Study on Asymmetric Supramolecular Assembly by a Series of Bile Acid Derivatives with Different Side-Chain Length. Cryst. Growth Des. 2004, 4, 263–272. [Google Scholar] [CrossRef]
- Kato, K.; Tohnai, N.; Miyata, M. Hierarchical Prediction Process of Cholic Acid Crystal Structures Based on Characteristic Helical Assemblies. Mol. Cryst. Liq. Cryst. 2005, 440, 125–132. [Google Scholar] [CrossRef]
- Watabe, T.; Kato, K.; Tohnai, N.; Miyata, M. An approach to the Qualitative Prediction of Crystal Structures; Hierarchical Crystal Structures of Inclusion Compounds of Steroids and Alkaloids. In Structure and Dynamics in Macromolecular Systems with Specific Interactions; Adachi, K., Sato, T., Eds.; Osaka University Press: Osaka, 2005; pp. 157–167. [Google Scholar]
- Miyata, M. Expression of Molecular Information of Organic Molecules through Flexible Bilayered Assemblies. Biophysics 1992, 32, 169–173. (in Japanese). [Google Scholar]
- Miyata, M. Expression of Molecular Information of Cholic Acid and Its Derivatives. J. Chem. Soc. Jpn. Chem. Ind. Chem. 1993, 128–138. (in Japanese). [Google Scholar]
- Miyata, M.; Miki, K. Reversible Intercalation of Guest Molecules in Crystals of Cholic Acid. In Reactivity in Molecular Crystals; Ohashi, Y., Ed.; VCH: Weinheim, 1993; pp. 153–164. [Google Scholar]
- Miyata, M. Kamachi, M., Nakamura, A., Eds.; Springer Verlag: Berlin, 1995; pp. 21–30.
- Sada, K.; Miyata, M. Inclusion Compounds of Bile Acids and Their Derivatives. J. Synth. Org. Chem. Jpn. 1996, 54, 113–121. (in Japanese). [Google Scholar]
- Kitaigorodskii, A. I. Molecular Crystals and Molecules; Academic Press: New York, 1973. [Google Scholar]
- Hisaki, I.; Watabe, T.; Kogami, Y.; Tohnai, N.; Miyata, M. 21 Helical Assemblies of Cinchona Alkaloids in Crystals: Definition of Their Handedness Based on the Molecular Tilt. Chem. Lett. 2006, 35, 1274–1275. [Google Scholar] [CrossRef]
- Hisaki, I.; Tohnai, N.; Miyata, M. Supramolecular Tilt Chirality in Crystals of Steroids and Alkaloids. Chirality. Published Online: 5 Jul 2007.
- Watabe, T.; Hisaki, I.; Tohnai, N.; Miyata, M. Four Kinds of 21 Helical Assemblies with the Molecular Tilt as Well as Three-Directional and Facial Chirality. Chem. Lett. 2007, 36, 234–235. [Google Scholar] [CrossRef]
- Gardner, M. The New Ambidextrous Universe; W. H. Freeman: New York, 1999. [Google Scholar]
- Yao, J.W.; Cole, J.C.; Pidcock, E.; Allen, F.H.; Howard, J.A.K.; Motherwell, W.D.S. CSD Symmetry: the Definitive Database of Point-Group and Space-Group Symmetry Relationships in Small-Molecule Crystal Structures. Acta Cryst., Sect B 2002, 58, 640–646. [Google Scholar]
- Fischer, E. Influence of Configuration on the Action of Enzymes. Ber. Dtsch. Chem. Ges. 1894, 27, 2985–2995. [Google Scholar]
- Tanaka, A.; Hisaki, I.; Tohnai, N.; Miyata, M. Supramolecular Tilt-chirality Derived from Symmetric Benzene Molecules: Handedness of the 21 Helical Assembly. Chem. Asian J. 2007, 2, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Sada, K.; Miyake, Y. Chiral Recognition of Organic Substances by Using Bile Acids/Salts and Their Derivatives. In Organized Assemblies in Chemical Analysis; Hinze, W., Ed.; JAI Press: Greenwich, CT, 2000; Vol.2, Chap. 7; pp. 205–228. [Google Scholar]
- Sada, K.; Kondo, T.; Miyata, M. Resolution of Aliphatic Alcohols by Hydrogen Bond ”Double Hooks” of Cholanamide Inclusion Compounds. Tetrahedron: Asymmetry 1995, 6, 2655–2656. [Google Scholar]
- Briozzo, P.; Kondo, T.; Sada, K.; Miyata, M.; Miki, K. Structures of Three Inclusion Compounds of Cholanamide with Either (S)-Enantiomer, (R)-Enantiomer or an Optically Resolved Mixture of Butan-2-ol. Acta Cryst. 1996, B52, 728–733. [Google Scholar] [CrossRef]
- Aburaya, K.; Hisaki, I.; Tohnai, N.; Miyata, M. Dependence of the Enantioselectivity on Reversion of Layer Directions in Cholamide Inclusion Compounds. Chem. Commun. Published Online 9 Aug 2007.
- Aoki, Y.; Hishikawa, Y.; Sada, K.; Miyata, M. Enantioresolution of Aliphatic Alcohols by Lithocholamide. Enantiomer 2000, 5, 95–104. [Google Scholar] [PubMed]
- Kato, K.; Aoki., Y.; Sugahara, M.; Tohnai, N.; Sada, K.; Miyata, M. Interpretation of Enantioresolution in Nordeoxycholic Acid Channels Based on the Four-Location Model. Chirality 2003, 15, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Aburaya, K.; Miyake, Y.; Sada, K.; Tohnai, N.; Miyata, M. Excellent Enantio-Selective Enclathration of (2R, 3S)-3-Methyl-2-pentanol in Channel-like Cavity of 3-Epideoxycholic Acid, Interpreted by the Four-location Model for Chiral Recognition. Chem. Commun. 2003, 2872–2873. [Google Scholar] [CrossRef]
- Miyata, M.; Shibakami, M.; Takemoto, K. Optical Resolution of Lactones by an Inclusion Method Using Cholic Acid as the Host. J. Chem. Soc. Chem. Commun. 1988, 655–656. [Google Scholar] [CrossRef]
- Miki, K.; Kasai, N.; Shibakami, M.; Takemoto, K.; Miyata, M. New Chiral Recognition Ability of a Steroidal Bile Acid; Direct Evidence fro Efficient Optical Resolution of Racemic Lactones by Cholic Acid Inclusion Crystals. J. Chem. Soc. Chem. Commun. 1991, 1757–1759. [Google Scholar] [CrossRef]
- Sada, K.; Nakano, K.; Hirayama, K.; Miyata, M.; Sasaki, S.; Takemoto, K.; Kasai, N.; Kato., K.; Shigesato, M.; Miki, K. Supramol. Chem. 2001, 13, 35–44.
- Nakamura, S.; Imashiro, F.; Takegoshi, K.; Terao, T. Sequential Arrangement of Gamma-Valerolactone Enantiomers Enclathrated in Cholic Acid Channels as Studied by 13C Solid-State NMR: Elucidation of the Optical Resolution Mechanism. J. Am. Chem. Soc. 2004, 126, 8769–8776. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, O.; Fantin, G.; Fogagnolo, M.; Medici, Z.; Pedrini, P. Resolution of Unfunctionalized Epoxides by Cholic Acid Inclusion Compounds. Chem. Lett. 2000, 1246–1247. [Google Scholar] [CrossRef]
- Bortolini, O.; Fantin, G.; Fogagnolo, M.; Medici, A.; Pedrini, P. Optical Resolution of Sulfoxides by Inclusion in Host Dehydrocholic Acid. Chem. Commun. 2000, 365–366. [Google Scholar] [CrossRef]
- Bertolasi, V.; Bortolini, O.; Fogagnolo, M.; Fantin, G.; Pedrini, P. Enantioselective Inclusion in Bile Acids: Resolution of Cyclic Ketones. Tetrahedron: Asymmetry 2001, 12, 1479–1483. [Google Scholar]
- Bortolini, O.; Fogagnolo, M.; Fantin, G.; Medici, A. Optical Resolution of Cyclic Amides by Inclusion in Dehydrocholic Acid. Chem. Lett. 2003, 32, 206–207. [Google Scholar] [CrossRef]
- Bortolini, O.; Fantin, G.; Fogagnolo, M. Bile Acid Derivatives as Enantiodifferentiating Host Molecules in Inclusion Processes. Chirality 2005, 17, 121–130. [Google Scholar]
- Gdaniec, M.; Polonski, T. Generation of Chirality in Guest Aromatic Ketones Included in the Crystals of Steroidal Bile Acids. J. Am. Chem. Soc. 1998, 120, 7353–7354. [Google Scholar]
- Gdanice, M.; Milewska, M.; Polonski, T. Enantioselective Inclusion Complexation of N-Nitrosopiperidines by Steroidal Bile Acids. Angew. Chem. Int. Ed. 1999, 38, 392–395. [Google Scholar] [CrossRef]
- Polonski, T.; Szyrszyng, M.; Gdanice, M.; Nowak, E.; Herman, A. Absolute Sense of Twist of the Benzil Molecule in Its Enantiomorphous Crystals and Inclusion Complexes with Optically Active Hosts. Tetrahedron: Asymmetry 2001, 12, 797–800. [Google Scholar]
- Ogston, A. G. Interpretation of Experiments on Metabolic Processes, Using Isotopic Tracer Elements. Nature 1948, 162, 963. [Google Scholar] [CrossRef] [PubMed]
- Mesecar, A. D.; Koshland, D. E., Jr. Structural Biology: A New Model for Protein Stereospecificity. Nature 2000, 403, 614–615. [Google Scholar] [PubMed]
- Matsuura, T.; Koshima, H. Introduction to Chiral Crystallization of Achiral Organic Compounds. J. Photochem. Photobio. C: Photo. Chem. Rev 2005, 6, 7–24. [Google Scholar]
- Watabe, T.; Kobayashi, K.; Hisaki, I.; Tohnai, N.; Miyata, M. Guest-induced Supramolecular Isomerism and Chirality of Brucine Inclusion Crystals with Aliphatic Alcohols: A Hierarchical Interpretation. Bull. Chem. Soc. Jpn. 2007, 80, 464–475. [Google Scholar] [CrossRef]
- Tanaka, A.; Inoue, K.; Hisaki, I.; Tohnai, N.; Miyata, M.; Matsumoto, A. Supramolecular Chirality in Layered Crystals of Achiral Ammonium Salts and Fatty Acids: A Hierarchical Interpretation. Angew. Chem. Int. Ed. 2006, 45, 4142–4145. [Google Scholar] [CrossRef]
- Tohnai, N.; Mizobe, Y.; Doi, M.; Sukata, S.; Hinoue, T.; Yuge, T.; Hisaki, I.; Matsukawa, Y.; Miyata, M. Well-Designed Supramolecular Clusters Comprising Triphenylmethylamine and Various Sulfonic Acids. Angew. Chem. Int. Ed. 2007, 46, 2220–2223. [Google Scholar] [CrossRef]
- Yuge, T.; Tohnai, N.; Fukuda, T.; Hisaki, I.; Miyata, M. Topological Study of Pseudo-Cubic Hydrogen-Bond Networks in a Binary System Composed of Primary Ammonium Carboxylates: An Analogue of an Ice Cube. Chem. Eur. J. 2007, 13, 4163–4168. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: not available.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Miyata, M.; Tohnai, N.; Hisaki, I. Supramolecular Chirality in Crystalline Assemblies of Bile Acids and Their Derivatives; Three-Axial, Tilt, Helical, and Bundle Chirality. Molecules 2007, 12, 1973-2000. https://doi.org/10.3390/12081973
Miyata M, Tohnai N, Hisaki I. Supramolecular Chirality in Crystalline Assemblies of Bile Acids and Their Derivatives; Three-Axial, Tilt, Helical, and Bundle Chirality. Molecules. 2007; 12(8):1973-2000. https://doi.org/10.3390/12081973
Chicago/Turabian StyleMiyata, Mikiji, Norimitsu Tohnai, and Ichiro Hisaki. 2007. "Supramolecular Chirality in Crystalline Assemblies of Bile Acids and Their Derivatives; Three-Axial, Tilt, Helical, and Bundle Chirality" Molecules 12, no. 8: 1973-2000. https://doi.org/10.3390/12081973




