Acetylcholinesterase Inhibition by Flavonoids from Agrimonia pilosa
Abstract
:Introduction
Results and Discussion
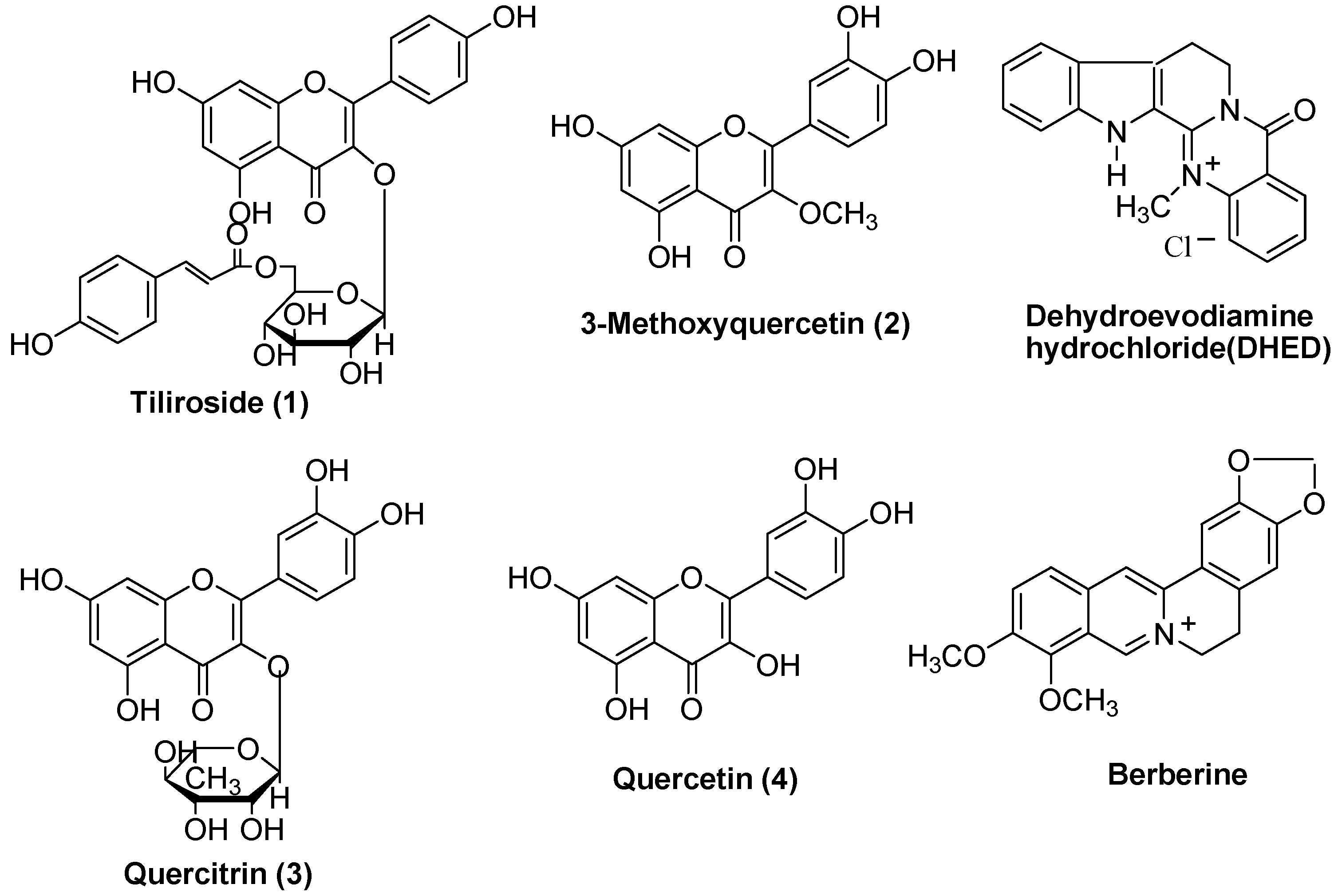
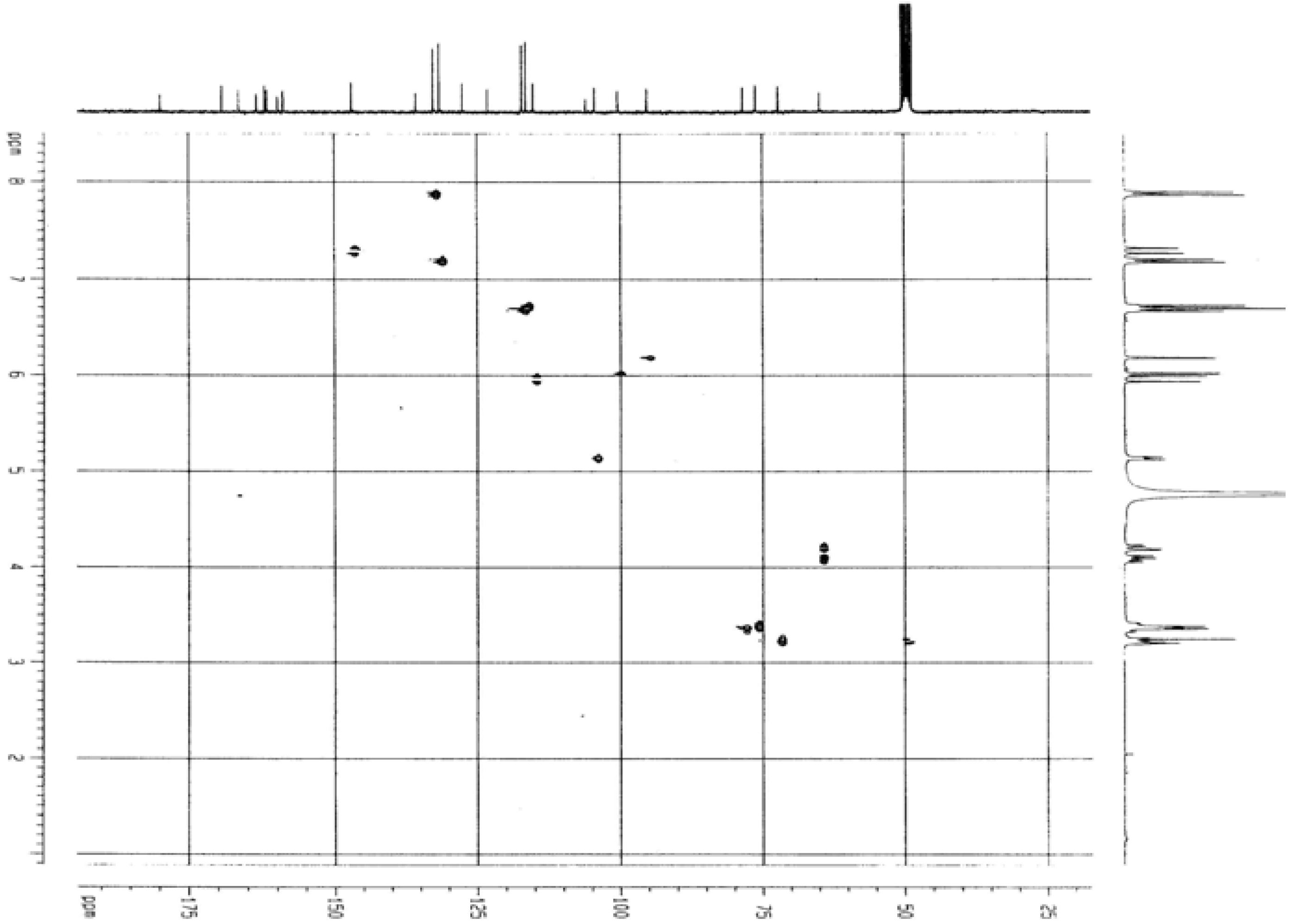
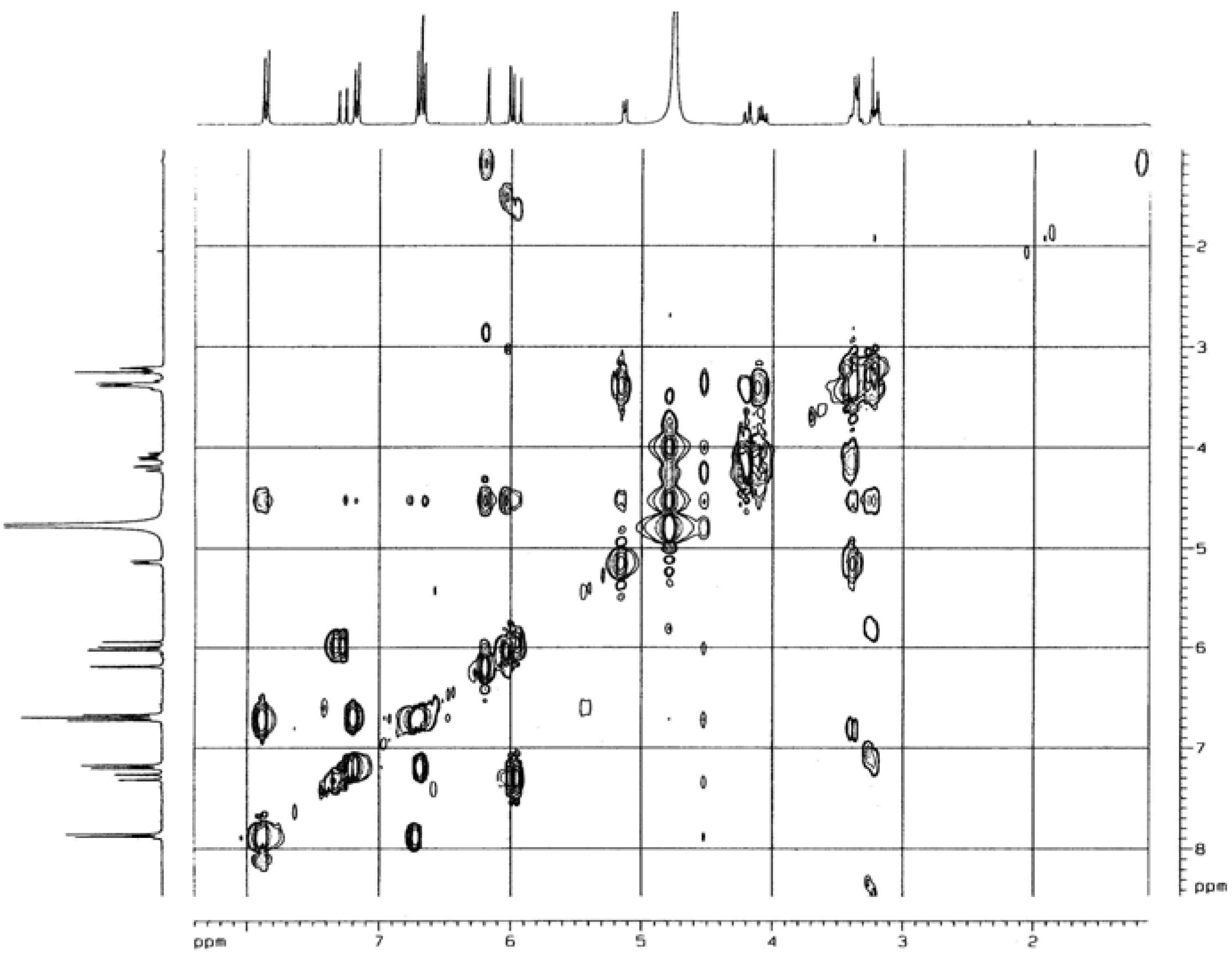
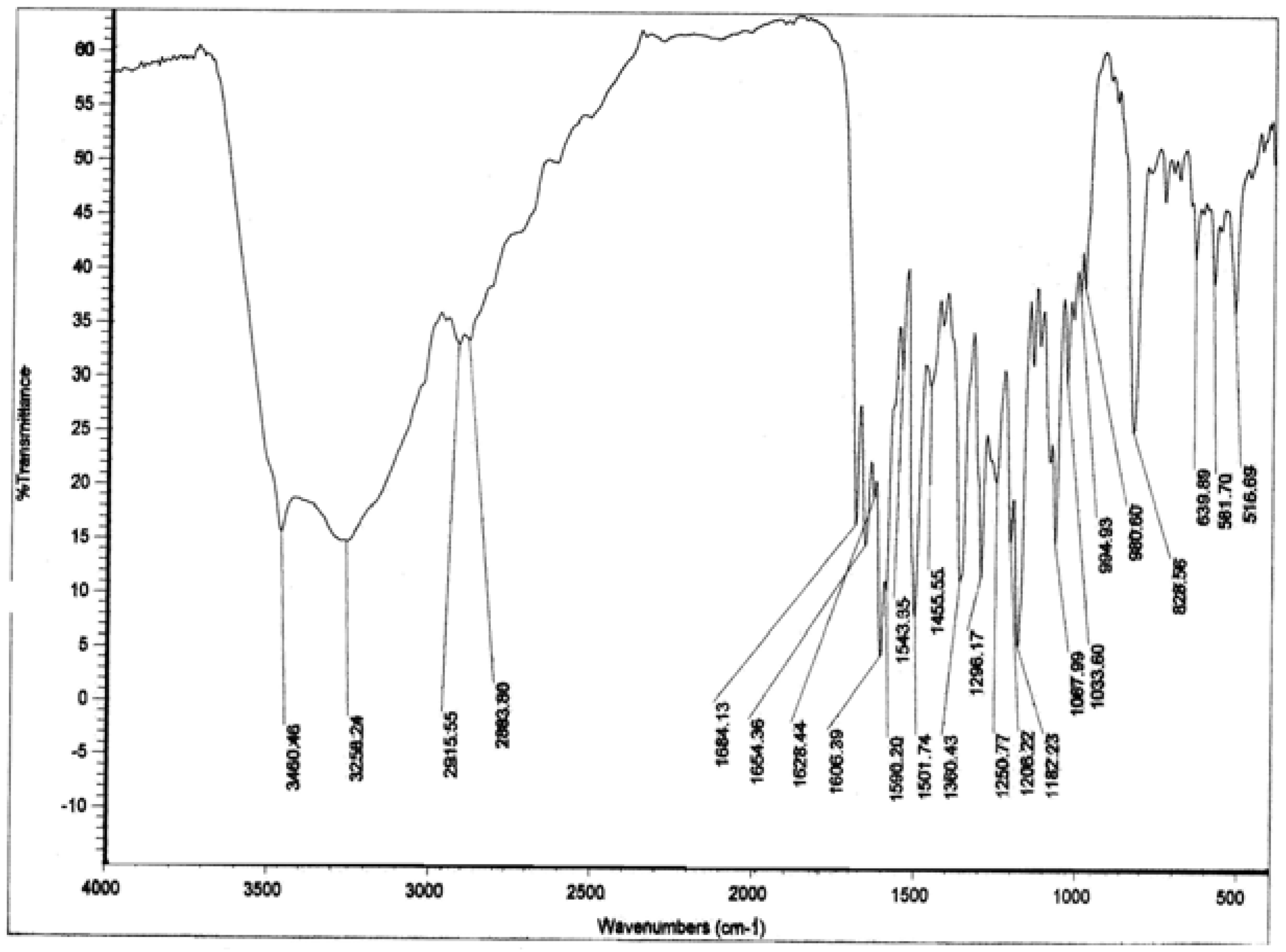
Biological activity
| Compound | +IC50 (μM) |
| Tiliroside (1) | 23.5 |
| 3-Methoxy quercetin (2) | 37.9 |
| Quercitrin (3) | 66.9 |
| Quercetin (4) | 19.8 |
| Dehydroevodiamine(DHED) | 37.8 |
Conclusions
Experimental
General
Plant material
Extraction, Screening and Isolation
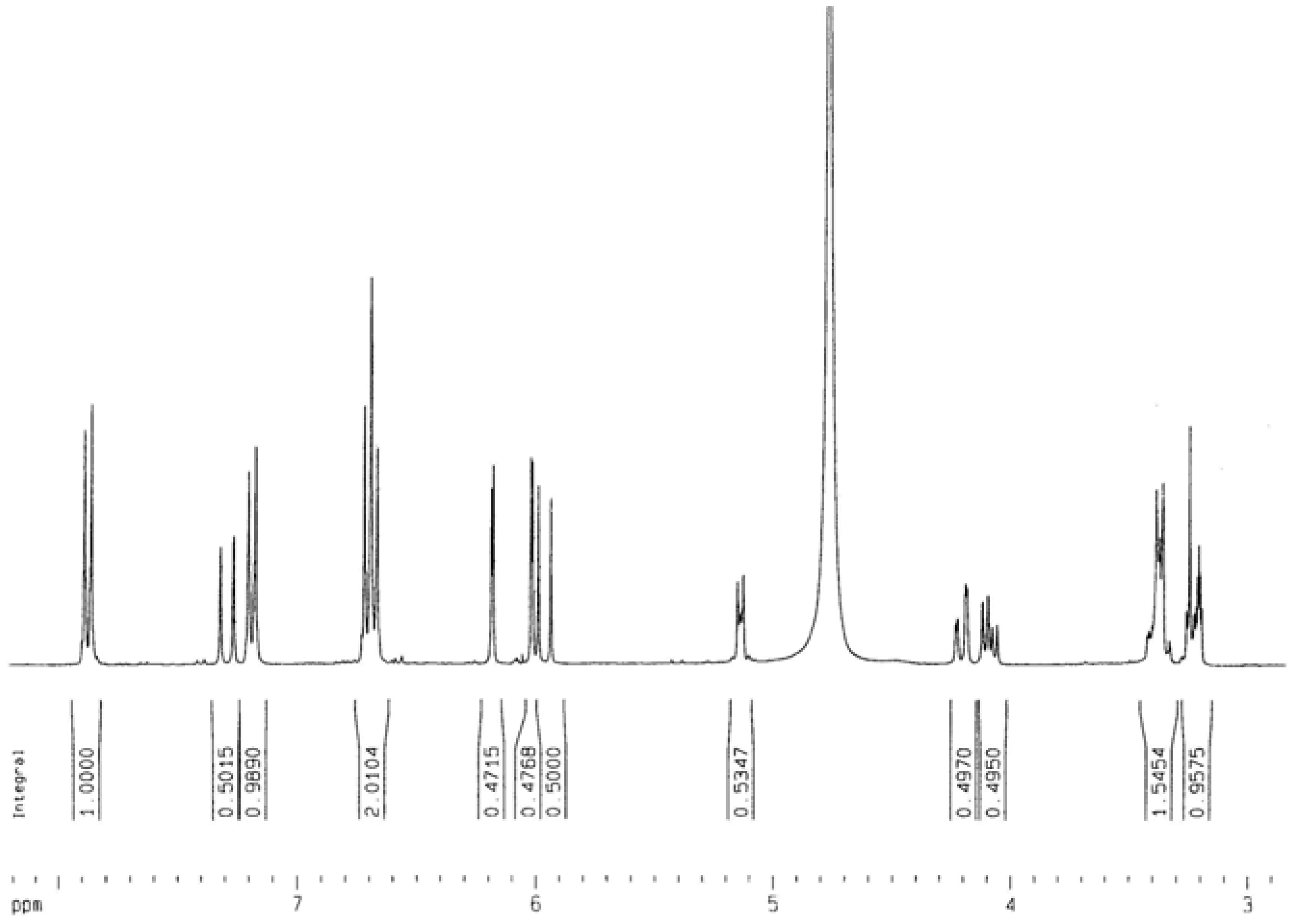
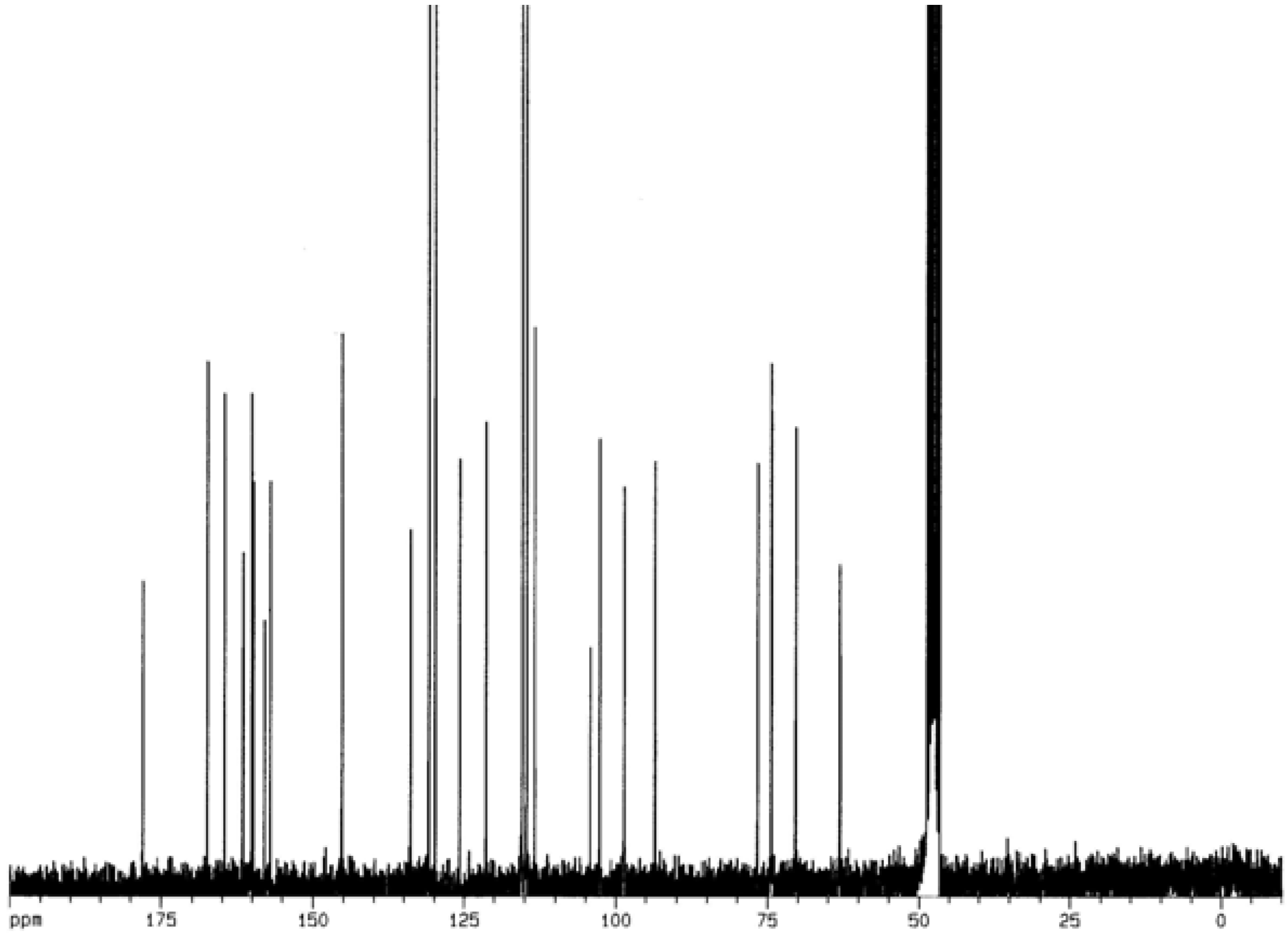
 = -62.5 °(C. 0.5 MeOH), C30H26O13; 1H-NMR (CD3OD) δ: 3.21 (2H, m, C3″,4″), 3.37 (1H, m, C2″), 3.38 (1H, m, C5″), 4.19 (2H, m, C6″), 5.13 (1H, m, C1″), 5.99 (1H, d, Cγ), 6.02 (1H, d, C6), 6.18 (1H, d, C8), 6.71 (4H, t, C3′,5′,3″′,5″′), 7.20 ( 2H, d, C2″’,6″′), 7.31 (1H, Cß ), 7.86 (2H, d, C2′,6′); 13C NMR: 63.06 (C6″), 70.42 (C4″), 74.44 (C3″), 74.50 (C2″), 76.71 (C5″), 93.57 (C8), 98.70 (C6), 102.72 (C1″), 104.28 (C10), 113.44 (Cß), 114.74 (C3′, 5′), 115.48 (C3″′, 5″′), 121.40 (C1’), 125.78 (C1″′), 129.89 (C2″′, 6″″), 130.93 (C2′, 6′), 133.93 (C3), 145.26 (Cγ), 157.07 (C9), 158.01 (C2), 159.87 (C4″′), 160.21 (C4′), 161.63 (C5), 164.65 (C7), 167.53 (Cα), 178.09 (C4); IR υmax 3262 (OH), 1654 (C=O), 1606 (C=C) cm-1.
= -62.5 °(C. 0.5 MeOH), C30H26O13; 1H-NMR (CD3OD) δ: 3.21 (2H, m, C3″,4″), 3.37 (1H, m, C2″), 3.38 (1H, m, C5″), 4.19 (2H, m, C6″), 5.13 (1H, m, C1″), 5.99 (1H, d, Cγ), 6.02 (1H, d, C6), 6.18 (1H, d, C8), 6.71 (4H, t, C3′,5′,3″′,5″′), 7.20 ( 2H, d, C2″’,6″′), 7.31 (1H, Cß ), 7.86 (2H, d, C2′,6′); 13C NMR: 63.06 (C6″), 70.42 (C4″), 74.44 (C3″), 74.50 (C2″), 76.71 (C5″), 93.57 (C8), 98.70 (C6), 102.72 (C1″), 104.28 (C10), 113.44 (Cß), 114.74 (C3′, 5′), 115.48 (C3″′, 5″′), 121.40 (C1’), 125.78 (C1″′), 129.89 (C2″′, 6″″), 130.93 (C2′, 6′), 133.93 (C3), 145.26 (Cγ), 157.07 (C9), 158.01 (C2), 159.87 (C4″′), 160.21 (C4′), 161.63 (C5), 164.65 (C7), 167.53 (Cα), 178.09 (C4); IR υmax 3262 (OH), 1654 (C=O), 1606 (C=C) cm-1.Determination of inhibitory activity against acetylcholinesterase
Acknowledgments
References
- Parnetti, L.; Senin, U.; Mecocci, P. Cognitive enhancement therapy for Alzheimer’s disease. The way forward. Drugs 1997, 53, 752–768. [Google Scholar]
- Brinton, R.D.; Yamazaki, R.S. Advances and Challenges in the Prevention and Treatment of Alzeimer’s Disease. Pharmaceut. Res. 1998, 15, 386–398. [Google Scholar] [CrossRef]
- Kim, W.G.; Cho, K.M.; Lee, C.K.; Yoo, I.D. Terreulactone A, a novel meroterpenoid with anti-acetylcholinesterase activity from Aspergillus terreus. Tetrahedron Lett. 2002, 43, 3197–3198. [Google Scholar] [CrossRef]
- Schneider, L.S. J. Clin. New therapeutic approaches to Alzeimer’s Disease. Psychiatry 1996, 57, 30–36. [Google Scholar]
- Sala, A.; Recio, M.C.; Schinella, G.R.; Manez, S.; Giner, R.M.; Cerda-Nicolas, M.; Rios, J-L. Assessment of the anti-inflammatory activity and free radical scavenger activity of tiliroside. Eur. J. Pharmacol. 2003, 461, 53–61. [Google Scholar]
- a) Pei, Y.H.; Li, X.; Zhu, T.R. Studies on the chemical constituents from the root-sprouts of Agrimonia pilosa Ledeb. Yao Hsueh Hsueh Pao 1989, 24, 431–437. [Google Scholar] b) Pei, Y.H.; Li, X.; Zhu, T.R.; Wu, L.J. Studies on the structurs of a new flavanonol glucoside of the root-sprouts of Agrimonia pilosa ledeb. Yao Xue Xue Bao 1990, 25, 267–270. [Google Scholar]
- Isao, K.; Naosuke, B.; Yumiko, O.; Nobusuke, K. Triterpenoids from Agrimonia pilosa. Phytochemistry 1988, 27, 297–299. [Google Scholar] [CrossRef]
- Su., G.; Su, S.; Zhu, T. Studies on bacteriostatic components from Agrimonia pilosa Ledeb. Shenyang Yaoxueyuan Xuebao 1984, 1, 44–50. [Google Scholar]
- Powell, R.G.; Smith, C.R.; Bajaj, R.; Chang, C.J.; McLaughlin, J. Tiliroside from the seeds of Eremocarpus setigerus. J. Nat. Prod. 1986, 49, 1174–1175. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.Y.; Oh, S.R.; Lee, I.S.; Ahn, K.S.; Lee, J.J.; Lee, H.K. Anti-complement activity of tiliroside from the flower buds of Magnolia fargesii. Biol. Pharm. Bull. 1998, 21, 1077–1078. [Google Scholar] [PubMed]
- Liu, H.; Orjala, J.; Sticher, O.; Rali, T. Acylated flavonol glycosides from the leaves of Stenocleane palustris. J. Nat. Prod. 1999, 62, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Dimas, K.; Demetzos, C.; Mitaku, S.; Marselos, M.; Tzavaras, T.; Kokkinopoulos, D. Cytotoxic activity of Kaempferol glycosides against human leukaemic cell lines in vitro. Pharmacol Res. 2000, 41, 85–88. [Google Scholar]
- Matsuda, H.; Ninomiya, K.; Shimoda, H.; Yoshikawa, M. Hepatoprotective principles from the flowers of Tila argentea (Linden): Structure requirements of tiliroside and mechanisms of action. Bioorg. Med. Chem. 2002, 10, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.F.; Strack, D.; Baumert, A.; Subramaniam, R.; Goh, N.K.; Chia, T.F.; Tan, S.N.; Chia, L. S. Antioxidant flavonoids from leaves of Polygonum hydropiper L. Phytochemistry 2003, 62, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Buckova, A.; Eisenreichova, E.; Homola, V.; Leifertova, I.; Licha, K.; Natherova, L. Acta Facult. Pharm. Univ. Comenianae. 1972, 22, 47–73.
- Park, C.H.; Kim, S.H.; Choi, W.; Lee, Y.J.; Kim, J.S.; Kang, S.S.; Suh, Y.H. Novel Anticholinesterase and antiamnestic activites of dehydroevodiamine, a constituent of Evodia rutaecarpa. Planta Med. 1996, 62, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Milligram quantities of compounds 1 and 2 are available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jung, M.; Park, M. Acetylcholinesterase Inhibition by Flavonoids from Agrimonia pilosa. Molecules 2007, 12, 2130-2139. https://doi.org/10.3390/12092130
Jung M, Park M. Acetylcholinesterase Inhibition by Flavonoids from Agrimonia pilosa. Molecules. 2007; 12(9):2130-2139. https://doi.org/10.3390/12092130
Chicago/Turabian StyleJung, Mankil, and Moonso Park. 2007. "Acetylcholinesterase Inhibition by Flavonoids from Agrimonia pilosa" Molecules 12, no. 9: 2130-2139. https://doi.org/10.3390/12092130




