Experimental
General
NMR spectra were recorded on a 400 MHz Bruker Digital FT-NMR ‘Avance 400’ spectrometer. Chemical shifts (δ) are on parts per million (ppm), with CDCl3 as solvent and relative to tetramethylsilane (TMS) as the internal reference. FT-IR spectra were recorded on Perkin Elmer FT-IR spectrometer (KBr pellets). GC-EIMS spectra were measured on a Varian SAT2100T mit GC3900 spectrometer using ionization by FAB. Melting points were measured on a Gallenkamp melting point apparatus. Silica gel 60 (Merck) was used for column separations. TLC was conducted on standard conversion aluminium sheets pre-coated with a 0.2 mm layer of silica gel. Elemental analyses were measured with a Thermo Flash Flash EA 1112 Series apparatus.
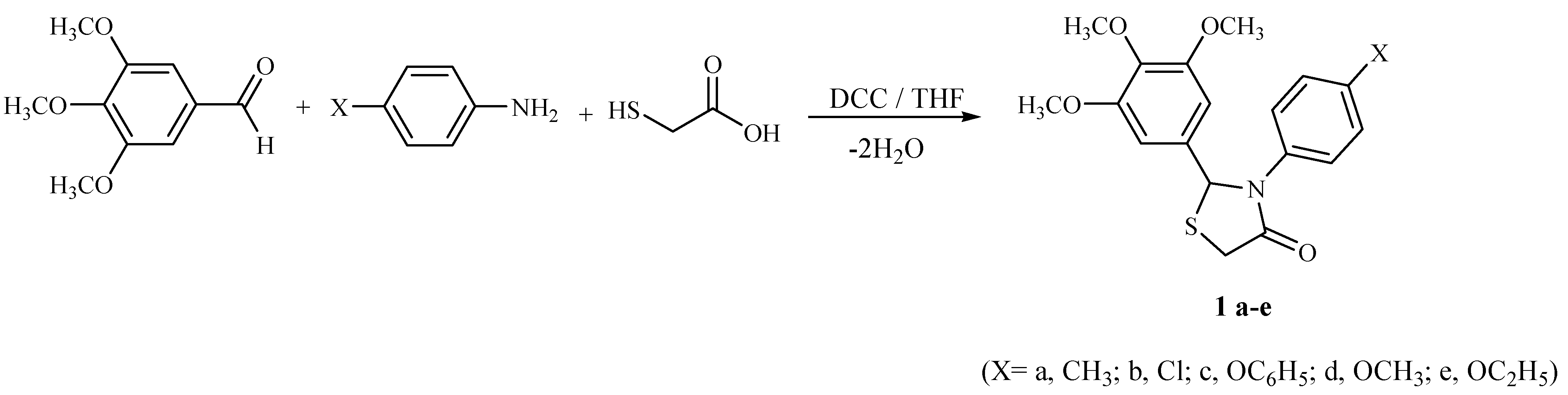
General procedure for the synthesis of 4-thiazolidinones 1a-e
p-Substituted aniline (1 mmol) and 3,4,5-trimethoxybenzaldehyde (2 mmol) were stirred in THF at an ice-bath for 5 min, followed by addition of mercaptoacetic acid (3 mmol). After 5 min DCC (1.2 mmol) was added to the reaction mixture at 0°C and the reaction mixture stirred for additional 1-3 hours at room temperature. Formed DCU was removed by filtration, filtrate was concentrated to dryness under reduced pressure and the residue was taken up with ethyl acetate. The organic layer was washed with 5 % aq. citric acid, water, 5 % aq. sodium hydrogen carbonate and then with brine. The organic layer was dried over sodium sulfate and the solvent removed under vacuum to give the crude product, which was purified by recrystallization from 2:1 petroleum ether-diethyl ether.
2-(3,4,5-Trimethoxyphenyl)-3-(4-methylphenyl)-4-thiazolidinone (1a). Light yellow crystals; yield 59 %; mp. 161 oC; 1H-NMR δ: 2.29 (s, 3H, CH3), 3.78 (s, 9H, OCH3), 3.87, 3.93 (AB system, J= 9.2 Hz, 2H, 5-CH2), 6.47 (s, 1H, 2-CH), 7.01-7.38 (m, 6H, aromatic); FT-IR: 1702 (N-C=O) cm-1; Anal. Calcd for C19H21NO4S (359.12): C: 63.49; H, 5.89; N, 3.90; S, 8.92. Found: C, 63.59; H, 5.92; N, 3.84; S, 8.89; MS: m/z 359.
3-(4-Chlorophenyl)-2-(3,4,5-trimethoxyphenyl)-4-thiazolidinone (1b). Yellow crystals, yield 52 %; mp. 161-162 oC; 1H-NMR δ: 3.78 (s, 9H, OCH3), 3.86, 3.91 (AB system, J= 9.2 Hz, 2H, 5-CH2), 6.01 (s, 1H, 2-CH), 6.57-7.33 (m, 6H, aromatic) ppm; FT-IR: 1682 (N-C=O) cm-1; Anal. Calcd for C18H18ClNO4S (379.86): C, 56.91, H: 4.77, N: 3.68, S: 8.44. Found: C:56.89, H: 4.79, N: 3.70, S: 8.47; MS: m/z 380.
2-(3,4,5-Trimethoxyphenyl)-3-(4-phenoxyphenyl)-4-thiazolidinone (1c). Light yellow crystals, yield 73 %; mp. 141-142 oC; 1H-NMR δ: 3.78 (s, 9H, OCH3), 3.86, 3.93 (AB system, J= 9.1 Hz, 2H, 5-CH2), 5.96 (s, 1H, 2-CH), 6.47-7.33 (m, 11H, aromatic) ppm; FT-IR: 1683 (N-C=O) cm-1; Anal. Calcd. for C24H23 NO5S (437.51): C: 65.88; H: 5.30; N: 3.20; S: 7.33. Found: C: 65.89, H: 5.29, N: 3.22, S: 7.36; MS: m/z 438.
3-(4-Methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)-4-thiazolidinone (1d). Light yellow crystals, yield 68 %; mp. 144-145 oC; 1H-NMR δ: 3.64 (s, 3H, OCH3), 3.78 (s, 9H, OCH3), 3.81, 3.93 (AB system, J= 9.2 Hz, 2H, 5-CH2), 5.91 (s, 1H, 2-CH), 6.47-7.24 (m, 6H, aromatic) ppm; FT-IR: 1685 (N-C=O) cm-1; Anal. Calcd. for C19H21NO5S (375.44): C: 60.78, H: 5.64, N: 3.73, S: 8.54. Found: C: 60.81, H: 5.67, N: 3.76, S: 8.58, MS: m/z 375.
3-(4-Ethoxyphenyl)-2-(3,4,5-trimethoxyphenyl)-4-thiazolidinone (1e). Light yellow crystals, yield 69 %; mp. 156-157 oC; 1H-NMR δ: 1.23-1.36 (t, J= 7.2 Hz, 3H, CH3), 3.77 (s, 9H, OCH3), 3.81-3.99 (m, 4H, 5-CH2, OCH2), 5.91 (s, 1H, 2-CH), 6.38-7.33 (m, 6H, aromatic) ppm; FT-IR: 1702 (N-C=O) cm-1; Anal. Calcd. for C20H23NO5S (389.47): C: 61.68, H: 5.95, N: 3.60, S: 8.23. Found: C: 61.70, H: 5.97, N: 3.63, S: 8.25, MS: m/z 389.
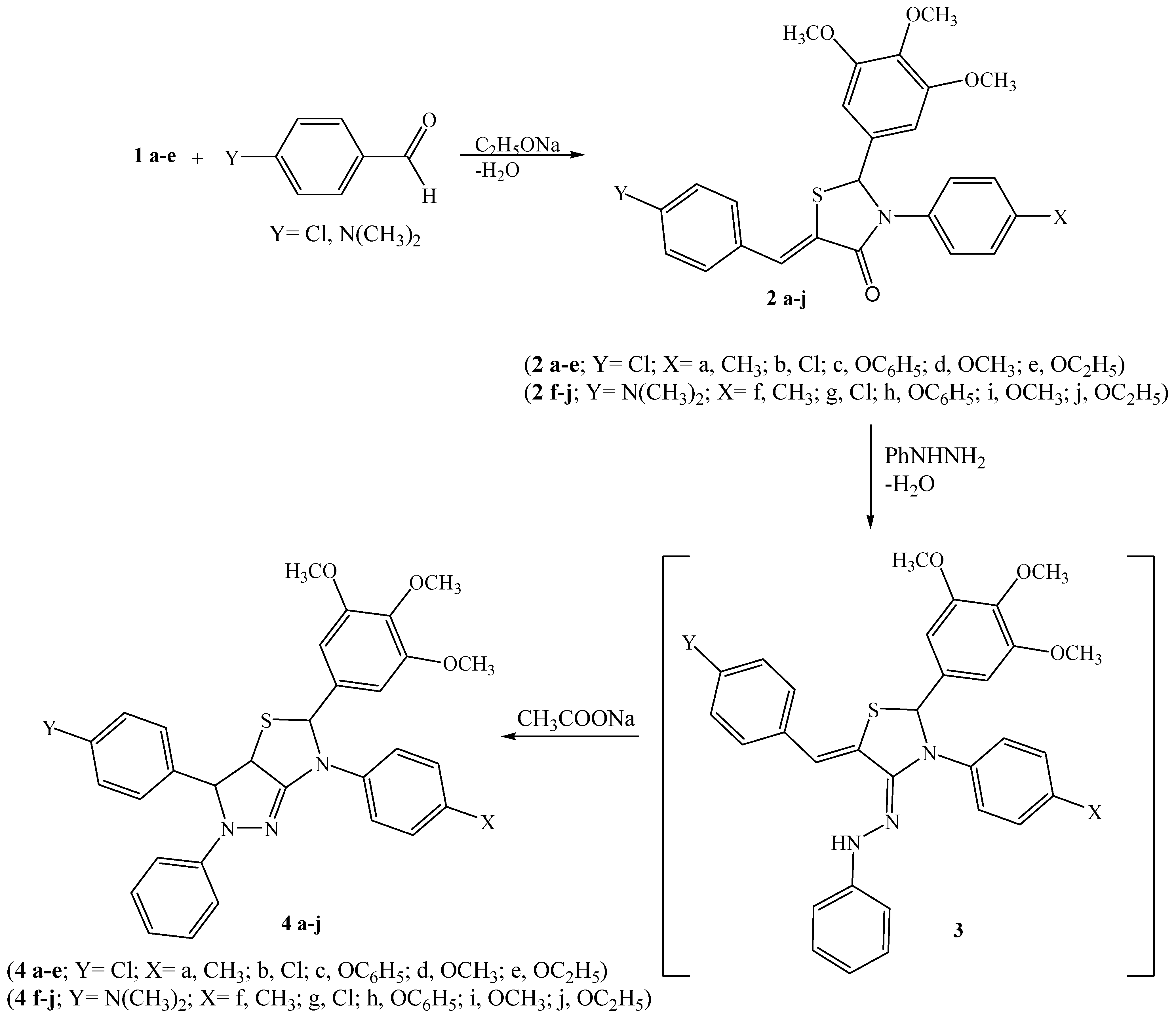
General procedure for the preparation of 5-arylidine-4-thiazolidinones 2a-j
A solution of 1a-e (1 mmol) and 4-chlorobenzaldehyde (1 mmol) or 4-dimethylaminoaniline (1 mmol) in dry benzene (25 mL) was refluxed for about 10-12 h, in the presence of sodium ethoxide (1 mmol), cooled, poured into ice cold water and then acidified with glacial acetic acid. The benzene layer was separated, dried over CaCl2 and evaporated in vacuo to give crude product that was purified by recrystallization.
5-(4-Chlorobenzylidene)-2-(3,4,5-trimethoxyphenyl)-3-(4-methylphenyl)-4-thiazolidinone (2a). Orange crystals; yield 53 %; mp. 174 oC (from petroleum ether-diethyl ether, 4:1); 1H-NMR δ: 2.29 (s, 3H, CH3), 3.74 (s, 9H, OCH3), 6.46 (s, 1H, =CH), 6.74-6.83 (m, 4H, aromatic), 7.18-7.43 (m, 3H, aromatic and 2-CH), 7.51-7.53 (m, 2H, aromatic), 7.64-7.77 (m, 2H, aromatic) ppm; FT-IR: 1683 (N-C=O) cm-1; Anal. Calcd. for C26H24ClNO4S (481.11): C: 64.79, H: 5.02; N: 2.91, S: 6.65. Found: C: 64.74, H: 5.07; N: 2.94, S: 6.61; MS: m/z 481.
5-(4-Chlorobenzylidene)-3-(4-chlorophenyl)-2-(3,4,5-trimethoxyphenyl)-4-thiazolidinone (2b). Red-dish crystals; yield 41 %; mp. 184-186 oC (from petroleum ether-diethyl ether, 4:1); 1H-NMR δ: 3.78 (s, 9H, OCH3), 6.46 (s, 1H, =CH), 6.96-7.21 (m, 4H, aromatic), 7.33-7.51 (m, 3H, aromatic and 2-CH), 7.63-7.66 (m, 2H, aromatic), 7.83-7.87 (m, 2H, aromatic) ppm; FT-IR: 1691 (N-C=O) cm-1; Anal. Calcd. for C25H21Cl2 NO4S (502.45): C: 59.77, H: 4.21, N: 2.79, S: 6.38. Found: C: 59.80, H: 4.23, N: 2.81, S: 6.40; MS: m/z 502.
5-(4-Chlorobenzylidene)-2-(3,4,5-trimethoxyphenyl)-3-(4-phenoxyphenyl)-4-thiazolidinone (2c). Dark orange crystals; yield 44 %; mp. 168-170 oC (from petroleum ether-diethyl ether, 3:1); 1H NMR (CDCl3) δ: 3.78 (s, 9H, OCH3), 6.52 (s, 1H, =CH), 6.96-7.15 (m, 8H, aromatic), 7.18-7.29 (m, 4H, aromatic and 2-CH), 7.63-7.66 (m, 2H, aromatic), 7.83-7.87 (m, 2H, aromatic) ppm; FT IR (KBr): 1685 (N-C=O) cm-1; Anal. Calcd. for C31H26ClNO5S (560.06): C: 66.48, H: 4.79, N: 2.50, S: 5.72. Found: C: 66.50, H: 4.81, N: 2.51, S: 5.75; MS: m/z 560.
5-(4-Chlorobenzylidene)-3-(4-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)-4-thiazolidinone (2d). Orange crystals; yield 48 %; mp. 181-182 oC (from petroleum ether-diethyl ether, 4:1); 1H-NMR δ: 3.63 (s, 3H, OCH3), 3.79 (s, 9H, OCH3), 6.47 (s, 1H, =CH), 6.96-7.09 (m, 4H, aromatic), 7.19-7.52 (m, 3H, aromatic and 2-CH), 7.63-7.67 (m, 2H, aromatic), 7.83-7.86 (m, 2H, aromatic) ppm; FT-IR: 1689 (N-C=O) cm-1; Anal. Calcd. for C26H24ClNO5S (497.99): C: 62.71, H: 4.86, N: 2.81, S: 6.44. Found: C: 62.74, H: 4.87, N: 2.81, S: 6.47; MS: m/z 498.
5-(4-Chlorobenzylidene)-3-(4-ethoxyphenyl)-2-(3,4,5-trimethoxyphenyl)-4-thiazolidinone (2e). Orange crystals; yield 51 %; mp. 173 oC (from petroleum ether-diethyl ether, 3:1); 1H-NMR δ: 1.22-1.36 (t, J= 7.2 Hz, 3H, CH3), 3.78 (s, 9H, OCH3), 3.93-3.97 (q, J= 7.2 Hz, 2H, OCH2), 6.45 (s, 1H, =CH), 6.94-7.11 (m, 4H, aromatic), 7.19-7.53 (m, 3H, aromatic and 2-CH), 7.63-7.68 (m, 2H, aromatic), 7.83-7.85 (m, 2H, aromatic) ppm; FT-IR: 1687 (N-C=O) cm-1; Anal. Calcd. for C27H26ClNO5S (512.02): C: 63.33, H: 5.12, N: 2.73, S: 6.26. Found: C: 63.36, H: 5.15, N: 2.75, S: 6.29; MS: m/z 512.
5-(4-Dimethylaminobenzylidene)-2-(3,4,5-trimethoxyphenyl)-3-(4-methylphenyl)-4-thiazolidinone (2f). Yellow crystals; yield 42 %; mp. 162 oC (from petroleum ether-diethyl ether, 4:1); 1H- NMR δ: 2.31 (s, 3H, CH3), 3.05 (s, 6H, CH3), 3.76 (s, 9H, OCH3), 6.42 (s, 1H, =CH), 6.76-7.22 (m, 4H, aromatic), 7.29-7.38 (m, 3H, aromatic and 2-CH), 7.45-7.49 (m, 2H, aromatic), 7.77-7.81 (m, 2H, aromatic) ppm; FT-IR: 1683 (N-C=O) cm-1; Anal. Calcd. for C28H30N2O4S (490.62): C: 68.55, H: 6.16, N: 5.71, S: 6.53. Found: C: 68.56, H: 6.19, N: 5.73, S: 6.58; MS: m/z 491.
3-(4-Chlorophenyl)-5-(4-dimethylaminobenzylidene)-2-(3,4,5-trimethoxyphenyl)-4-thiazolidinone (2g). Red crystals; yield 32 %; mp. 156-157 oC (from petroleum ether-diethyl ether, 3:1); 1H-NMR δ: 3.05 (s, 6H, CH3), 3.78 (s, 9H, OCH3), 6.42 (s, 1H, =CH), 6.87-6.94 (m, 4H, aromatic), 7.18-7.22 (m, 3H, aromatic and 2-CH), 7.33-7.37 (m, 2H, aromatic), 7.47-7.50 (m, 2H, aromatic) ppm; FT-IR: 1684 (N-C=O) cm-1; Anal. Calcd. for C27H27 ClN2O4S (511.04): C: 63.46, H: 5.32, N: 5.48, S: 6.27. Found: C: 63.49, H: 5.33, N: 5.50, S: 6.31; MS: m/z 511.
5-(4-Dimethylaminobenzylidene)-2-(3,4,5-trimethoxyphenyl)-3-(4-phenoxyphenyl)-4-thiazolidinone (2h). Orange crystals; yield 31 %; mp. 152 oC (from petroleum ether-diethyl ether, 2:1); 1H-NMR δ: 3.02 (s, 6H, CH3), 3.81 (s, 9H, OCH3), 6.43 (s, 1H, =CH), 6.92-7.05 (m, 8H, aromatic), 7.17-7.23 (m, 3H, aromatic), 7.39-7.52 (m, 3H, aromatic and 2-CH), 7.63-7.71 (m, 2H, aromatic) ppm; FT-IR: 1691 (N-C=O) cm-1; Anal. Calcd. for C33H32 N2O5S (568.69): C: 69.71, H: 5.67, N: 4.93, S: 5.63. Found: C: 69.74, H: 5.69, N: 4.97, S: 5.67; MS: m/z 569.
5-(4-Dimethylaminobenzylidene)-3-(4-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)-4-thiazolidinone (2i). Orange crystals; yield 43 %; mp. 156-158 oC (from petroleum ether-diethyl ether, 2:1); 1H-NMR δ: 3.02 (s, 6H, CH3), 3.59 (s, 3H, OCH3), 3.81 (s, 9H, OCH3), 6.47 (s, 1H, =CH), 6.82-7.09 (m, 4H, aromatic), 7.19-7.53 (m, 3H, aromatic and 2-CH), 7.63-7.68 (m, 2H, aromatic), 7.78-7.81 (m, 2H, aromatic) ppm; FT-IR: 1691 (N-C=O) cm-1; Anal. Calcd. for C28H30N2O5S (506.62): C: 66.38, H: 5.97, N: 5.53, S: 6.33. Found: C: 66.41, H: 5.99, N: 5.56, S: 6.37; MS: m/z 507.
5-(4-Dimethylaminobenzylidene)-3-(4-ethoxyphenyl)-2-(3,4,5-trimethoxyphenyl)-4-thiazolidinone (2j). Orange crystals; yield 36 %; mp. 162 oC (from petroleum ether-diethyl ether, 4:1); 1H- NMR δ: 1.24-1.38 (t, J= 7.2 Hz, 3H, CH3), 3.01 (s, 6H, CH3), 3.81 (s, 9H, OCH3), 3.91-3.95 (q, J= 7.2 Hz, 2H, OCH2), 6.43 (s, 1H, =CH), 6.94-7.15 (m, 4H, aromatic), 7.23-7.49 (m, 3H, aromatic and 2-CH), 7.58-7.62 (m, 2H, aromatic), 7.77-7.81 (m, 2H, aromatic) ppm; FT-IR: 1682 (N-C=O) cm-1; Anal. Calcd. for C29H32N2O5S (520.65): C: 66.91, H: 6.20, N: 5.38, S: 6.16. Found: C: 66.95, H: 6.22, N: 5.41, S: 6.19; MS: m/z 521.
General method for tetrahydro-2H-pyrazolo[3,4-d]thiazoles 4a-j
The respective benzylidene derivative, 2a-j (1 mmol) in glacial acetic acid (10 mL), sodium acetate (1 g) and phenyl hydrazine (1 mL) were heated for 7 h. The mixture was filtered hot to remove any insoluble material, cooled, and then water was added and boiled for few minutes, then it was cooled to afford the crude product which was purified by column chromatography from n-hexane-ethyl acetate (2:1).
![Molecules 12 02151 i001]()
3-(4-Chlorophenyl)-5-(3,4,5-trimethoxyphenyl)-2-phenyl-6-p-tolyl-3,3a,5,6-tetrahydro-2H-pyrazolo-[3,4-d]thiazole (4a). Light yellow crystals; yield 49 %; mp. 143 oC; 1H-NMR δ: 2.31 (s, 3H, CH3), 3.72 (s, 9H, OCH3), 4.58 (d, J= 11.0 Hz, 1H, 3-CH), 5.82 (d, J= 11.0 Hz, 1H, 3a-CH), 6.01 (s, 1H, 5-CH), 6.90-7.13 (m, 7H, aromatic), 7.17-7.38 (m, 4H, aromatic), 7.45-7.48 (m, 2H, aromatic), 7.64-7.77 (m, 2H, aromatic) ppm; FT-IR: 3005-3011 (aromatic), 1598 (C=N), 1256 (C-O) cm-1; Anal. Calcd. for C32H30ClN3O3S (572.12): C, 67.18; H, 5.29; N, 7.34; S, 5.60. Found: C, 67.23; H, 5.21; N, 7.31; S, 5.57; MS: m/z 572.
3,6-Bis(4-Chlorophenyl)-5-(3,4,5-trimethoxyphenyl)-2-phenyl-3,3a,5,6-tetrahydro-2H-pyrazolo[3,4-d]thiazole (4b). Yellow crystals; yield 51 %; mp. 152 oC; 1H-NMR δ: 3.73 (s, 9H, OCH3), 4.52 (d, J= 11.0 Hz, 1H, 3-CH), 5.79 (d, J= 11.0 Hz, 1H, 3a-CH), 5.99 (s, 1H, 5-CH), 6.92-7.12 (m, 7H, aromatic), 7.18-7.37 (m, 4H, aromatic), 7.46-7.51 (m, 2H, aromatic), 7.71-7.81 (m, 2H, aromatic) ppm; FT-IR: 3005-3010 (aromatic), 1596 (C=N), 1248 (C-O) cm-1; Anal. Calcd. for C31H27Cl2N3O3S (592.54): C, 62.83; H, 4.59; N, 7.09; S, 5.41. Found: C, 62.84; H, 4.61, N: 7.11, S: 5.43; MS: m/z 593.
3-(4-Chlorophenyl)-5-(3,4,5-trimethoxyphenyl)-2-phenyl-6-(4-phenoxyphenyl)-3,3a,5,6-tetrahydro-2H-pyrazolo[3,4-d]thiazole (4c). Yellow crystals; yield 62 %; mp. 151 oC; 1H-NMR δ: 3.71 (s, 9H, OCH3), 4.56 (d, J= 11.0 Hz, 1H, 3-CH), 5.82 (d, J= 11.0 Hz, 1H, 3a-CH), 6.03 (s, 1H, 5-CH), 6.92-7.11 (m, 12H, aromatic), 7.17-7.23 (m, 4H, aromatic), 7.39-7.52 (m, 2H, aromatic), 7.63-7.71 (m, 2H, aromatic) ppm; FT-IR: 3002-3011 (aromatic), 1598 (C=N), 1250 (C-O) cm-1; Anal. Calcd. for C37H32ClN3O4S (650.19): C: 68.34, H: 4.96, N, 6.46, S: 4.93. Found: C, 68.37; H, 4.98, N: 6.47, S: 4.97; MS: m/z 650.
3-(4-Chlorophenyl)-6-(4-methoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-2-phenyl-3,3a,5,6-tetrahydro-2H-pyrazolo[3,4-d]thiazole (4d). Light yellow crystals; yield 49 %; mp. 143 oC; 1H-NMR δ: 3.61 (s, 3H, OCH3), 3.72 (s, 9H, OCH3), 4.58 (d, J= 11.0 Hz, 1H, 3-CH), 5.81 (d, J= 11.0 Hz, 1H, 3a-CH), 6.05 (s, 1H, 5-CH), 6.90-7.08 (m, 7H, aromatic), 7.17-7.38 (m, 4H, aromatic), 7.47-7.52 (m, 2H, aromatic), 7.68-7.81 (m, 2H, aromatic) ppm; FT-IR: 3000-3010 (aromatic), 1602 (C=N), 1256 (C-O) cm-1; Anal. Calcd. for C32H30ClN3O4S (588.12): C: 65.35, H: 5.14, N, 7.15, S: 5.45. Found: C, 65.37; H, 5.16, N: 7.19, S: 5.49; MS: m/z 588.
3-(4-Chlorophenyl)-6-(4-ethoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-2-phenyl-3,3a,5,6-tetrahydro-2H-pyrazolo[3,4-d]thiazole (4e). Yellow crystals; yield 41 %; mp. 138 oC; 1H-NMR δ: 1.23-1.37 (t, J= 7.2 Hz, 3H, CH3), 3.71 (s, 9H, OCH3), 3.91-3.95 (q, J= 7.2 Hz, 2H, OCH2), 4.58 (d, J= 11.0 Hz, 1H, 3-CH), 5.82 (d, J= 11.0 Hz, 1H, 3a-CH), 6.07 (s, 1H, 5-CH), 6.92-7.11 (m, 7H, aromatic), 7.17-7.39 (m, 4H, aromatic), 7.47-7.52 (m, 2H, aromatic), 7.68-7.81 (m, 2H, aromatic) ppm; FT-IR: 3000-3009 (aromatic), 1599 (C=N), 1245 (C-O) cm-1; Anal. Calcd. for C33H32ClN3O4S (602.15): C: 65.82, H: 5.36, N, 6.98, S: 5.33. Found: C, 65.84; H, 5.37, N: 6.99, S: 5.38; MS: m/z 602.
5-(3,4,5-Trimethoxyphenyl)-3-(4-dimethylaminophenyl)-2-phenyl-6-p-tolyl-3,3a,5,6-tetrahydro-2H-pyrazolo[3,4-d]thiazole (4f). Yellow crystals; yield 51 %; mp. 161 oC; 1H-NMR δ: 2.32 (s, 3H, CH3), 2.85 (s, 6H, CH3), 3.76 (s, 9H, OCH3), 4.56 (d, J= 11.0 Hz, 1H, 3-CH), 5.81 (d, J= 11.0 Hz, 1H, 3a-CH), 6.02 (s, 1H, 5-CH), 6.86-7.21 (m, 11H, aromatic), 7.30-7.51 (m, 4H, aromatic) ppm; FT-IR: 3004-3010 (aromatic), 1600 (C=N), 1246 (C-O) cm-1; Anal. Calcd. for C34H36N4O3S (580.75): C: 70.32, H: 6.25, N: 9.65, S: 5.52. Found: C, 70.34; H, 6.27, N: 9.67, S: 5.55; MS: m/z 581.
6-(4-Chlorophenyl)-5-(3,4,5-trimethoxyphenyl)-3-(4-dimethylaminophenyl)-2-phenyl-3,3a,5,6-tetra-hydro-2H-pyrazolo[3,4-d]thiazole (4g). Yellow crystals; yield 38 %; mp. 150 oC; 1H-NMR δ: 2.89 (s, 6H, CH3), 3.76 (s, 9H, OCH3), 4.57 (d, J= 11.0 Hz, 1H, 3-CH), 5.81 (d, J= 11.0 Hz, 1H, 3a-CH), 6.05 (s, 1H, 5-CH), 6.83-7.26 (m, 11H, aromatic), 7.27-7.53 (m, 4H, aromatic) ppm; FT-IR: 3000-3008 (aromatic), 1600 (C=N), 1257 (C-O) cm-1; Anal. Calcd. for C33H33 ClN4O3S (601.16): C: 65.93, H: 5.53, N: 9.32, S: 5.33 Found: C, 65.97; H, 5.57, N: 9.37, S: 5.38; MS: m/z 601.
5-(3,4,5-Trimethoxyphenyl)-3-(4-dimethylaminophenyl)-6-(4-phenoxyphenyl)-2-phenyl-3,3a,5,6-tetra-hydro-2H-pyrazolo[3,4-d]thiazole (4h). Yellow crystals; yield 38 %; mp. 155 oC; 1H-NMR δ: 2.89 (s, 6H, CH3), 3.81 (s, 9H, OCH3), 4.56 (d, J= 11.0 Hz, 1H, 3-CH), 5.81 (d, J= 11.0 Hz, 1H, 3a-CH), 6.02 (s, 1H, 5-CH), 6.86-7.21 (m, 16H, aromatic), 7.30-7.51 (m, 4H, aromatic) ppm; FT-IR: 3004-3010 (aromatic), 1598 (C=N), 1258 (C-O) cm-1; Anal. Calcd. for C39H38 N4O4S (658.82): C: 71.10, H: 5.81, N: 8.50, S: 4.87 Found: C: 71.17; H, 5.83, N: 8.52, S:4.90; MS: m/z 659.
6-(4-Methoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-3-(4-dimethylaminophenyl)-2-phenyl-3,3a,5,6-tetra-hydro-2H-pyrazolo[3,4-d]thiazole (4i). Yellow crystals; yield 42 %; mp. 158 oC; 1H-NMR δ: 2.83 (s, 6H, CH3), 3.55 (s, 3H, OCH3), 3.80 (s, 9H, OCH3), 4.56 (d, J= 11.0 Hz, 1H, 3-CH), 5.81 (d, J= 11.0 Hz, 1H, 3a-CH), 6.01 (s, 1H, 5-CH), 6.91-7.21 (m, 11H, aromatic), 7.29-7.53 (m, 4H, aromatic) ppm; FT-IR: 3002-3011 (aromatic), 1596 (C=N), 1245 (C-O) cm-1; Anal. Calcd. for C34H36 N4O4S (596.75): C: 68.43, H: 6.08, N: 9.39, S: 5.37 Found: C: 68.45; H, 6.11, N: 9.34, S:5.39; MS: m/z 597.
6-(4-Ethoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-3-(4-dimethylaminophenyl)-2-phenyl-3,3a,5,6-tetra-hydro-2H-pyrazolo[3,4-d]thiazole (4j). Yellow crystals; yield 41 %; mp. 143 oC; 1H-NMR δ: 1.23-1.37 (t, J= 7.2 Hz, 3H, CH3), 2.81 (s, 6H, CH3), 3.83 (s, 9H, OCH3), 3.91-3.95 (q, J= 7.2 Hz, 2H, OCH2), 4.58 (d, J= 11.0 Hz, 1H, 3-CH), 5.81 (d, J= 11.0 Hz, 1H, 3a-CH), 6.03 (s, 1H, 5-CH), 6.89-7.23 (m, 11H, aromatic), 7.29-7.53 (m, 4H, aromatic) ppm; FT-IR: 3000-3007 (aromatic), 1600 (C=N), 1249 (C-O) cm-1; Anal. Calcd. for C35H38N4O4S (610.77): C: 68.83, H: 6.27, N: 9.17, S: 5.25. Found: C: 68.85; H, 6.29, N: 9.20, S: 5.29; MS: m/z 611.