H14[NaP5W30O110] as a Heterogeneous Recyclable Catalyst for the Air Oxidation of Thiols Under Solvent Free Conditions
Abstract
:Introduction
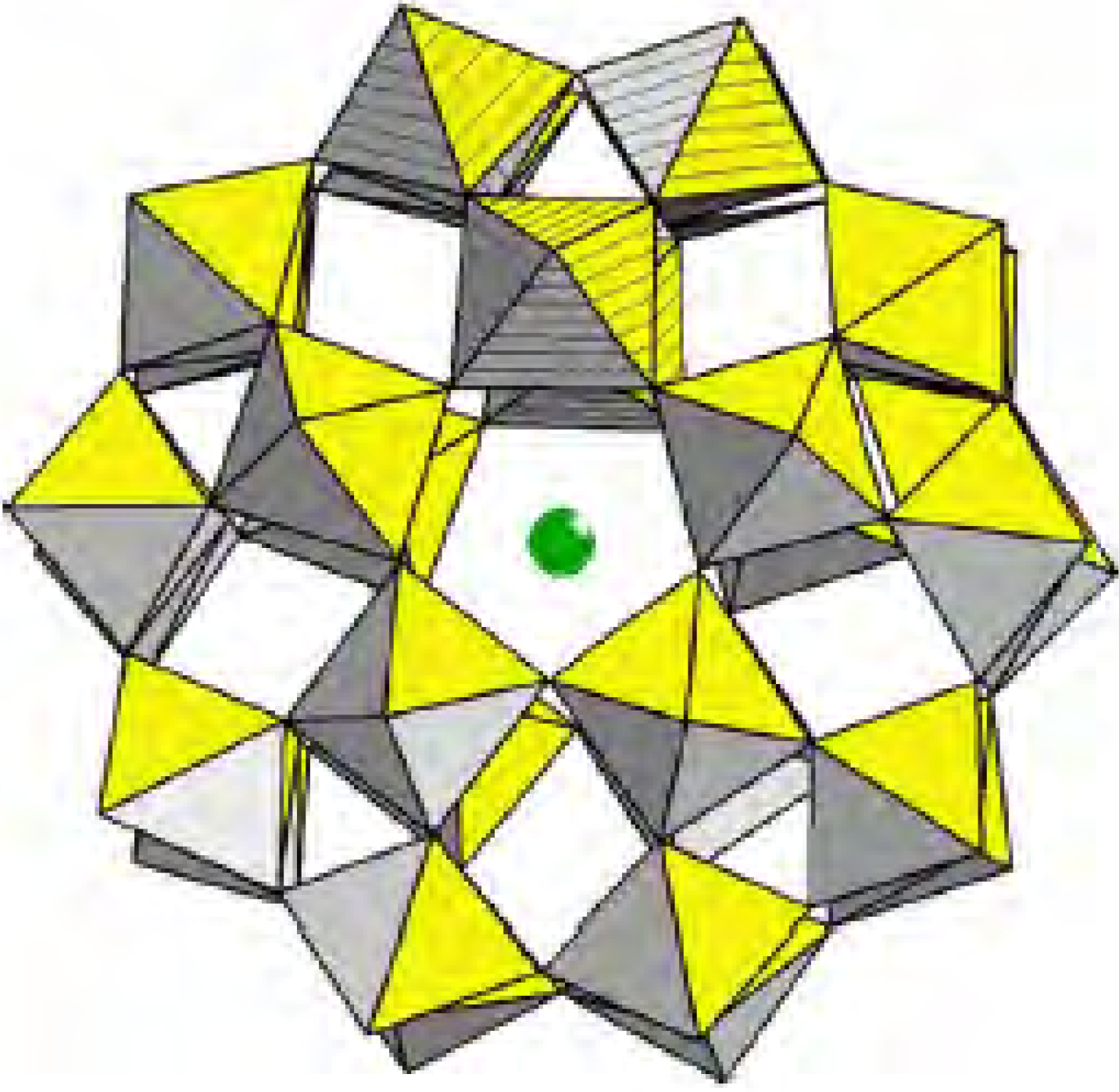
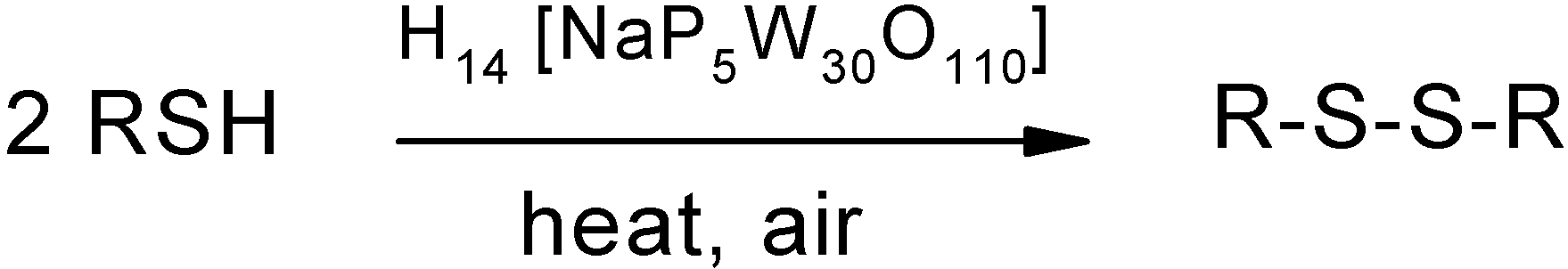
Results and Discussion
| Entry | Thiol | Disulfide | Yield/% |
|---|---|---|---|
| 1 | p-Tolylthiol | Di-p-ditolyldisulfide | 98 |
| 2 | Thiophenol | Diphenyldisulfide | 97 |
| 3 | Naphtylthiol | Di-naphtyldisulfide | 91 |
| 4 | Cyclohexylthiol | Dicyclohexyldisulfide | 95 |
| 5 | p-Chlorophenylthiol | Bis[p-chlorophenyl]disulfide | 97 |
| 6 | Ethanethiol | Diethyldisulfide | 92 |
| 7 | Propylthiol | Dipropyldisulfide | 95 |
| 8 | Octylthiol | Dioctyldisulfide | 98 |
| 9 | Phenyl-methanethiol | Dibenzyldisulfide | 94 |
| 10 | sec-Butylthiol | Di-sec-butyldisulfide | 97 |
Reusability of the Catalyst

Conclusions
Experimental
General
Catalyst Preparation
Oxidation of Thiols: General Procedure
Acknowledgments
References
- Karami, B.; Montazerozohori, M.; Moghadam, M.; Habibi, M.H.; Niknam, Kh. Selective Oxidation of Thiols to Disulfides Catalyzed by Iron (III) {Tetra Phenyl Porphyrin} Using Urea-Hydrogen Peroxide as Oxidizing Reagent. Turk. J. Chem. 2005, 29, 539–546. [Google Scholar]
- Akdag, A.; Webb, T.; Worley, S.D. Oxidation of thiols to disulfides with monochloropoly (styrene hydantoin) beads. Tetrahedron Lett. 2006, 47, 3509–3510. [Google Scholar] [CrossRef]
- Chauhan, S.M.S.; Kumar, A.; Srinivas, K.A. Oxidation of thiols with molecular oxygen catalyzed by cobalt (II) phthalocyanines in ionic liquid. Chem. Commun. 2003, 2348–2349. [Google Scholar] [CrossRef]
- Hashmat Ali, M.; McDermott, M. Oxidation of thiols to disulfides with molecular bromine on hydrated silica gel support. Tetrahedron Lett. 2002, 43, 6271–6273. [Google Scholar] [CrossRef]
- Walters, M.A.; Chaparro, J.; Siddiqui, T.; Williams, F.; Ulku, C.; Rheingold, A.L. The formation of disulfides by the [Fe(nta)Cl2]2_ catalyzed airoxidation of thiols and dithiols. Inorg. Chim. Acta 2006, 359, 3996–4000. [Google Scholar] [CrossRef]
- Izumi, Y.; Urabe, K.; Onaka, M. Zeolites Clay and Heteropolyacid in Organic Reactions; Kodansha: Tokyo, 1992; Vol. 99. [Google Scholar]
- Pope, M.T.; Muller, A. Polyoxometalates: From Platonic Solid to Anti-Retroviral Activity; Kluwer Academic Publishing: Dorderecht, The Netherlands, 1994. [Google Scholar]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Jahangir, M.; Gharib, A. Preyssler catalyst, [NaP5W30O110]14- : A green, efficient and reusable catalyst for esterification of salicylic acid with aliphatic and benzylic alcohols. J. Appl. Catal. A: Gen. 2006, 302, 42–47. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Jahangir, M.; Gharib, Jahangir M. A catalytic method for synthesis of γ-butyrolactone, ε-caprolactone and 2-cumaranone in the presence of Preyssler’s anion, [NaP5W30O110]14-, as a green and reusable catalyst. J. Mol. Catal A: Chem. 2006, 252, 90–95. [Google Scholar] [CrossRef]
- Heravi, M.M.; Motamedi, R.; Seifi, N.; Bamoharram, F.F. Catalytic synthesis of 6-aryl-1H-pyrazolo[3,4-d]pyrimidin-4[5H]-ones by heteropolyacid: H14[NaP5W30O110] and H3PW12O40. J. Mol. Catal. A: Chem. 2006, 249, 1–3. [Google Scholar] [CrossRef]
- Heravi, M.M.; Bakhtiari, Kh.; Bamoharram, F.F. 12-Molybdophosphoric acid: A recyclable catalyst for the synthesisof Biginelli-type 3,4-dihydropyrimidine-2(1H)-ones. Catal. Commun. 2006, 7, 373. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Tavakoli, N. N-oxidation of pyridine carboxylic acids using hydrogen peroxide catalyzed by a green heteropolyacid catalyst: Preyssler’s anion, [NaP5W30O110]14-. J. Mol. Catal A: Chem. 2006, 252, 219–225. [Google Scholar] [CrossRef]
- Heravi, M.M.; Bakhtiari, Kh.; Bamoharram, F.F. An efficient and chemoselective synthesis of acylals from aromatic aldehydes and their regeneration, catalyzed by 12-molybdophosphoric acid. Catal. Commun. 2006, 7, 499–501. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Bakhtiari, Kh.; Oskooie, H.A.; Bamoharram, F.F. Green and reusable heteropolyacid catalyzed oxidation of benzylic, allylic and aliphatic alcohols to carbonyl compounds. Catal. Commun. 2007, 8, 315–318. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Akbarpour, M. Catalytic performance of Preyssler heteropolyacid as a green and recyclable catalyst in oxidation of primary aromatic amines. J. Mol. Catal A: Chem. 2006, 255, 193–198. [Google Scholar] [CrossRef]
- Sample Availability: Available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Hekmatshoar, R.; Sajadi, S.; Heravi, M.M.; Bamoharram, F.F. H14[NaP5W30O110] as a Heterogeneous Recyclable Catalyst for the Air Oxidation of Thiols Under Solvent Free Conditions. Molecules 2007, 12, 2223-2227. https://doi.org/10.3390/12092223
Hekmatshoar R, Sajadi S, Heravi MM, Bamoharram FF. H14[NaP5W30O110] as a Heterogeneous Recyclable Catalyst for the Air Oxidation of Thiols Under Solvent Free Conditions. Molecules. 2007; 12(9):2223-2227. https://doi.org/10.3390/12092223
Chicago/Turabian StyleHekmatshoar, Rahim, Sodeh Sajadi, Majid M. Heravi, and Fatemeh F. Bamoharram. 2007. "H14[NaP5W30O110] as a Heterogeneous Recyclable Catalyst for the Air Oxidation of Thiols Under Solvent Free Conditions" Molecules 12, no. 9: 2223-2227. https://doi.org/10.3390/12092223




