General
Melting points were determined on a Gallenkamp melting point apparatus and are uncorrected. Infrared spectra were measured with a Shimadzu Model 470 spectrophotometer. The NMR spectra were recorded on a Bruker AM 400 spectrometer with CDCl3, DMSO-d6 as solvents and TMS as internal reference, chemical shifts are expressed as δ ppm. Mass spectra were measured on a GCMS-QP1000EX mass spectrometer operating at 70 eV. Analytical data were determined on the Microanalytical Data Unit at Cairo University. Analytical TLC was performed with silica gel plates using silica gel 60 PF254 (Merck).
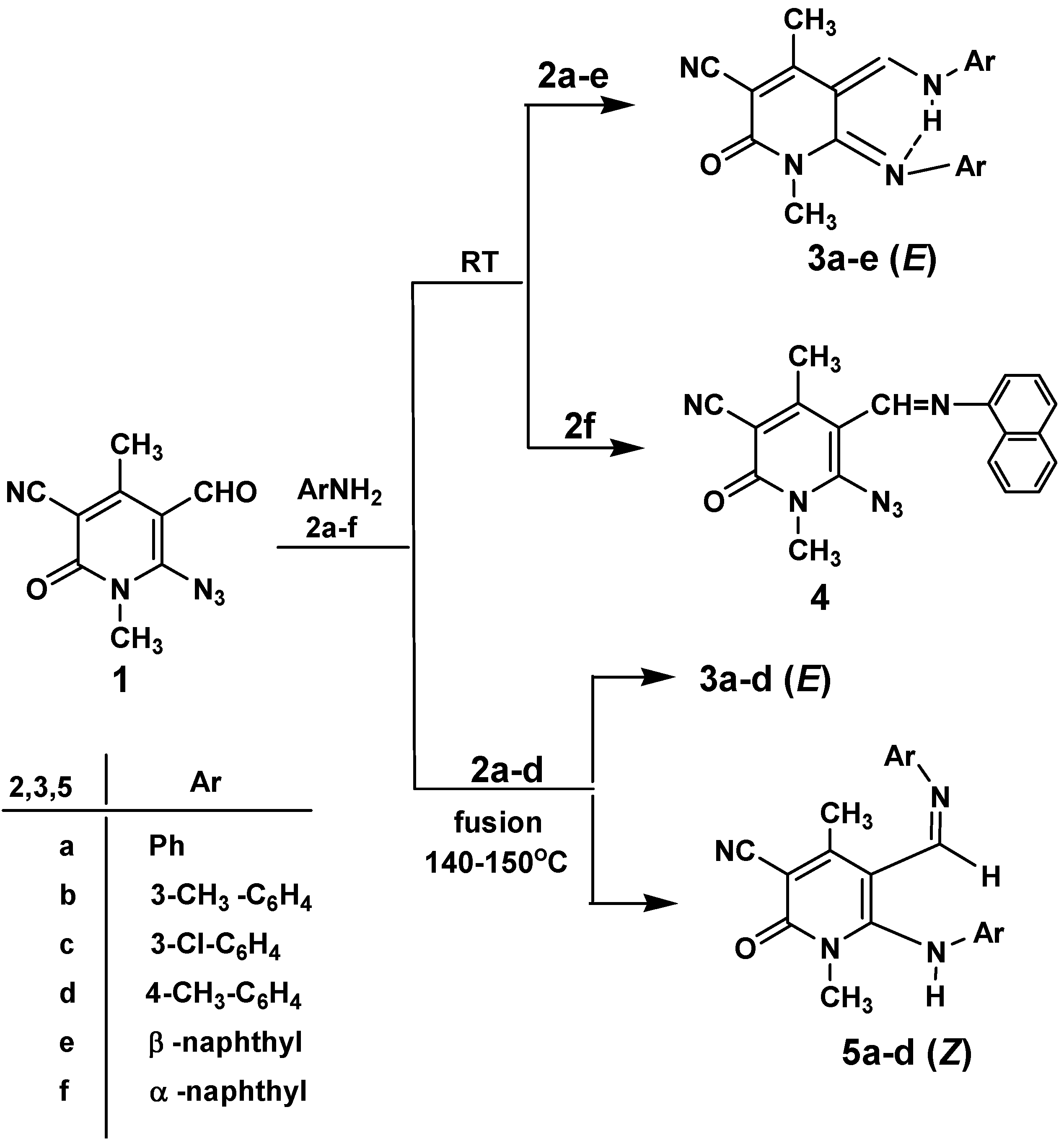
Synthesis of 5-Arylaminomethylene-6-(E)-arylimino-1,2,5,6-tetrahydro-1,4-dimethyl-2-oxo-pyridin-3-carbonitriles 3a-e, 6-arylamino-5-(Z)-aryliminomethyl-1,2-dihydro-1,4-dimethyl-2-oxo-pyridin-3-carbonitriles 5a-d and 6-azido-1,2-dihydro-1,4-dimethyl-5-(naphthalen-2-yl-iminomethyl)-2-oxo-pyridin-3-carbonitrile (4)
Method A for compounds (E)-3a-e and 4: Aromatic amines 2a-f (4.60 mmol) were added to a solution of compound 1 (0.50 g, 2.30 mmol) in absolute ethanol (10 mL) and the mixture was stirred for 2 h at room temperature (25°C). The resulting solid products were collected by filtration, washed with a small amount of EtOH, dried and recrystallized from EtOH to afford compounds (E)-3a-e and 4.
Method B for (E)-3a-d and (Z)-5a-d: A mixture of aromatic amine 2a-d (5 mL) and azidopyridone derivative 1 (0.50 g, 2.30 mmol) was heated at 140-150˚C for 10 min. After cooling at room temperature, the reaction mixture were treated with a small amount of EtOH and the resulting solid products were chromatographed on a preparative TLC plate using 10:2 toluene-acetone as eluent to give two zones. Extraction with acetone followed by recrystallization from EtOH gave compounds (E)-3a-d and (Z)-5a-d.
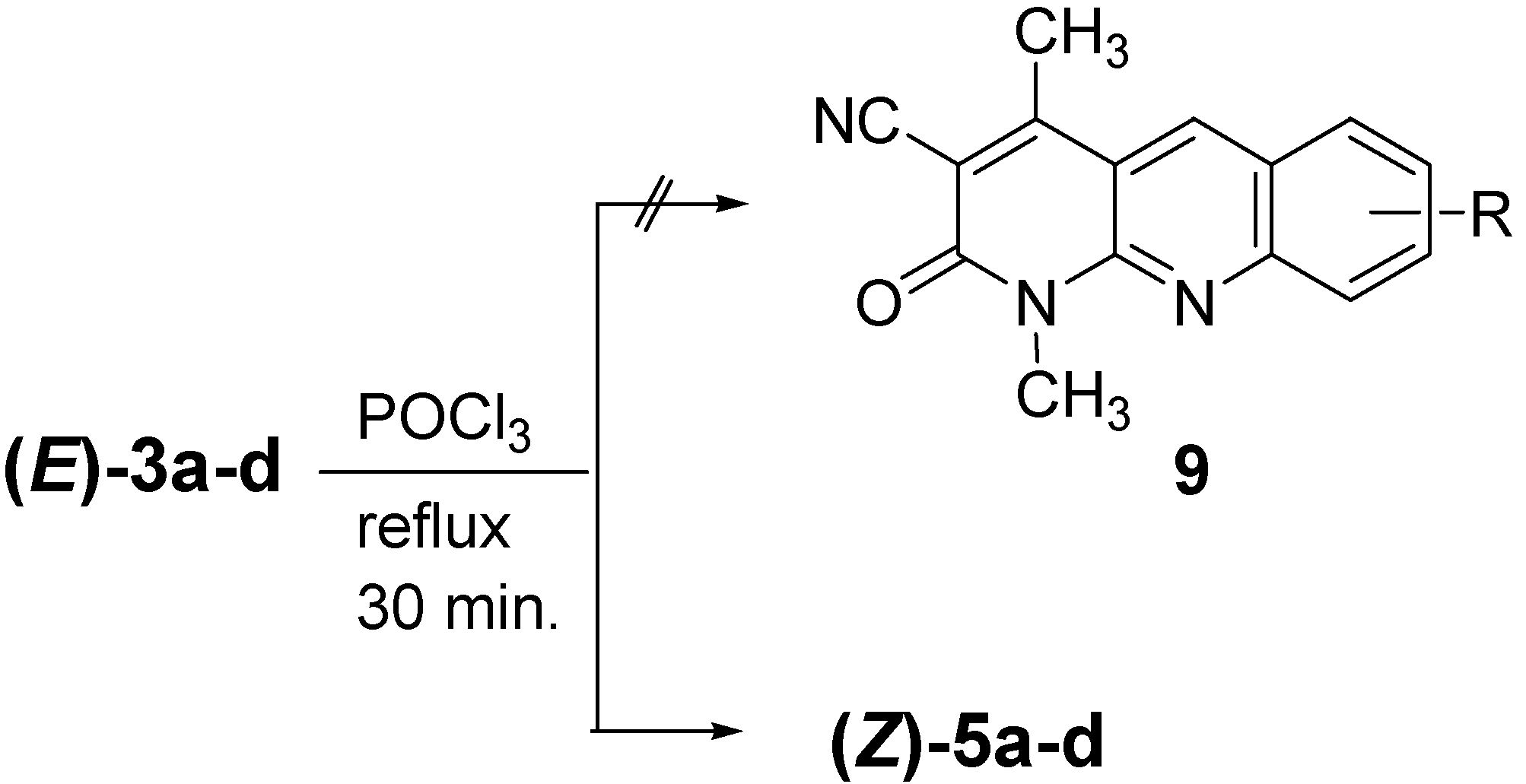
Method C for compounds (Z)-5a-d: A mixture of (E)-3a-d (1 mmol) and POCl3 (7 mL) was refluxed for 30 min. The excess of POCl3 was distilled off in vacuo and the residue was treated with cold H2O. The resulting solid product that formed was collected by filtration, washed well with H2O, dried and finally recrystallized from EtOH to afford (Z)-5a-d.
5-Anilinomethylene-1,2,5,6-tetrahydro-1,4-dimethyl-2-oxo-6-(E)-phenylimino-pyridin-3-carbonitrile (3a). Yield: 87% (route A) or 43% (route B); m.p. 236-238˚C; IR (νmax, KBr, cm-1): 3050, 2950, 2200, 1645; 1H-NMR (DMSO-d6): δ 2.42 (3H, s, CH3), 3.33 (3H, s, CH3), 6.75 (2H, d, J=8.0 Hz, ArH), 6.97 (1H, t, J=8.0 Hz, ArH), 7.21 (2H, t, J=8.0 Hz, ArH), 7.26 (1H, t, J=8.0 Hz, ArH), 7.45 (2H, t, J=8.0 Hz, ArH), 7.59 (2H, d, J=8.0 Hz, ArH), 8.44 (1H, d, J=13.0 Hz, =CH-N), 12.70 (1H, d, J=13.0 Hz, NH); 13C-NMR (DMSO-d6): δ 20.43, 27.36, 109.73, 112.81, 115.72, 119.01, 121.34, 122.56, 127.41, 128.26, 129.65, 130.59, 143.17, 146.24, 161.37, 162.81, 164.40; Anal. calcd. for C21H18N4O: C, 73.66; H, 5.30; N , 16.36. Found: C, 73.51; H, 5.44; N, 16.49.
1,2,5,6-Tetrahydro-1,4-dimethyl-2-oxo-5-(3-tolylaminomethylene)-6-(E)-(3-tolylimino)-pyridine-3-carbonitrile (3b). Yield: 85% (route A) or 45% (route B); m.p. 258-260˚C; IR (νmax, KBr, cm-1): 3050, 2950, 2900, 2200, 1645; 1H-NMR (DMSO-d6): δ 2.24 (3H, s, CH3), 2.34 (3H, s, CH3), 2.41 (3H, s, CH3), 3.31 (3H, s, CH3), 6.54 (1H, d, J=8.0 Hz, ArH), 6.57 (1H, s, ArH), 6.78 (1H, d, J=8.0 Hz, ArH), 7.07 (2H, d, J=8.0 Hz, ArH), 7.31 (2H, t, J=8.0 Hz, ArH), 7.42 (1H, s, 1ArH ), 8.42 (1H, d, J=13.0 Hz, =CH-N), 12.70 (1H, d, J=13.0 Hz, NH); MS m/z (rel. int. %): 371 (M+1, 21), 370 (M+, 86), 369 (100), 354 (4), 252 (19), 185 (16), 120 (5), 107 (33), 106 (31), 105 (11), 91 (13), 58 (3), 42 (10); Anal. calcd. for C23H22N4O: C, 74.57; H, 5.99; N, 15.12. Found: C, 74.43; H, 6.12; N, 15.34.
5-(3-Chlorophenylaminomethylene)-6-(E)-(3-Chlorophenylimino)-1,2,5,6-tetrahydro-1,4-dimethyl-2-oxo-pyridine-3-carbonitrile (3c). Yield: 89% (route A) or 41% (route B); m.p. 242-244˚C; IR (νmax, KBr, cm-1): 3050, 2200, 1645; 1H-NMR (DMSO-d6): δ 2.45 (3H, s, CH3), 3.36 (3H, s, CH3), 6.73 (1H, d, J=8.0 Hz, ArH), 6.83 (1H, s, ArH), 7.01 (1H, d, J=8.0 Hz, ArH), 7.22 (1H, t, J=8.0 Hz, ArH), 7.29 (1H, d, J=8.0 Hz, ArH), 7.45 (1H, t, J=8.0 Hz, ArH), 7.54 (1H, d, J=8.0 Hz, ArH), 7.87 (1H, s, ArH), 8.46 (1H, d, J=13.0 Hz, =CH-N), 12.72 (1H, d, J=13.0 Hz, NH); Anal. calcd. for C21H16Cl2N4O: C, 61.31; H, 3.92; Cl,17.26; N, 13.62. Found: C, 61.10; H, 4.12; Cl, 17.31; N, 13.43.
1,2,5,6-Tetrahydro-1,4-dimethyl-2-oxo-5-(4-tolylaminomethylene)-6-(E)-(4-tolylimino)-pyridine-3-carbonitrile (3d). Yield: 88% (route A) or 42% (route B); m.p. 252-254˚C; IR (νmax, KBr, cm-1): 3050, 2950, 2900, 2200, 1640; 1H-NMR (DMSO-d6): δ 2.25 (3H, s, CH3), 2.31 (3H, s, CH3), 2.40 (3H, s, CH3), 3.33 (3H, s, CH3), 6.63 (2H, d, J=8.0 Hz, ArH), 7.01 (2H, d, J=8.0 Hz, ArH), 7.25 (2H, d, J= 8.0 Hz, ArH), 7.47 (2H, d, J=8.0 Hz, ArH), 8.39 (1H, d, J=13.0 Hz, =CH-N), 12.72 (1H, d, J=13.0 Hz, NH); 13C-NMR (DMSO-d6): δ 20.41, 21.04, 21.33, 26.76, 109.45, 112.67, 115.74, 118.96, 121.22, 127.03, 128.14, 128.36, 129.63, 136.73, 139.82, 143.43, 162.23, 163.09, 164.46; MS m/z (rel. int. %): 371 (M+1, 26), 370 (M+, 97), 369 (100), 354 (5), 252 (22), 185 (23), 120 (5), 107 (26), 106 (34), 105 (22), 91 (13); Anal. calcd. for C23H22N4O: C, 74.57; H, 5.99; N, 15.12. Found: C, 74.44; H, 6.14; N, 14.95.
1,2,5,6-Tetrahydro-1,4-dimethyl-5-(naphthalen-3-yl-aminomethylene)-6-(E)-(naphthalen-3-yl-imino)-2-oxo-pyridine-3-carbonitrile (3e). Yield: 84% (route A); m.p. 292-294˚C; IR (νmax, KBr, cm-1): 3050, 2200, 1640; 1H-NMR (DMSO-d6): δ 2.44 (3H, s, CH3), 3.38 (3H, s, CH3), 7.04 (1H, d, J=8.0 Hz, ArH), 7.18 (1H, s, ArH), 7.31-7.57 (4H, m, ArH), 7.73-7.94 (6H, m, ArH), 8.01 (1H, d, J=8.0 Hz, ArH), 8.11 (1H, s, ArH), 8.59 (1H, d, J=13.0 Hz, =CH-N), 12.99 (1H, d, J=13.0 Hz, NH); MS m/z (rel. int. %): 442 (M+, 100), 441 (M-1, 91), 301 (7), 288 (14), 155 (4),142 (39), 141 (57), 127 (47); Anal. calcd. for C29H22N4O: C, 78.84; H, 4.98; N, 12.49. Found: C, 78.71; H, 5.01; N, 12.66.
6-Azido-1,2-dihydro-1,4-dimethyl-5-(naphthalen-2-yl-iminomethyl)-2-oxo-pyridine-3-carbonitrile (4).
Yield: 79%; m.p. 174-176˚C; IR (νmax, KBr, cm-1): 3050, 2925, 2220, 2140, 1660; 1H-NMR (DMSO-d6): δ 2.88 (3H, s, CH3), 3.45 (3H, s, CH3), 7.10 (1H, d, J=7.0 Hz, ArH), 7.50-7.58 (3H, m, ArH), 7.80 (1H, d, J=8.0 Hz, ArH), 7.93 (1H, d, J=7.0 Hz, ArH), 8.17 (1H, d, J=8.0 Hz, ArH), 8.82 (1H, s, =CH‑N); Anal. calcd. for C19H14N6O: C, 66.66; H, 4.12; N, 24.55. Found: C, 66.78; H, 4.28; N, 24.37.
6-Anilino-1,2-dihydro-1,4-dimethyl-2-oxo-5-(Z)-(phenyliminomethyl)-pyridine-3-carbonitrile (5a). Yield: 38% (route B) or 73% (route C); m.p. 256-258˚C; IR (νmax, KBr, cm-1): 3250, 3050, 2950, 1645; 1H-NMR, (DMSO-d6): δ 2.26 (3H, s, CH3), 3.24 (3H, s, CH3), 6.76 (1H, d, J=8.0 Hz, ArH), 6.84 (1H, d, J=8.0 Hz, ArH), 6.94-7.04 (2H, m, ArH), 7.19-7.39 (6H, m, ArH), 8.04 (1H, s, -CH=N-), 9.07 (1H, s, NH); 13C-NMR (DMSO-d6): δ 20.83, 27.61, 112.21, 113.34, 115.67, 120.08, 121.52, 123.02, 127.62, 128.73, ;130.94, 143.78, 146.54, 148.27, 151.84, 161.91, 163.37; MS m/z (rel. int. %): 42 (M+, 10), 341 (12), 239 (64), 210 (11), 147 (54), 93 (100), 91 (58), 78 (35), 77 (13); Anal. calcd. for C21H18N4O: C, 73.66; H, 5.30; N, 16.36. Found: C, 73.82; H, 5.16; N, 16.27.
1,2-Dihydro-1,4-dimethyl-2-oxo-6-(3-tolylamino)-5-(Z)-(3-tolyliminomethyl)-pyridine-3-carbonitrile (5b). Yield: 28% (route B) or 78% (route C); m.p. 288-290˚C; IR (νmax, KBr, cm-1): 3250, 3050, 2900, 2200, 1640; 1H-NMR (DMSO-d6): δ 2.02 (3H, s, CH3). 2.24 (3H, s, CH3), 2.29 (3H, s, CH3), 3.37 (3H, s, CH3), 6.59-7.30 (8H, m, ArH), 8.37 (1H, s, -CH=N-), 9.19 (1H, s, NH); Anal. calcd. for C23H22N4O: C, 74.57; H, 5.99; N, 15.12. Found: C, 74.38; H, 5.82; N, 15.01.
6-(3-Chlorophenylamino)-5-(Z)-(3-chlorophenyliminomethyl)-1,2-dihydro-1,4-dimeth-yl-2-oxo-pyridine-3-carbonitrile (5c). Yield: 29% (route B) or 73% (route C); m.p. 195-197˚C; IR (νmax, KBr, cm-1): 3250, 3050, 2200, 1640; 1H-NMR (DMSO-d6): δ 2.31 (3H, s, CH3), 3.31 (3H, s, CH3), 6.74- 7.41 (8H, m, ArH), 8.13 (1H, s, -CH=N-), 9.26 (1H, s, NH); 13C-NMR (DMSO-d6): δ 20.88, 27.69, 114.10, 115.65, 117.20, 124.91, 125.93, 127.06, 127.44, 129.02, 129.52, 130.32, 131.06, 134.23, 135.10, 143.38, 145.09, 148.34, 152.30, 161.97, 164.08; MS (70 eV); m/z (rel int. %) 410/414 (M+, 6), 273 (44), 147 (39), 127 (100); Anal. calcd. for C21H16Cl2N4O: C, 61.31; H, 3.92; Cl, 17.26; N, 13.62. Found: C, 61.46; H, 4.05; Cl, 17.36; N, 13.49.
1,2-Dihydro-1,4-dimethyl-2-oxo-6-(4-tolylamino)-5-(Z)-(4-tolyliminomethyl)pyridine-3-carbonitrile (5d). Yield: 30% (route B) or 72% (route C); m.p. 236-238˚C; IR (νmax, KBr, cm-1): 3250, 3050, 2900, 2200, 1645; 1H-NMR (DMSO-d6): δ 2.23 (3H, s, CH3), 2.25 (3H, s, CH3), 2.27 (3H, s, CH3), 3.35 (3H, s, CH3), 6.67 (2H, d, J=7.0 Hz, ArH), 6.79 (2H, d, J=7.0 Hz, ArH), 7.03 (2H, d, J=8.0 Hz, ArH), 7.08 (2H, d, J=8.0 Hz, ArH), 8.00 (1H, s, -CH=N-), 9.00 (1H, s, NH); MS m/z (rel. int. %) 370 (M+, 17), 369 (M-1, 19), 253 (35), 147 (27), 106 (100), 105 (46), 91 (18); Anal. calcd. for C23H22N4O: C, 74.57; H, 5.99; N, 15.12. Found: C, 74.64; H, 5.78; N, 15.01.
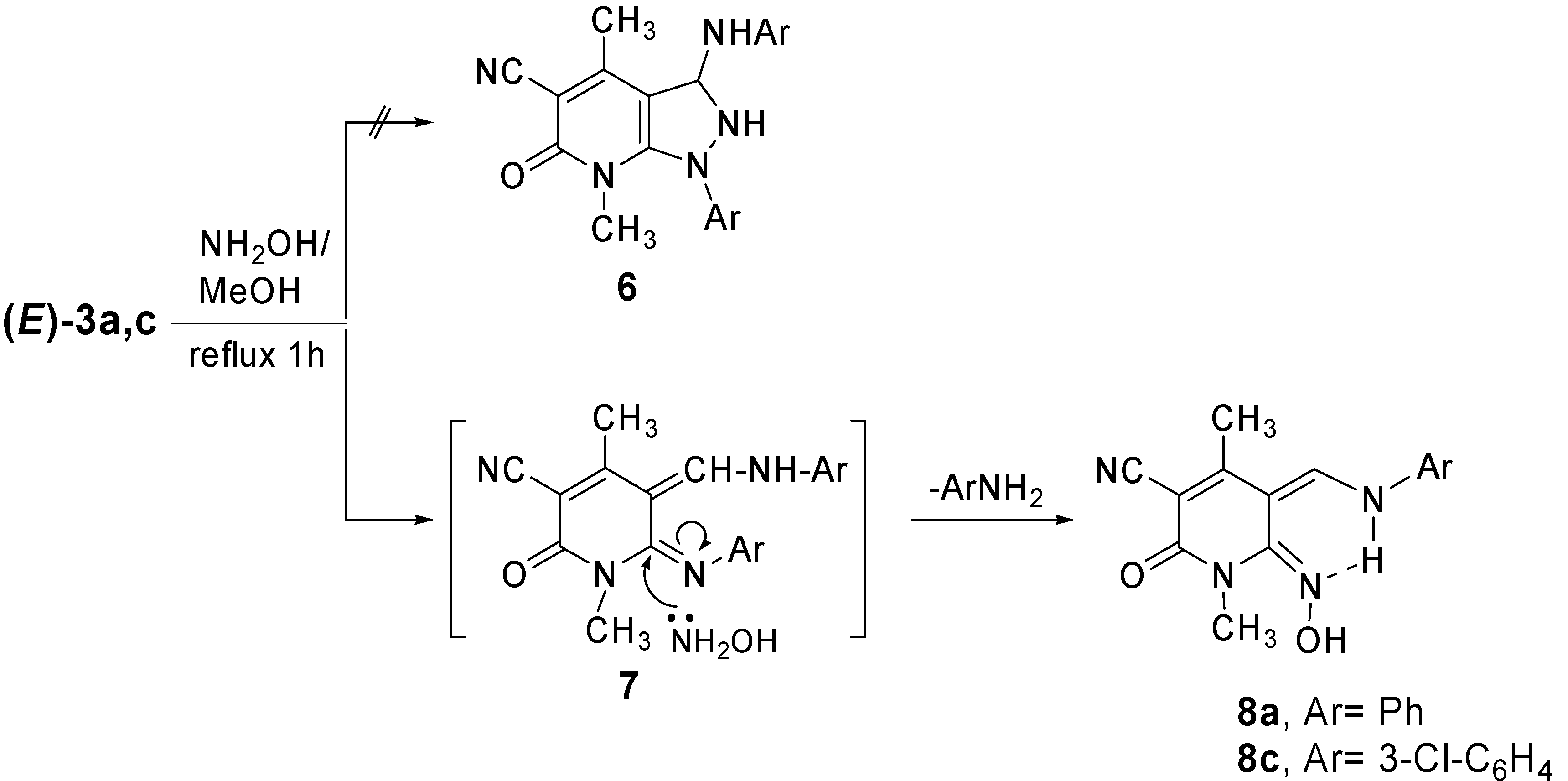
Synthesis of 5-arylaminomethylene-1,2,5,6-tetrahydro-6-hydroxyimino-1,4-dimethyl-2-oxo-pyridine-3-carbonitrile (8a,c)
A mixture of 3a,c (1.44 mmol) and hydroxylamine hydrochloride (0.10 g, 1.44 mmol) in MeOH (15 mL) was refluxed for 1 h. After cooling and concentration, the resulting solid products were collected by filtration, washed with a small amount of EtOH, dried and recrystallized from EtOH to afford compounds 8a,c.
5-Anilinomethylene-1,2,5,6-tetrahydro-6-hydroxyimino-1,4-dimethyl-2-oxo-pyridine-3-carbonitrile (8a). Yield: 91%; m.p. 260-262˚C; IR (νmax, KBr, cm-1): 3336, 2220, 1739, 1660; 1H-NMR (DMSO-d6): δ 2.28 (3H, s, CH3), 3.35 (3H, s, CH3), 7.23-7.63 (5H, m, ArH), 8.46 (1H, d, J=20.0 Hz, CH), 11.19 (1H, s, OH), 12.73 (1H, d, J=20.0 Hz, NH); Anal. calcd. for C15H14N4O2: C, 63.82; H, 5.00; N, 19.85. Found: C, 63.75; H, 5.12; N, 19.68.
5-(3-Chlorophenylaminomethylene)-1,2,5,6-tetrahydro-6-hydroxyimino-1,4-dimethyl-2-oxo-pyridine-3-carbonitrile (8c). Yield: 82%; m.p. 270-272˚C; IR (νmax, KBr, cm-1): 3370, 2220, 1735, 1660; 1H-NMR (DMSO-d6): δ 2.29 (3H, s, CH3), 3.34 (3H, s, CH3), 7.11-7.84 (4H, m, ArH), 8.43 (1H, d, J= 20.0 Hz, CH), 11.18 (1H, s, OH), 12.67 (1H, d, J= 20 Hz, NH); MS m/z (rel int. %) 316/318 (M+, 25), 303 (52), 302 (100), 276 (31), 246 (57), 204 (1), 139 (2), 127 (11). Anal. calcd. for C15H13ClN4O2: C, 56.87; H, 4.14; Cl, 11.21; N, 17.68. Found: C, 56.74; H, 4.23; Cl, 11.36; N, 17.57.