Antiparkinson Prodrugs
Abstract
:Introduction
Dopamine prodrugs
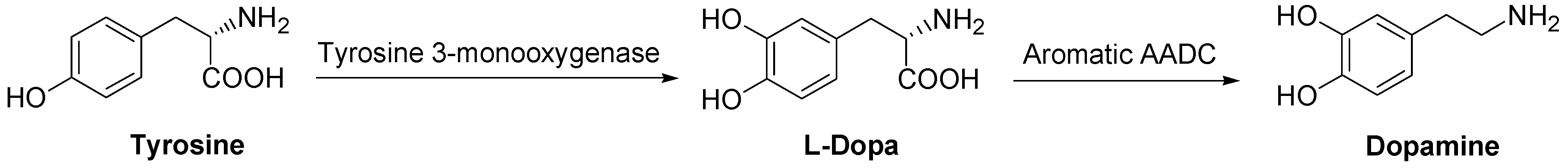
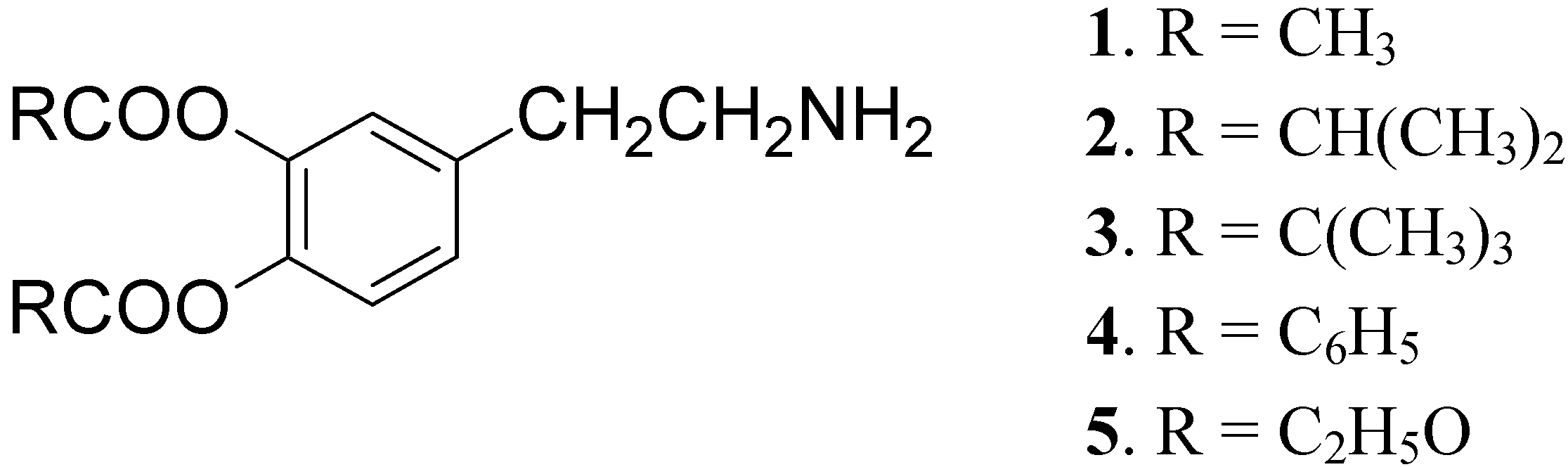

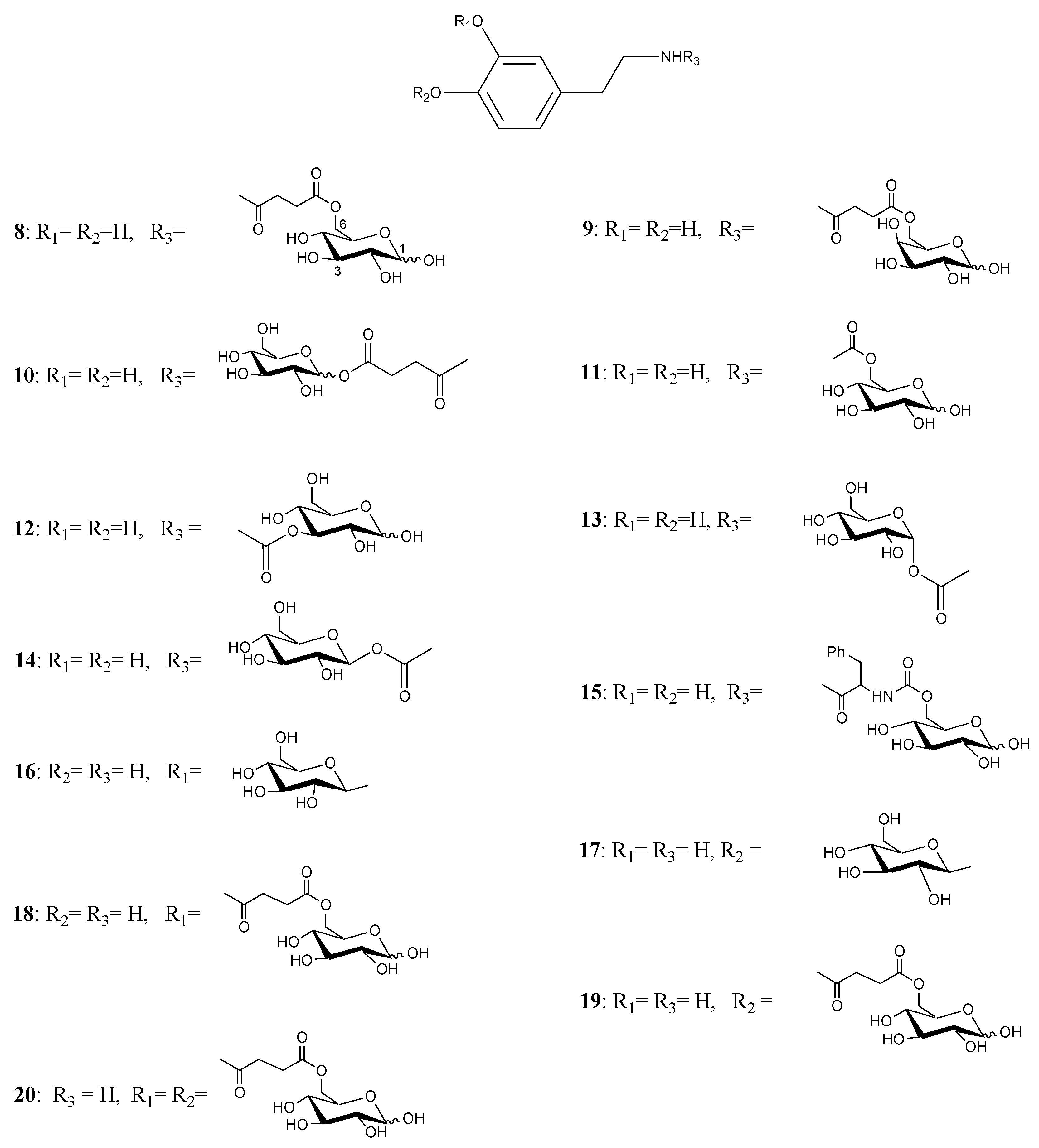
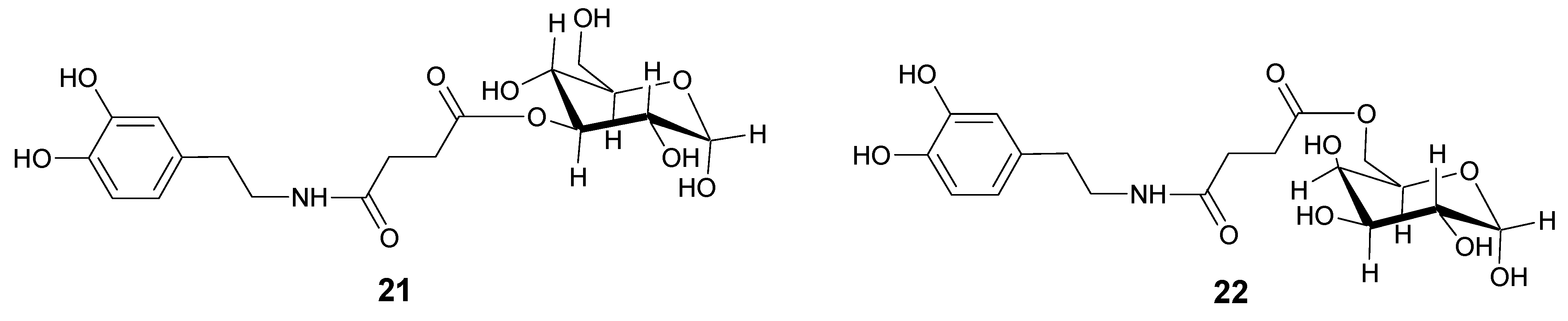
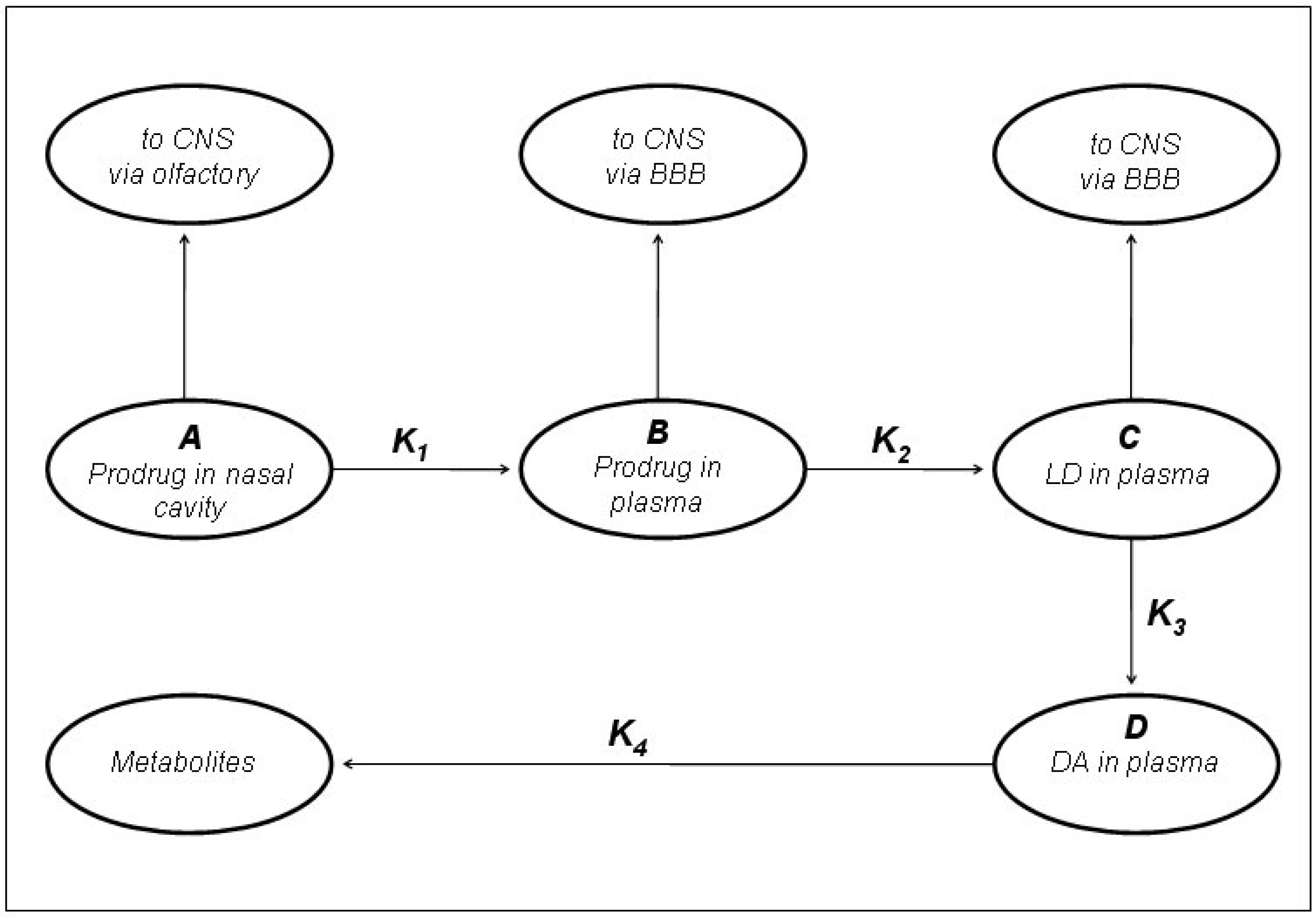
L-Dopa prodrugs
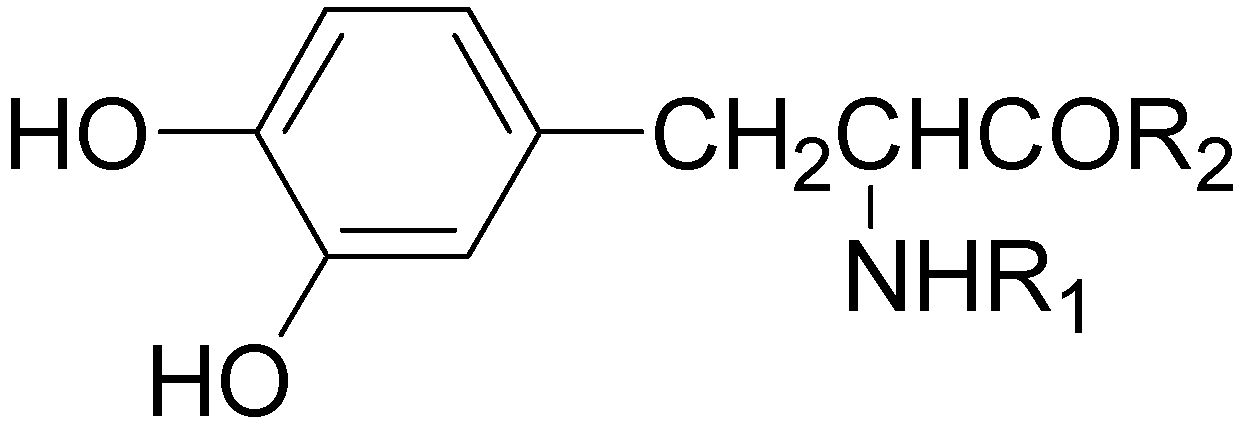
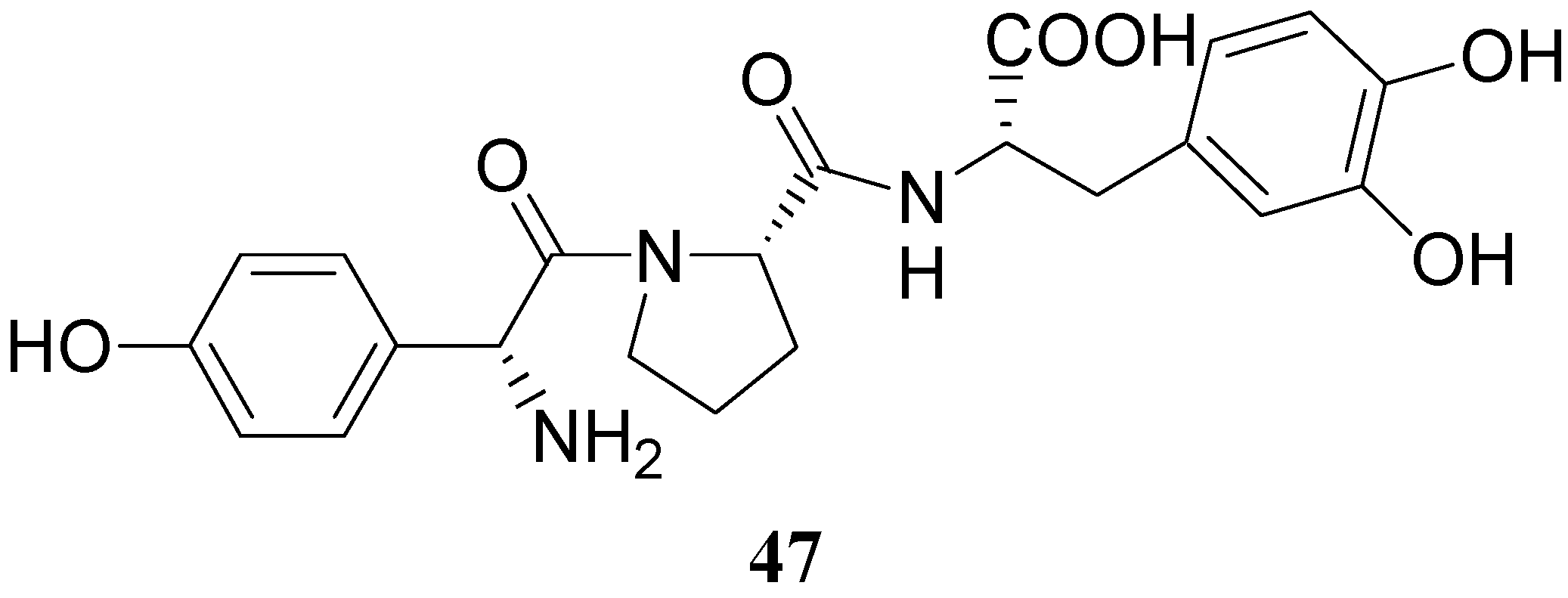
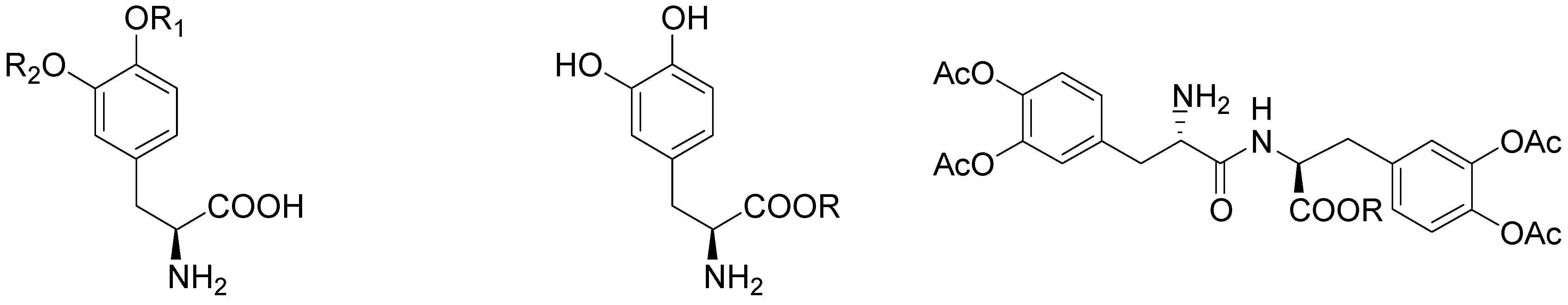
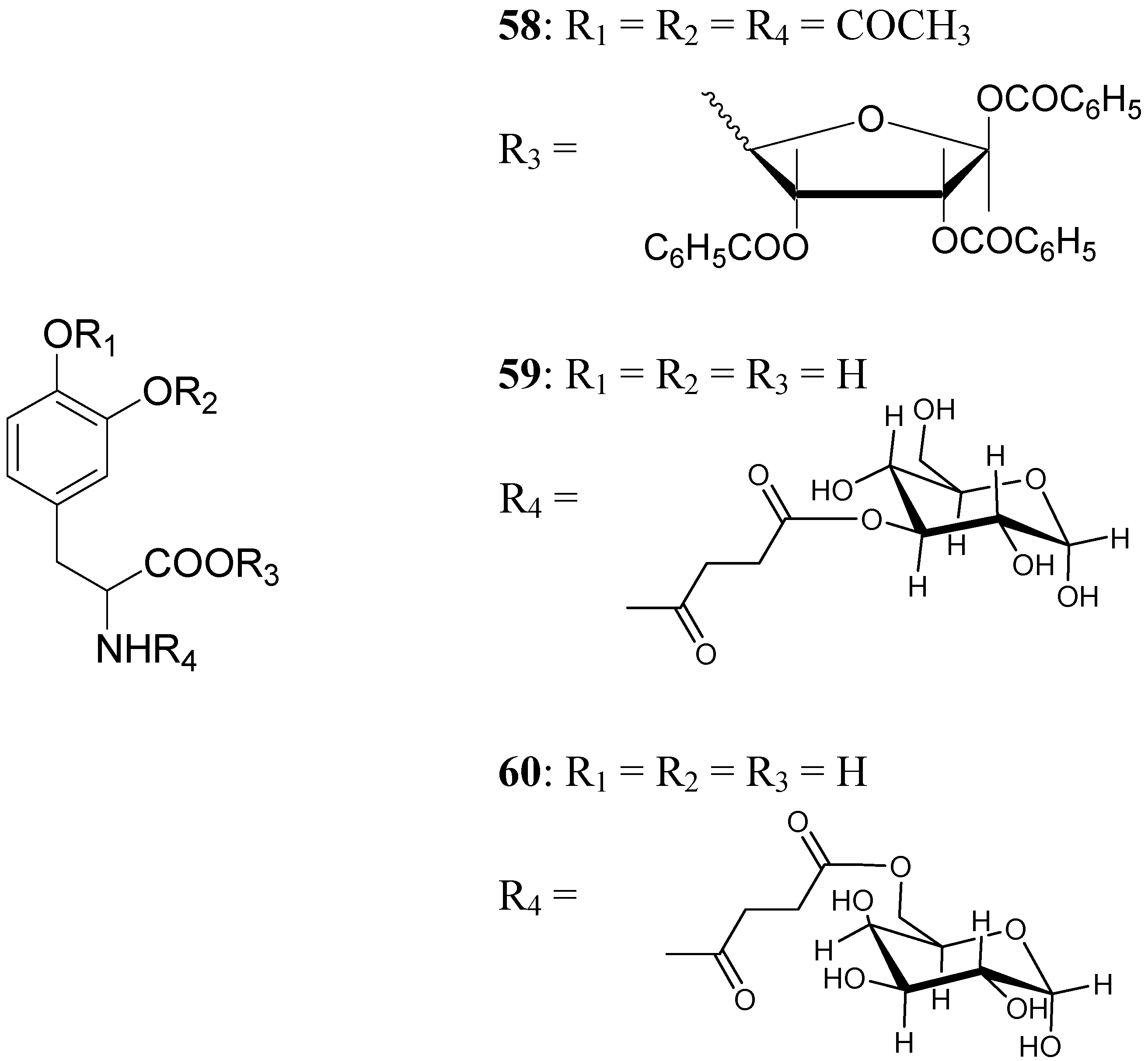
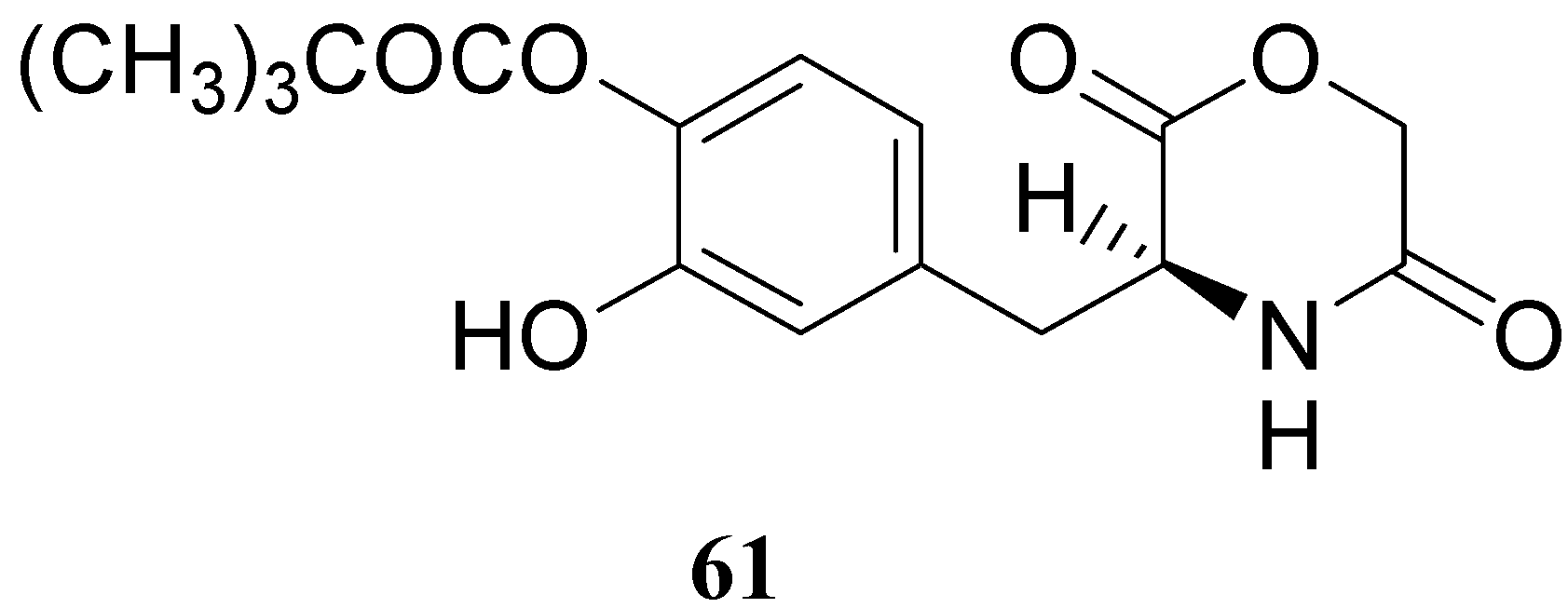
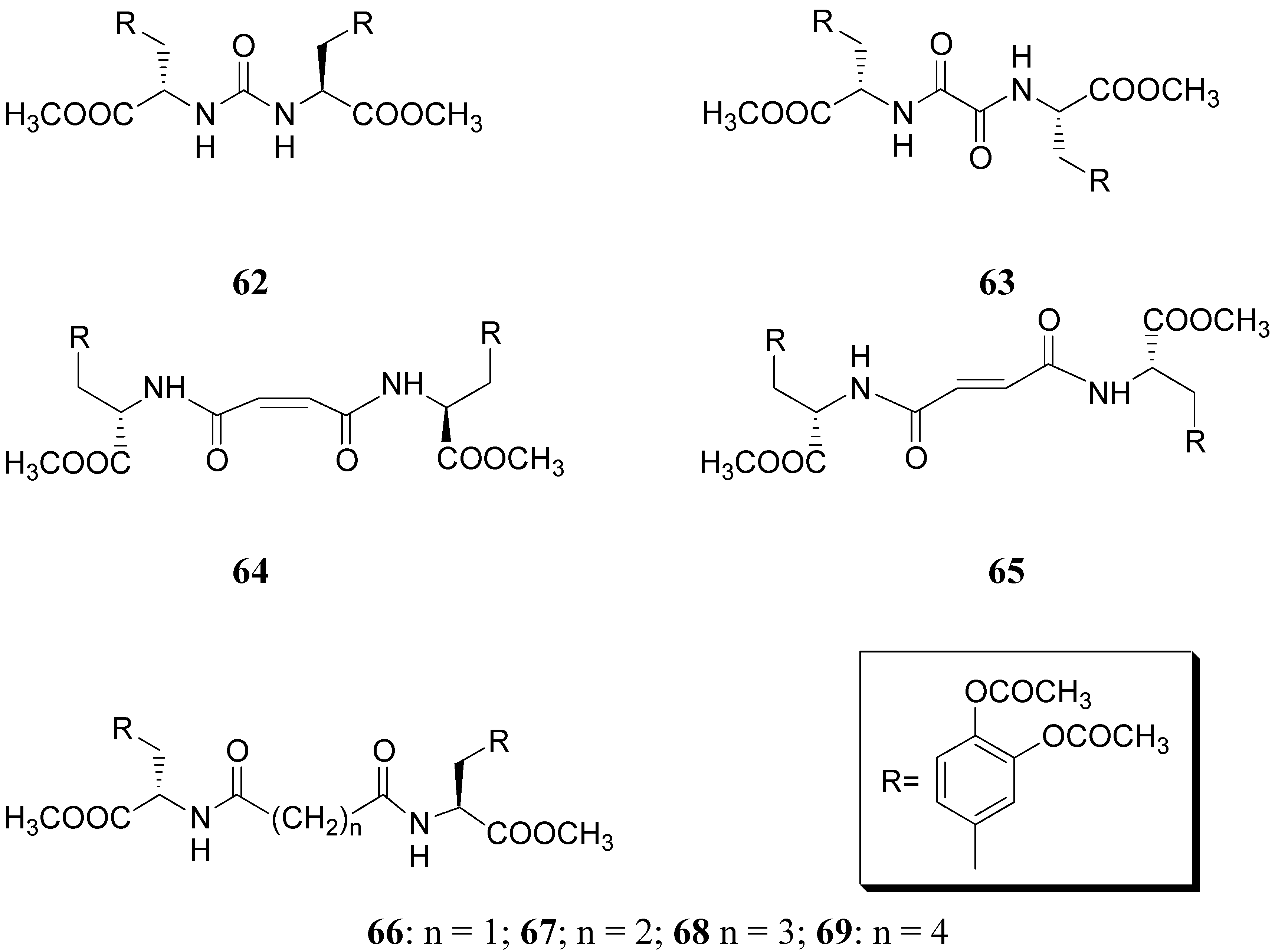
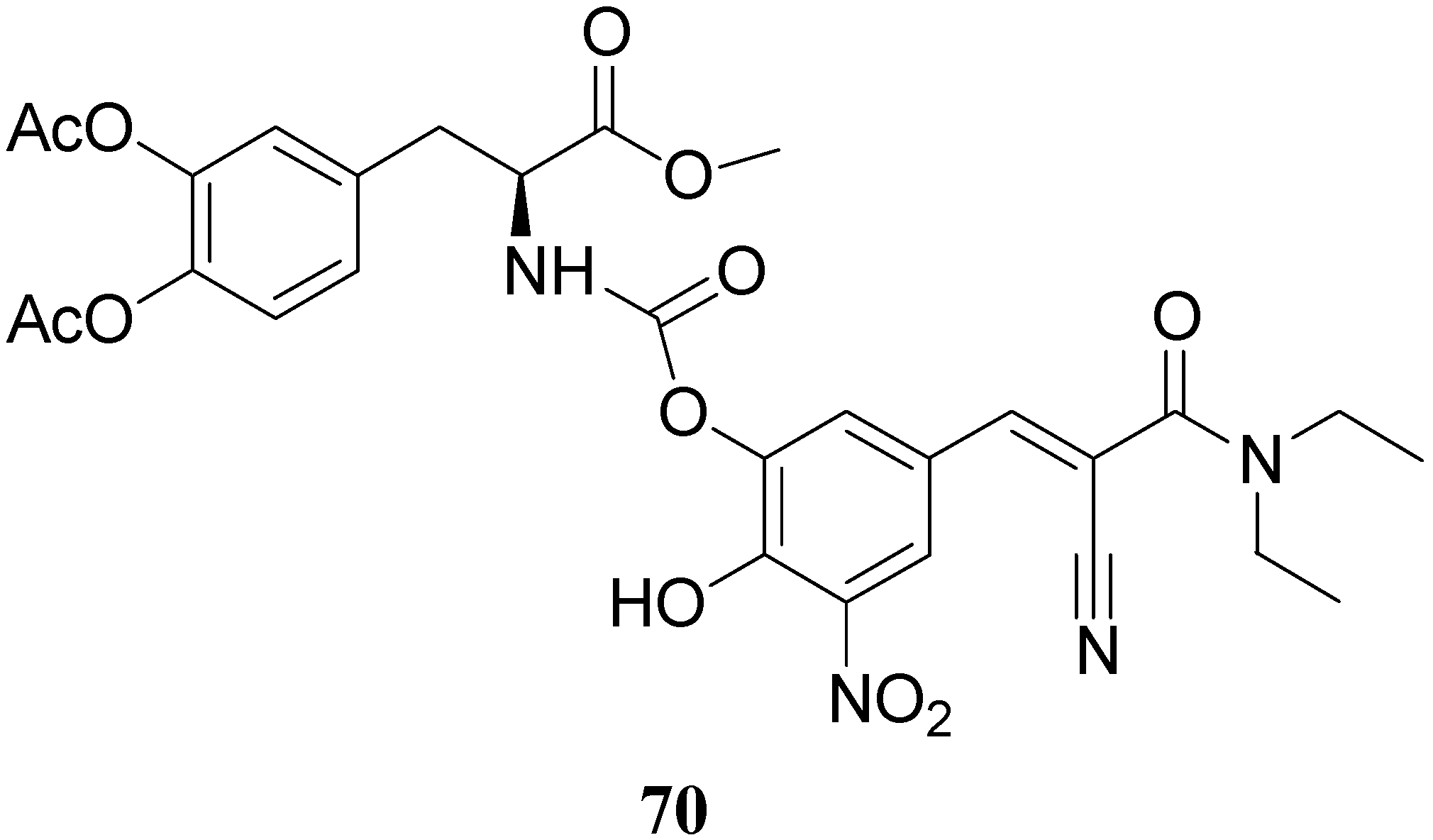
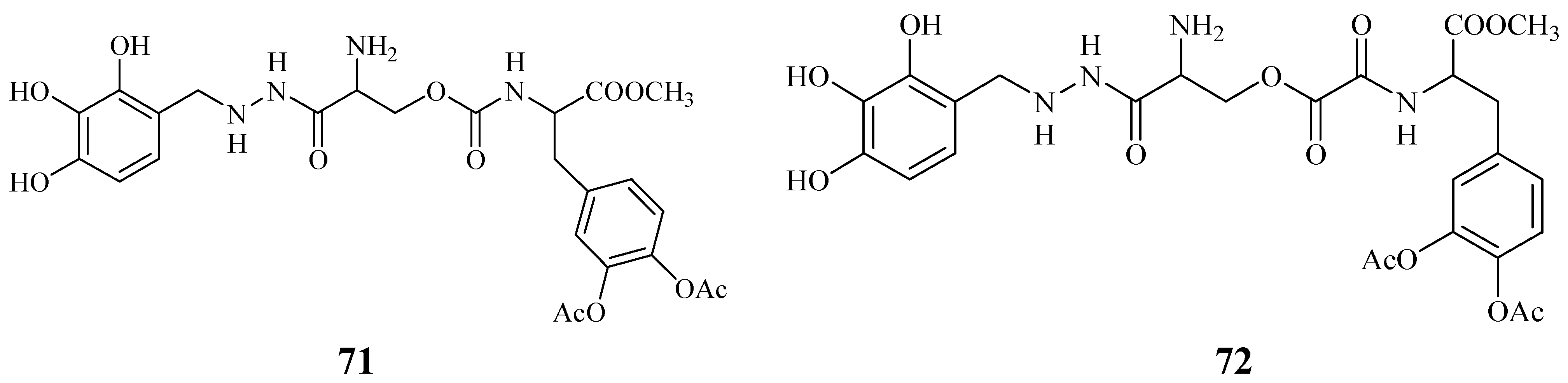
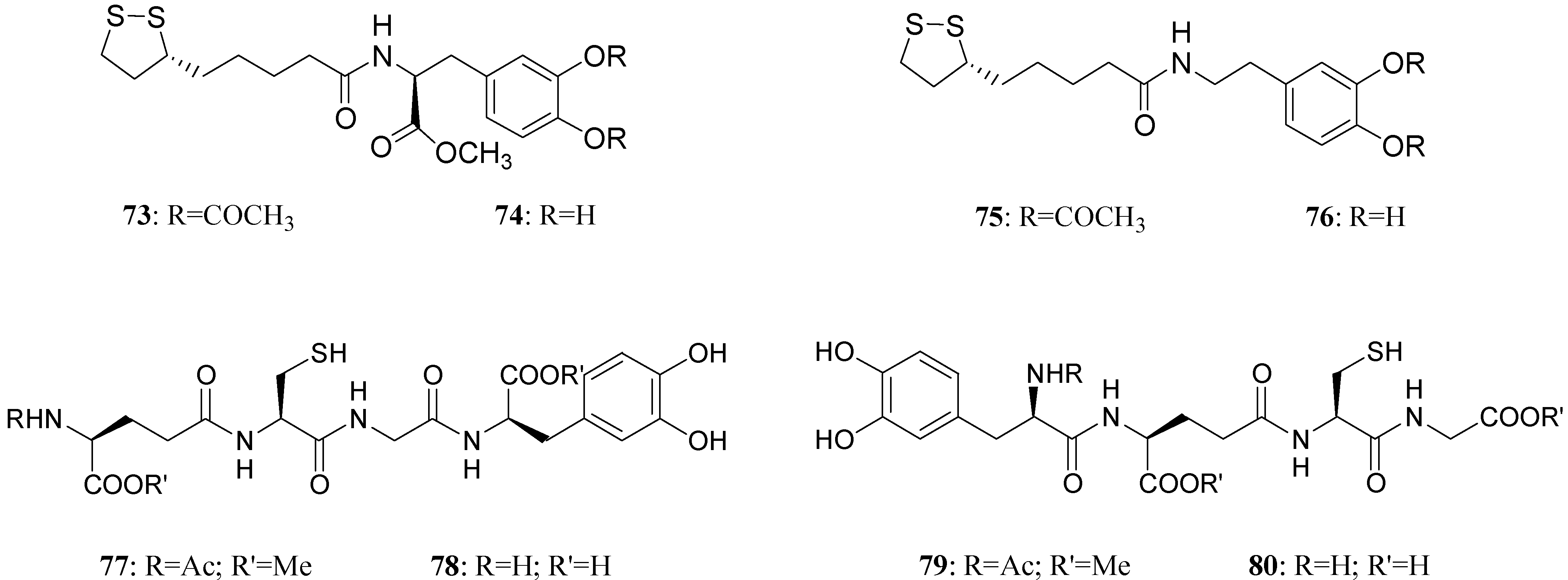
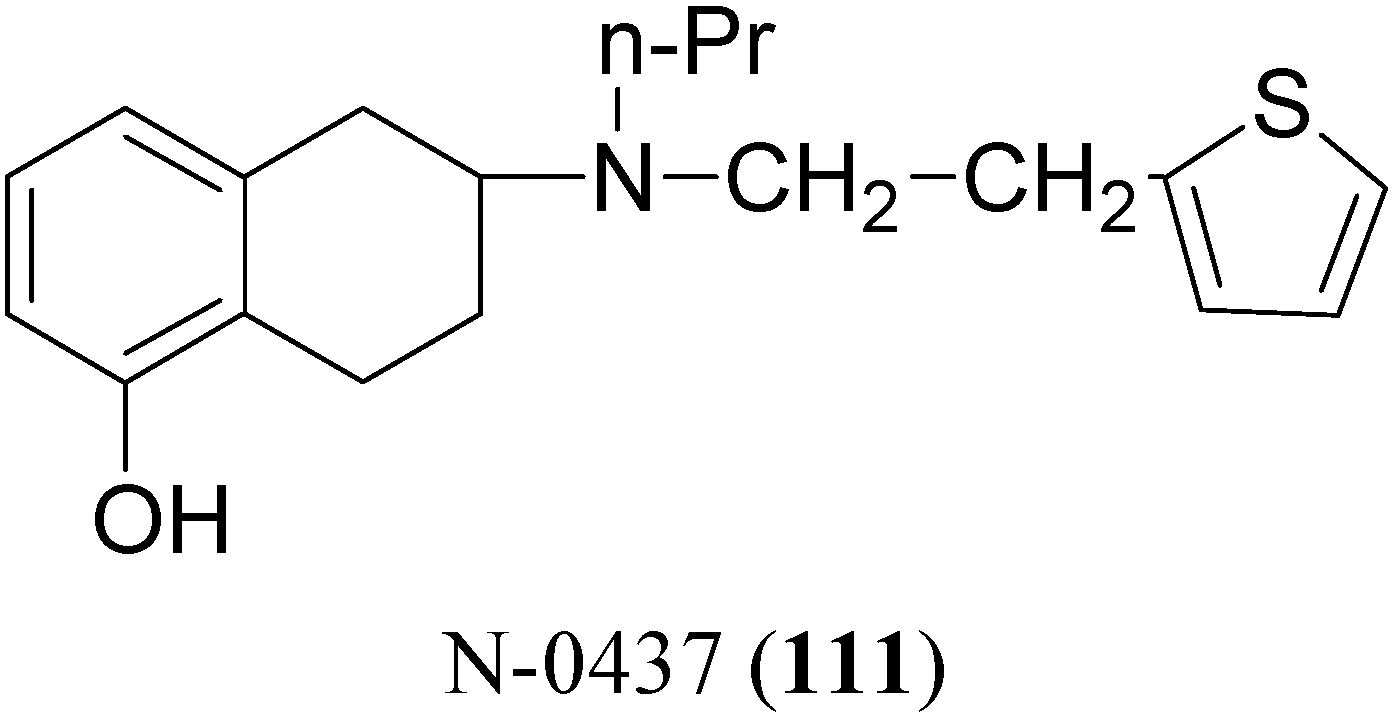
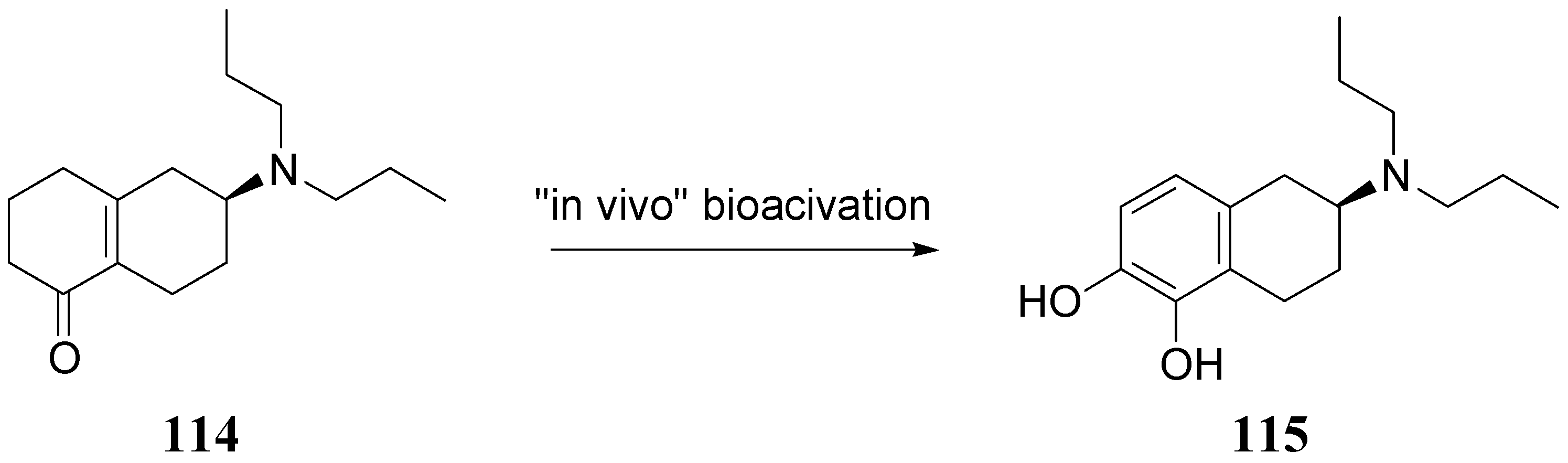
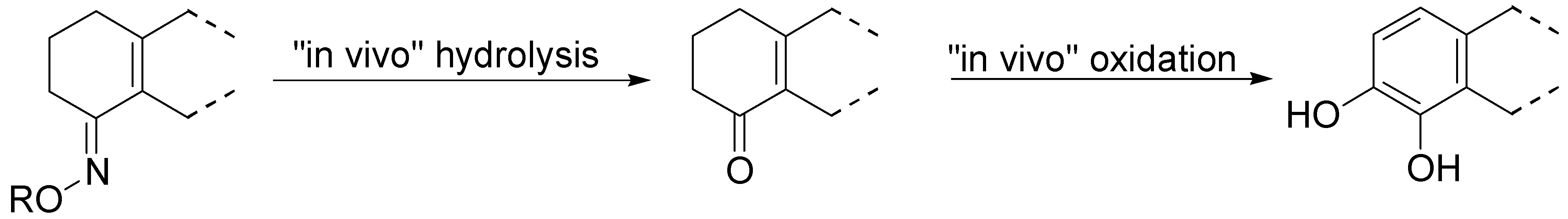
Dopamine receptor agonist prodrugs
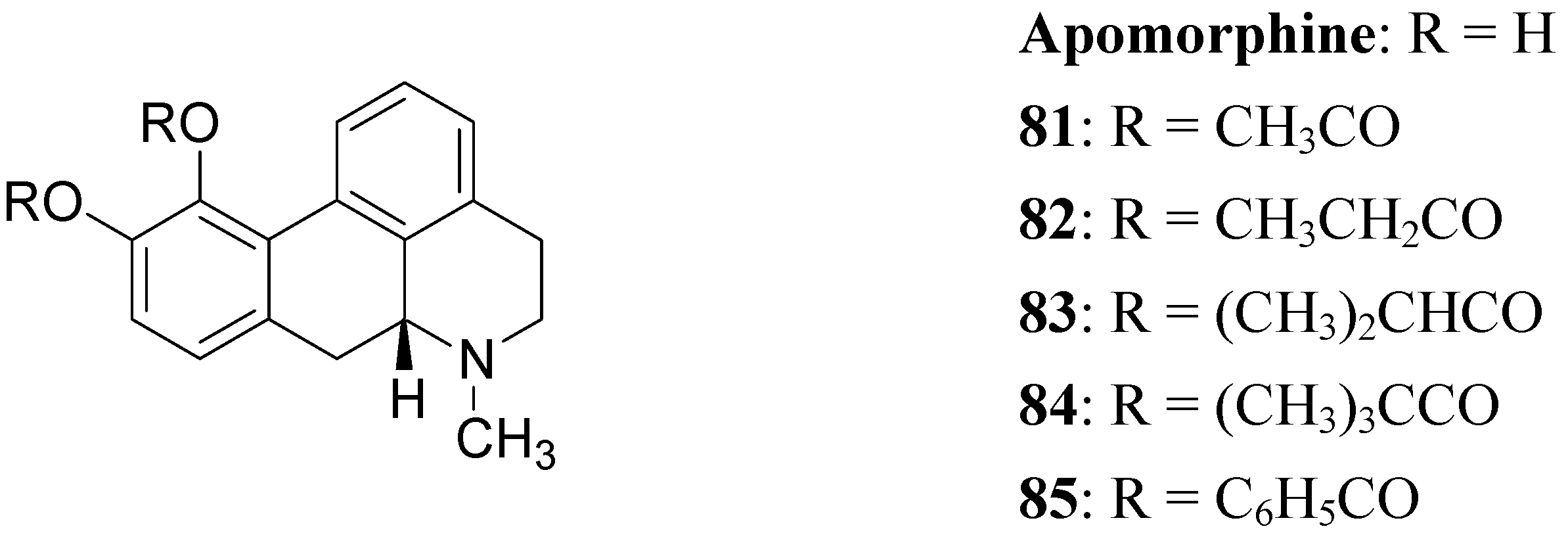

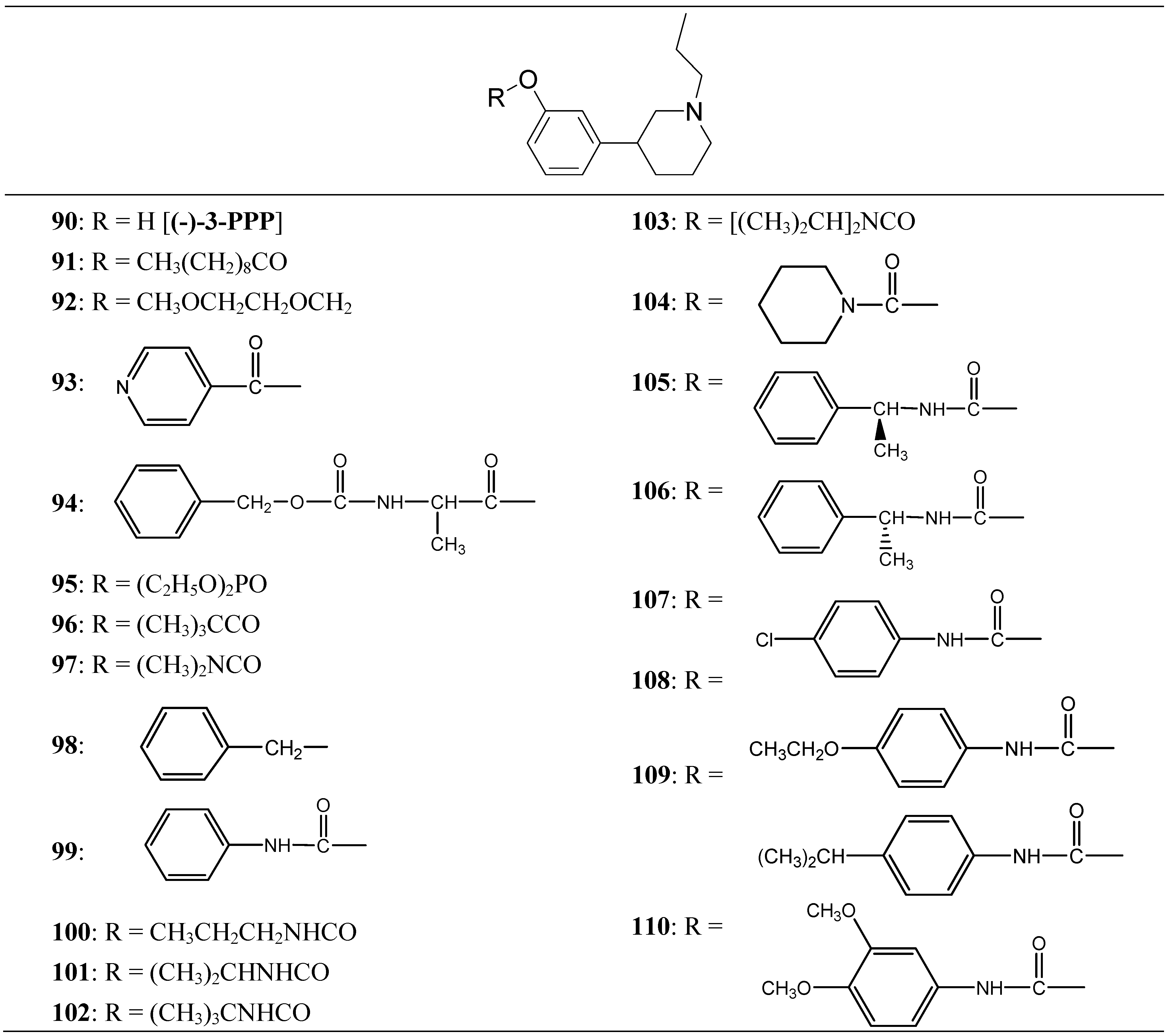

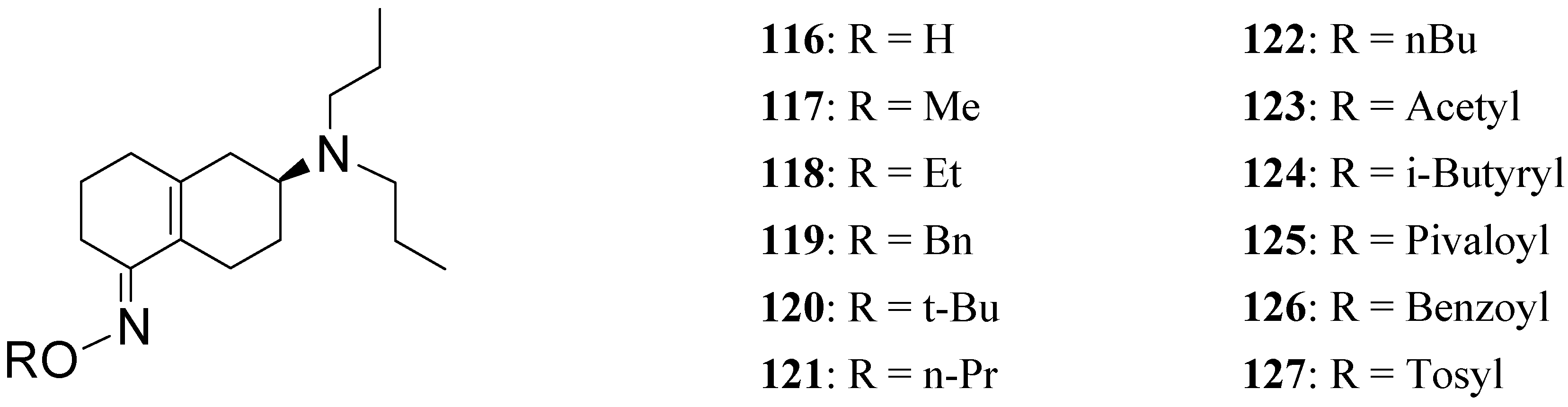
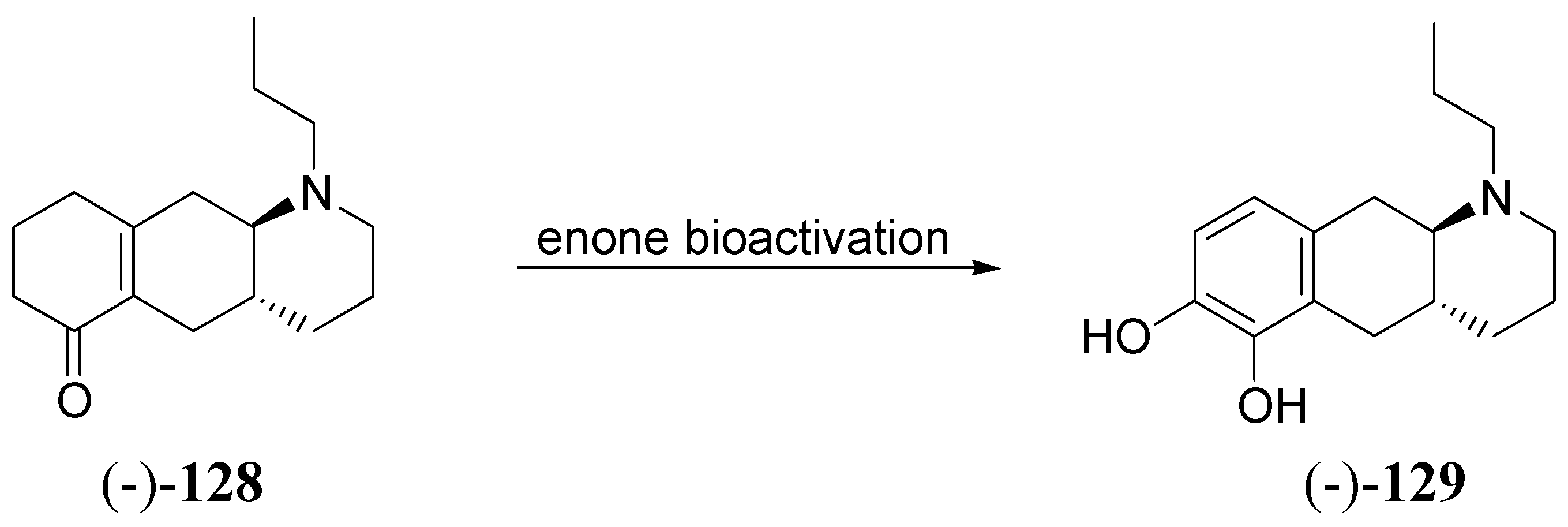
Conclusions
References
- Abou-Sleiman, P.M.; Muqit, M.M.K.; Wood, N.W. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat. Rev. Neurosci. 2006, 7, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V; Bezard, E.; Brotchie, J.; Calon, F.; Collingridge, G.L.; Ferger, B.; Hengerer, B.; Hirsch, E.; Jenner, P.; Le Novère, N.; Obes, J.A; Schwarzschild, M.A.; Spampinato, U.; Davidai, G. Novel pharmacological targets for the treatment of Parkinson’s disease. Nat. Rev. Drug Discov. 2006, 5, 845–854. [Google Scholar]
- Blandini, F.; Greenamyre, J.T. Protective and Symptomatic Strategies for Therapy of Parkinson's. Drugs Today 1999, 35, 473–483. [Google Scholar]
- Bodor, N; Farag, H.H. Improved delivery through biological membranes. 13. Brain specific delivery of dopamine with a dihydropyridine-pyridinium salt type redox delivery system. J. Med. Chem 1983, 26, 528–534. [Google Scholar]
- Kao, H.D.; Traboulsi, A.; Itoh, S.; Dittert, L.; Hussain, A. Enhancement of the Systemic and CNS Specific Delivery of L-Dopa by the Nasal Administration of Its Water. Soluble Prodrugs. Pharmaceut. Res. 2000, 17, 978–984. [Google Scholar]
- Standaert, D.; Young, A. Treatment of Central Nervous System Degenerative Disorders. In The Pharmacological Basis of Therapeutics; Gilman, A. G., Hardman, J. G., Limbird, L. E., Molinoff, P. B., Ruddon, R.W., Eds.; McGraw Hill: New York, 1996; pp. 503–519. [Google Scholar]
- Hagan, J.J.; Middlemiss, D.N.; Sharpe, P.C; Poste, G.H. Parkinson’s disease: prospects for improved drug therapy. TIPS 1997, 18, 156–163. [Google Scholar]
- Sasahara, K.; T. Nitanai, T.; Habara, T.; Morioka, T.; Nakajimai, E. Dosage form design for improvement of bioavailability of levodopa III: Influence of dose on pharmacokinetic behaviour of levo-dopa administration in dogs and parkinsonian patients. J. Pharm. Sci. 1980, 69, 1374–1378. [Google Scholar]
- Nutt, J.G.; Woodward, W.R.; Anderson, J.L. The Effect of carbidopa on the pharmacokinetics of intravenously administered levodopa: The mechanism of action in the treatment of parkinsonism. Annal. Neurol. 1986, 18, 537–543. [Google Scholar]
- Wingard, L.B.; Brody, T.M.; Larner, J.; Schwartz, A. Human Pharmacology; Mosby Year Book: St Louis, 1991. [Google Scholar]
- Cedarbaum, J.M. Antiparkinsonian drugs. Clin. Pharmacokin. 1987, 13, 141–178. [Google Scholar] [CrossRef]
- Nutt, J.G.; Woodward., W.R. Levodopa pharmacokinetics and pharmacodynamics in fluctuating parkinsonian patients. Neurology 1986, 36, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Hardie, R.; Lees, A.J.; Stern, G.M. On-off fluctuations in parkinson’s disease. Brain 1984, 107, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Quinn, N.; Parkes, J.D.; Marsden, C.D. Control of on/off phenomenon by continuous intravenous infusion of levodopa. Neurology 1984, 34, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Birkmayer, W.; Danielcyk, W.; Neumayer, E.; Riederer, P. L-dopa level in plasma, primary condition for the kinetic effect. J. Neural Trans. 1973, 34, 133–143. [Google Scholar] [CrossRef]
- Leppert, P.S.; Cortese, M.; Fix, J.A. The Effects of Carbidopa Dose and Time and Route of Administration on Systemic L-Dopa Levels in Rats. Pharmaceut. Res. 1988, 5, 587–591. [Google Scholar] [CrossRef]
- Sasahara, K.; Nitanai, T.; Habara, T.; Morioka, T.; Kawahara, Y.; Nakajima, E. Dosage form design for improvement of bioavailability of levodopa IV: Possible causes of low bioavailability of oral levodopa in dogs. J. Pharm. Sci. 1981, 70, 730–733. [Google Scholar]
- Hutton, J.H.; Morris, J.L.; Gustavo, C.R. Treatment of chronic parkinson’s disease with controlled-release carbidopa/ levodopa. Neurology 1988, 45, 861–864. [Google Scholar]
- Bordor, N.; Sloan, K.B.; Higuchi, T.; Sasahara, K. Improved delivery through biological membranes 4: Prodrugs of L-dopa. J. Med. Chem. 1977, 20, 1435–1445. [Google Scholar]
- Goole, J.; Vanderbist, F.; Amighi, K. Development and evaluation of new multiple-unit levodopa sustained-release floating dosage forms. Int. J. Pharm. 2007, 334, 35–41. [Google Scholar]
- Di Stefano, A.; Mosciatti, B.; Cingolani, G.M.; Giorgioni, G.; Ricciutelli, M.; Cacciatore, I.; Sozio, P.; Claudi, F. Dimeric L-Dopa derivatives as potential prodrugs. Bioorg. Med. Chem. Lett. 2001, 11, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Chemuturi, N.V.; Donovan, M.D. Role of Organic Cation Transporters in Dopamine Uptake across Olfactory and Nasal Respiratory Tissues. Mol. Pharmaceut. 2007, 4, 936–942. [Google Scholar]
- Casagrande, C.; Ferrari, G. 3,4-0-diacyl derivatives of dopamine. Farmaco 1973, 28, 143–148. [Google Scholar]
- Borgman, R.J; McPhillips, J.J.; Stitzel, R.E. Synthesis and pharmacology of centrally acting dopamine derivatives and analogs in relation to Parkinson’s disease. J. Med. Chem. 1973, 16, 630–633. [Google Scholar]
- Bodor, N; Farag, H.H.; Brewster, M.E. Site-specific, sustained release of drugs to the brain. Science 1981, 214, 1370–1372. [Google Scholar]
- Fernández, C.; Nieto, O.; Rivas, E.; Montenegro, G.; Fontenla, J. A.; Fernández-Mayoralas, A. Carbohydr. Res. 2000, 327, 353–365.
- Fernández, C.; Nieto, O.; Fontenla, J. A.; Rivas, E.; de Ceballos, M.L.; Fernández-Mayoralas, A. Org. Biomol. Chem. 2003, 1, 767–771. [CrossRef] [PubMed]
- Bonina, F.; Puglia, C.; Rimoli, M.G.; Melisi, D.; Boatto, G.; Nieddu, M.; Malignano, A.; La Rana, G.; De Caprariis, P. J. Drug Target 2003, 11, 25–36.
- Oldendorf, W.H. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am. J. Physiol. 1971, 221, 1629–1639. [Google Scholar]
- Mueckler, M. Facilitative glucose transporters. Eur. J. Biochem. 1994, 219, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Prokai, L. Peptide drug delivery into the central nervous system. Prog. Drug Res. 1998, 51, 95–131. [Google Scholar] [PubMed]
- Carlsson, A; Lindqvist, M; Magnusson, T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature 1957, 180, 1200. [Google Scholar] [PubMed]
- Birkmayer, W.; Hornykiewicz, O. The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia. Wien Klin. Wochenschr. 1961, 10, 787–788. [Google Scholar]
- Felix, A.M.; Winter, D.P.; Wang, S.S.; Kulesha, I.D.; Pool, W.R.; Hane, D.L.; Sheppard, H. Synthesis and antireserpine activity of peptides of L. Dopa. J. Med. Chem. 1974, 17, 422–426. [Google Scholar] [CrossRef]
- Wang, H.; Lee, J.; Tsai, M.; Lu, H.; Hsu, W. Synthesis and Pharmacological activities of a novel tripeptide mimetic dopamine prodrug. Bioorg. Med. Chem. Lett. 1995, 5, 2195–2198. [Google Scholar] [CrossRef]
- Marrel, C.; Boss, G.; Van De Waterbeemd, H.; Testa, B.; Cooper, D.; Jenner, P.; Marsden, C.D. L-Dopa esters as potential prodrugs. Eur. J. Med. Chem.-Chim. Ther 1985, 20, 459–465. [Google Scholar]
- Cooper, D.R.; Marrel, C.; Van De Waterbeemd, H.; Quinn, N.; Testa, B.; Jenner, P.; Marsden, C.D. L-Dopa methyl ester-a candidate for chronic systemic delivery of L-Dopa in Parkinson's disease. Clin. Neuropharmacol. 1984, 89–98. [Google Scholar] [CrossRef]
- Garzon-Aburbeh, A; Poupaert, JH; Claesen, M; Dumont, P. A lymphotropic prodrug of L-dopa: synthesis, pharmacological properties, and pharmacokinetic behavior of 1,3-dihexadecanoyl-2-[(S)-2-amino-3-(3,4-dihydroxyphenyl)prop anoyl] propane-1,2,3-triol. J. Med. Chem. 1986, 29, 687–691. [Google Scholar]
- Cooper, D.R.; Marrel, C.; Van De Waterbeemd, H.; Testa, B.; Jenner, P.; Marsden, C.D. L-dopa esters as potential prodrugs: effect on brain concentration of dopamine metabolites in reserpinized mice. J. Pharm. Pharmacol. 1987, 39, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.R.; Marrel, C.; Van De Waterbeemd, H.; Testa, B.; Jenner, P.; Marsden, C.D. L-dopa esters as potential prodrugs: behavioural activity in experimental models of Parkinson's disease. J. Pharm. Pharmacol. 1987, 39, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Ihara, M.; Tsuchiya, Y.; Sawasaki, Y.; Hisaka, A.; Takehana, H.; Tomimoto, K.; Yano, M.A. New potential prodrug to improve the duration of L-dopa: L-3-(3-hydroxy-4-pivaloyloxyphenyl)-alanine. J. Pharm. Sci. 1989, 78, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Ihara, M; Nakajima, S; Hisaka, A; Tsuchiya, Y; Sakuma, Y; Suzuki, H; Kitani, K; Yano, M. Hydrolysis and acyl migration of a catechol monoester of L-dopa: L-3-(3-hydroxy-4-pivaloyloxyphenyl)alanine. J. Pharm. Sci. 1990, 79, 703–708. [Google Scholar]
- Brime, B.; Ballesteros, M.P.; Frutos, P. Preparation and in vitro characterization of gelatin microspheres containing Levodopa for nasal administration. J. Microencapsul. 2000, 17, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Chavis, C.; Grodenic, F.; Imbach, J.L. Ribosides et dérivés de la L-méthyl dopa et de la L-dopa. Eur. J. Med. Chem.-Chim. Ther. 1981, 16, 219–227. [Google Scholar]
- Cingolani, G.M.; Di Stefano, A.; Mosciatti, B.; Napolitani, F.; Giorgioni, G.; Ricciutelli, M.; Claudi, F. Bioorg. Med. Chem. Lett. 2000, 10, 1385–1388. [CrossRef]
- Langlois, M.; Quintard, D.; Abalain, C. Synthesis of symmetrical pseudopeptides as potential inhibitors of the human immunodeficiency virus-1 protease. Eur. J. Med. Chem. 1994, 29, 639–647. [Google Scholar]
- Christiaans, J. A. M.; Timmerman, H. Cardiovascular hybrid drugs: combination of more than one pharmacological property in one single molecule. Eur. J. Pharm. Sci. 1996, 4, 1–22. [Google Scholar] [CrossRef]
- Mahfouz, N.M.; Aboul-Fadl, T.; Diab, A. K. Metronidazole twin ester prodrugs: synthesis, physicichemical properties, hydrolysis kinetics and antigiardial activity. Eur. J. Med. Chem. 1998, 33, 675–683. [Google Scholar] [CrossRef]
- Giannola, L. I.; Giammona, G.; Alotta, R. Pro-drugs of isoniazid: synthesis and diffusion characteristics of acyl derivatives. Pharmazie 1992, 47, 423–425. [Google Scholar] [PubMed]
- Ducho., C.; Görbig, U.; Jessel, S.; Gisch, N.; Balzarini, J.; Meier, C. Bis-cycloSal-d4T-monophosphates: drugs that deliver two molecules of bioactive nucleotides. J. Med. Chem. 2007, 50, 1335–1346. [Google Scholar] [PubMed]
- Cannazza, G.; Di Stefano, A.; Mosciatti, B.; Braghiroli, D.; Baraldi, M.; Pinnen, F.; Sozio, P.; Benatti, C.; Parenti, C. Detection of levodopa, dopamine and its metabolites in rat striatum dialysates following peripheral administration of l-DOPA prodrugs by mean of HPLC–EC. J. Pharm. Biomed, Anal. 2005, 36, 1079–1084. [Google Scholar]
- Di Stefano, A.; Carafa, M.; Sozio, P.; Pinnen, F.; Braghiroli, D.; Orlando, G.; Cannazza, G.; Ricciutelli, M.; Marianecci, C.; Santucci, E. Evaluation of rat striatal l-dopa and DA concentration after intraperitoneal administration of l-dopa prodrugs in liposomal formulations. J. Control. Release 2004, 99, 293–300. [Google Scholar]
- Di Stefano, A.; Sozio, P.; Iannitelli, A.; Marianecci, C.; Santucci, E.; Carafa, M. Maleic- and fumaric-diamides of (O,O-diacetyl)-L-Dopa-methylester as anti-Parkinson prodrugs in liposomal formulation. J. Drug Target 2006, 14, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Menger, F. M.; Rourk, M.J. Synthesis and Reactivity of 5-Fluorouracil/Cytarabine Mutual Prodrugs. J. Org. Chem. 1997, 62, 9083–9088. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, P.D. Mutual prodrugs : a recent trend in prodrug design. Indian J. Pharm. Sci. 1994, 56, 69–79. [Google Scholar]
- Savolainen, J.; Leppänen, J.; Forsberg, M.; Taipale, H.; Nevalainen, T.; Huuskonen, J.; Gynther, J.; Männistö, P. T.; Järvinen, T. Synthesis and in vitro/in vivo evaluation of novel oral N-alkyl- and N,N-dialkyl-carbamate esters of entacapone. Life Sci. 2000, 67, 205. [Google Scholar]
- Leppanen, J.; Huuskonen, J.; Nevalainen, T.; Gynther, J.; Taipale, H.; Jarvinen, T. Design and Synthesis of a Novel L-Dopa-Entacapone Codrug. J. Med. Chem. 2002, 45, 13791382. [Google Scholar] [CrossRef]
- Di Stefano, A.; Sozio, P.; Iannitelli, A.; Cocco, A.; Orlando, G.; Ricciutelli, M. Synthesis and Preliminary Evaluation of L-Dopa/Benserazide Conjugates as Dual Acting Codrugs. Lett. Drug Des. Discovery 2006, 3, 747–752. [Google Scholar]
- Rosini, M.; Andrisano, V.; Bartolini, M.; Bolognesi, M. L.; Hrelia, P.; Minarini, A.; Tarozzi, A.; Melchiorre, C. Rational Approach to discover multipotent anti-Alzheimer drugs. J. Med. Chem. 2005, 48, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Bharath, S.; Cochran, B. C.; Hsu, M.; Ames, B. N.; Andersen, J. K. Pretreatment with R-Lipoic acid alleviates the effects of GSH depletion in PC12 cells: implications for Parkinson’s disease therapy. Neurotoxicology 2002, 23, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Sozio, P.; Cocco, A.; Iannitelli, A.; Santucci, E.; Costa, M.; Pecci, L.; Nasuti, C.; Cantalamessa, F.; Pinnen, F. L-Dopa and DA/(R)-α-lipoic acid conjugates as multifunctional codrugs with antioxidant properties. J. Med. Chem. 2006, 49, 1486–1493. [Google Scholar]
- Pinnen, F.; Cacciatore, I.; Cornacchia, C.; Sozio, P.; Iannitelli, A.; Costa, M.; Pecci, L.; Nasuti, C.; Cantalamessa, F.; Di Stefano, A. Synthesis and Study of L-Dopa-Glutathione Codrugs as New Anti-Parkinson Agents with Free Radical Scavenging Properties. J. Med. Chem. 2007, 50, 2506–2515. [Google Scholar]
- Seeman, P.; Bzowej, N.H.; Guan, H.C.; Bergeon, C.; Reynolds, G.P.; Bird, E.D. Human brain D1 and D2 dopamine receptors in schizophrenia, Alzheimer’s, Parkinson’s and Huntington’s diseases. Neuropsycopharmacology 1987, 1, 5–15. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.; Jaber, M.; Caron, M.G. Dopamine receptors: from structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [PubMed]
- Uitti, R.Y.; Ahlskog, J.E. Comparative review of dopamine receptor agonists in Parkinson's disease. CNS Drugs 1996, 5, 369–388. [Google Scholar] [CrossRef]
- Maratos, E.C.; Jackson, M.J.; Pearce, R.K.; Jenner, P. Antiparkinsonian activity and dyskinesia risk of ropinirole and L-DOPA combination therapy in drug naive MPTP-lesioned common marmosets (Callithrix jacchus). Mov. Disord. 2001, 16, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Baldessarini, R.J.; Walton, K.G.; Borgman, R.J. Esters of apomorphine and N,N-dimethyldopamine as agonists of dopamine receptors in the rat brain in vivo. Neuropharmacology 1975, 14, 725–731. [Google Scholar]
- Borgman, R.J.; Baldessarini, R.J.; Walton, K.G. Diester derivatives as apomorphine prodrugs. J. Med. Chem. 1976, 19, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Baldessarini, R.J.; Kula, N.S.; Walton, K.G.; Borgman, R.J. Hydrolysis of diester prodrugs of apomorphine. Biochem. Pharmaco. 1977, 26, 1749–1756. [Google Scholar] [CrossRef]
- Woodruff, G.N.; Elkhawad, A.O.; Crossman, A.R.; Walker, R.J. Further evidence for the stimulation of rat brain dopamine. J. Pharm. Pharmac. 1974, 26, 740–741. [Google Scholar] [CrossRef]
- Woodruff, G.N.; Elkhawad, A.O.; Pinder, R.M. Long lasting stimulation of locomotor activity produced by the intraventricular injection of a cyclic analogue of dopamine into conscious mice. Eur. J. Pharmac. 1974, 25, 80–86. [Google Scholar] [CrossRef]
- Costall, B.; Naylor, R. J.; Cannon, J. G.; Lee, T. Differential activation by some 2-aminotetralin derivatives of the receptor mechanisms in the nucleus accumbens of rats which mediate hyperactivity and stereotyped biting. Eur. J. Pharmac. 1977, 41, 307–319. [Google Scholar]
- Westerink, B.H.; Dijkstra, D.; Feenstra, M.G.; Grol, C.J.; Horn, A.S.; Rollema, H; Wirix, E. Dopaminergic prodrugs: brain concentrations and neurochemical effects of 5,6- and 6,7-ADTN after administration as dibenzoyl esters. Eur. J. Pharmac. 1980, 61, 7–15. [Google Scholar] [CrossRef]
- Houwing, H.A.; van Oene, J.C.; Horn, A.S. 3,4-disubstituted phenyliminoimidazolidines as potential prodrugs of the purported dopamine agonist 3,4-dihydroxyphenylimino-2-imidazolidine (DPI). Pharmaceut. Weekblad. Sci. 1983, 5, 177–181. [Google Scholar] [CrossRef]
- Thorberg, S.O.; Berg, S.; Lundstrom, J.; Pettersson, B.; Wijkstrom, A.; Sanchez, D.; Lindberg, P.; Nilsson, J.L. Carbamate ester derivatives as potential prodrugs of the presynaptic dopamine autoreceptor agonist (-)-3-(3-hydroxyphenyl)-N-propylpiperidine. J. Med. Chem. 1987, 30, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- den Daas, I.; Tepper, P.G.; Horn, A.S. Improvement of the oral bioavailability of the selective dopamine agonist N-0437 in rats: the in vitro and in vivo activity of eight ester prodrugs. N-S Arch. Pharmacol. 1990, 341, 186–191. [Google Scholar]
- den Daas, I.; Tepper, P.G.; Rollema, H.; Horn, A.S. Transdermal administration of the dopamine agonist N-0437 and seven ester prodrugs: comparison with oral administration in the 6-OHDA turning model. N-S Arch Pharmacol. 1990, 342, 655–659. [Google Scholar]
- den Daas, I.; de Boer, P.; Tepper, P.G.; Rollema, H.; Horn, A.S. Orally active carbamate prodrugs of the selective dopamine agonist N-0437: in-vivo activities in the 6-OHDA turning model and in-vitro activities. J. Pharm. Pharmacol. 1991, 43, 11–16. [Google Scholar]
- Hansen, K. T; Faarup, P; Bundgaard, H. Carbamate ester prodrugs of dopaminergic compounds: synthesis, stability, and bioconversion. J. Pharm. Sci. 1991, 80, 793–798. [Google Scholar]
- LeWitt, P.A.; Lyons, K.E.; Pahwa, R. Advanced Parkinson disease treated with rotigotine transdermal system. Neurology 2007, 68, 1262–1267. [Google Scholar]
- Michaelides, M.R.; Hong, Y.; DiDomenico, S.Jr.; Asin, K.E.; Britton, D.R.; Lin, C.W.; Williams, M.; Shiosaki, K. (5aR,11bS)-4,5,5a,6,7,11b-Hexahydro-2-propyl-3-thia-5-azacyclopent-1-ena[c]phenanthrene-9,10-diol (A-86929): a potent and selective dopamine D1 agonist that maintain behavioral efficacy following repeated administration and characterization of its diacetyl prodrug (ABT-431). J. Med. Chem. 1995, 38, 3445–3447. [Google Scholar] [CrossRef] [PubMed]
- Shiosaki, K.; Jenner, P.; Asin, K.E.; Britton, D.R.; Lin, C.W.; Michaelides, M.; Smith, L.; Bianchi, B.; DiDomenico, S.; Hodges, L.; Hong, Y.; Mahan, L.; Mikusa, J.; Miller, T.; Nikkel, A.; Stashko, M.; Witte, D.; Williams, M. ABT-431: the diacetyl prodrug of A-86929, a potent and selective dopamine D1 receptor agonist: in vitro characterization and effects in animal models of Parkinson's disease. J. Pharmacol. Exp. Ther. 1996, 276, 150–160. [Google Scholar]
- Rascol, O.; Blin, O.; Thalamas, C.; Descombes, S.; Soubrouillard, C.; Azulay, P.; Fabre, N.; Viallet, F.; Lafnitzegger, K.; Wright, S.; Carter, J. H.; Nutt, J. G. ABT-431, a D1 receptor agonist prodrug, has efficacy in Parkinson's disease. Ann. Neurol. 1999, 45, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Giardina, W. J.; Williams, M. Adrogolide HCl (ABT-431; DAS-431), a prodrug of the dopamine D1 receptor agonist, A-86929: Preclinical pharmacology and clinical data. Neurological and Urological Diseases Research. CNS Drug Rev. 2001, 7, 305–316. [Google Scholar]
- Dijkstra, D.; Venhuis, B. J.; Wikstro¨m, H. V.; Wise, L. D.; Wustrow, D. J.; Meltzer, L. T. Method for treating Parkinson’s disease by administering of (-)-5-keto-2-N,N-di-propylamino-tetrahydrotetralin. Patent WO 0128977 A1.
- Venhuis, B. J.; Wikstro¨m, H. V.; Rodenhuis, N.; Sundell, S.; Wustrow, D.; Meltzer, L. T.; Wise, L. D.; Johnson, S. J.; Dijkstra, D. A New Type of Prodrug of Catecholamines - An Opportunity to Improve the Treatment of Parkinsons Disease. J. Med. Chem. 2002, 45, 2349–2351. [Google Scholar] [CrossRef] [PubMed]
- Venhuis, B. J.; Dijkstra, D.; Wustrow, D. J.; Meltzer, L. T.; Wise, L. D.; Johnson, S. J.; Heffner, T. G.; Wikstro¨m, H. V. Orally Active Analogues of the Dopaminergic Prodrug 6-(N,N-di-npropylamino)-3,4,5,6,7,8-hexahydro-2H-naphthalen-1-one. Synthesis and Pharmacological Activity. J. Med. Chem. 2003, 46, 584–590. [Google Scholar] [CrossRef]
- Venhuis, B.J.; Dijkstra, D.; Wustrow, D.; Meltzer, L.T.; Wise, Lawrence D.; Johnson, S.J.; Wikstroem, H.V. Orally active oxime derivatives of the dopaminergic prodrug 6-(N,N-Di-n-propylamino)-3,4,5,6,7,8-hexahydro-2H-naphthalen-1-one. Synthesis and pharmacological activity. J. Med. Chem. 2003, 46, 4136–4140. [Google Scholar]
- Liu, D.; Wikstroem, H.V.; Dijkstra, D.; De Vries, J.B.; Venhuis, B. Extremely potent orally active benzo[g]quinoline analogue of the dopaminergic prodrug: 6-(N,N-Di-n-propyl)amino-3,4,5,6,7,8-hexahydro-2H-naphthalen-1-one. J. Med. Chem. 2006, 49, 1494–1498. [Google Scholar] [CrossRef] [PubMed]
- Juncos, J.L.; Mouradian, M.M.; Fabbrini, G.; Serrati, C.; Chase, T.N. Levodopa methyl ester treatment of Parkinson's disease. Neurology 1987, 37, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are not available.
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Di Stefano, A.; Sozio, P.; Cerasa, L.S. Antiparkinson Prodrugs. Molecules 2008, 13, 46-68. https://doi.org/10.3390/molecules13010046
Di Stefano A, Sozio P, Cerasa LS. Antiparkinson Prodrugs. Molecules. 2008; 13(1):46-68. https://doi.org/10.3390/molecules13010046
Chicago/Turabian StyleDi Stefano, Antonio, Piera Sozio, and Laura Serafina Cerasa. 2008. "Antiparkinson Prodrugs" Molecules 13, no. 1: 46-68. https://doi.org/10.3390/molecules13010046




