Evaluation of the Antioxidant Activity of Syzygium cumini Leaves
Abstract
:Introduction
Results and Discussion
Preparation of the methanolic extract and its fractions
Total phenolic and total flavonoid content
DPPH free radical-scavenging assay
| Concentration (μg/mL) | ME | HxF | WtF | EaF | CfF |
|---|---|---|---|---|---|
| DPPH a | |||||
| 15.63 | 92.78 ± 0.50D | 91.99 ± 0.37D | 91.03 ± 0.61D | 90.26 ± 3.55D | 88.42 ± 1.08D |
| 31.25 | 86.92 ± 0.78C | 90.10 ± 0.18C | 86.02 ± 1.82C | 81.73 ± 0.43C | 83.57 ± 0.98C |
| 62.5 | 74.25 ± 0.45B | 86.78 ± 0.84B | 75.72 ± 1.67B | 69.15 ± 0.30B | 73.52 ± 0.39B |
| 125 | 49.27 ± 3.35A | 79.09 ± 0.67A | 56.85 ± 2.66A | 45.95 ± 0.72A | 56.93 ± 1.02A |
| FRAP b | |||||
| 15.63 | 0.13 ± 0.01a | 0.06 ± 0.01a | 0.10 ± 0.00a | 0.11 ± 0.01a | 0.11 ± 0.01a |
| 31.25 | 0.19 ± 0.01b | 0.07 ± 0.00a | 0.16 ± 0.01b | 0.20 ± 0.00b | 0.18 ± 0.00b |
| 62.5 | 0.33 ± 0.02c | 0.10 ± 0.00b | 0.27 ± 0.01c | 0.35 ± 0.01c | 0.31 ± 0.01c |
| 125 | 0.60 ± 0.01d | 0.15 ± 0.00c | 0.51 ± 0.00d | 0.68 ± 0.01d | 0.55 ± 0.01d |
Ferric reducing antioxidant power (FRAP)
| Samples | Antioxidant activity | |
|---|---|---|
| IC50/DPPH (µg/mL) a | FRAP (mmol AAE/g) b | |
| ME | 125.39 ± 7.55b | 2.73 ± 0.06d |
| HxF | 479.56 ± 9.31d | 0.53 ± 0.05a |
| CfF | 149.33 ± 4.43c | 2.51 ± 0.05c |
| EaF | 112.79 ± 2.51b | 3.12 ± 0.07e |
| WtF | 150.66 ± 6.95c | 2.32 ± 0.02b |
| VitC | 71.30 ± 1.66a | -- |
| BHA | 114.69 ± 1.95b | 4.66 ± 0.10f |
Correlations between total phenolic content and antioxidative function


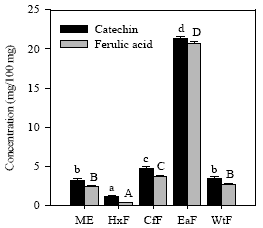
Determination of phenolic compounds


Conclusions
Experimental
Chemicals and plant materials
Extraction procedure
Determination of total phenolics and total flavonoid content
Free radical scavenging ability on 2,2-diphenyl-1-picrylhydrazyl
Ferric reducing/antioxidant power (FRAP) assay
HPLC analysis of phenolic compounds
Statistical analysis
Acknowledgements
References
- Yeum, K.J.; Aldini, G.; Chung, H.Y.; Krinsky, N.I.; Russell, R.M. The activities of antioxidant nutrients in human plasma depend on the localization of attacking radical species. J. Nutr. 2003, 133, 2688–2691. [Google Scholar]
- You, Y.L.; Duan, X.W.; Wei, X.Y.; Su, X.G.; Zhao, M.M.; Sun, J.; Ruenroengklin, N.; Jiang, Y.M. Identification of major phenolic compounds of Chinese water chestnut and their antioxidant activity. Molecules. 2007, 12, 842–852. [Google Scholar] [CrossRef]
- Warrier, P.K.; Nambiar, V.P.K.; Ramankutty, C. Indian Medicinal Plants.; Orient Longman Ltd.: Hyderabad, India, 1996; vol. 5, pp. 225–228. [Google Scholar]
- Bhandary, M.J.; Chandrashekar, K.R.; Kaveriappa, K.M. Medical ethnobotany of the siddis of Uttara Kannada district, Karnataka, India. J. Ethnopharmacol. 1995, 47, 149–158. [Google Scholar] [CrossRef]
- Rastogi, R.M.; Mehrotra, B.N. Compendium of Indian Medicinal Plants.; Central Drug Research Institute: Lucknow, India, 1990; vol. 1, pp. 388–389. [Google Scholar]
- Tanaka, M.; Kuei, C.W.; Nagashima, Y.; Taguchi, T. Application of antioxidative maillard reaction products from histidine and glucose to sardine products. Nippon Sui. Gakk. 1998, 54, 1409–1414. [Google Scholar]
- Banerjee, A.; Dasgupta, N.; De, B. In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chem. 2005, 90, 727–733. [Google Scholar] [CrossRef]
- Benherlal, P.S.; Arumughan, C. Chemical composition and in vitro antioxidant studies on Syzygium cumini fruit. J. Sci. Food Agric. 2007, 87, 2560–2569. [Google Scholar] [CrossRef]
- Lacikova, L.; Muselik, J.; Masterova, I.; Grancai, D. Antioxidant activity and total phenols in different extracts of four Staphylea L. species. Molecules 2007, 12, 98–102. [Google Scholar] [CrossRef]
- Hatano, T.; Edamatsu, R.; Hiramatsu, M.; Mori, A.; Fujita, Y.; Yasuhara, T.; Yoshida, T.; Okuda, T. Effect of interaction of tannins with co-existing substances VI. Effect of tannins and related polyphenols on superoxide anion radical and on DPPH radical. Chem. Pharm. Bull. 1989, 37, 2016–2021. [Google Scholar] [CrossRef]
- Duh, P.D.; Tu, Y.Y.; Yen, G.C. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat). Leb-ensm. Wiss. Technol. 1999, 32, 269–277. [Google Scholar] [CrossRef]
- Fenglin, H.; Ruili, L.; Bao, H.; Liang, M. Free radical scavenging activity of extracts prepared from fresh leaves of selected Chinese medicinal plants. Fitoterapia 2004, 75, 14–23. [Google Scholar]
- Yesilada, E.; Tsuchiya, K.; Takaishi, Y.; Kawazoe, K. Isolation and characterization of free radical scavenging flavonoid glycosides from the flowers of Spartium junceum by activity-guided fractionation. J. Ethnopharmacol. 2000, 73, 471–478. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Baliga, M.S. Syzygium cumini (Jamun) reduces the radiation-induced DNA damage in the cultured human peripheral blood lymphocytes: a preliminary study. Toxicol. Lett. 2002, 132, 19–25. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Paganga, G.; Miller, N.; Rice-Evans, C.A. The polyphenolic content of fruit and vegetables and their antioxidant activities. What does a serving constitute? Free Radical. Res. 1999, 30, 153–162. [Google Scholar] [CrossRef]
- Oszmianski, J.; Wojdylo, A.; Lamer-Zarawska, E.; Swiader, K. Antioxidant tannins from Rosaceae plant roots. Food Chem. 2007, 100, 579–583. [Google Scholar] [CrossRef]
- Moini, H.; Guo, Q.O.; Packer, L. Xanthine oxidase and xanthine dehydrogenase inhibition by the procyanidin-rich French maritime pine bark extract, pycnogenol: a protein binding effect. Adv. Exp. Med. Biol. 2002, 505, 141–149. [Google Scholar]
- Scott, B.C.; Butler, J.; Halliwell, B.; Aruoma, O.I. Evaluation of the antioxidant actions of ferulic acid and catechins. Free Radic. Res. Commun. 1993, 19, 241–253. [Google Scholar]
- Bors, W.; Michel, C. Antioxidant capacity of flavanols and gallate esters: pulse radiolysis studies. Free Radic. Biol. Med. 1999, 27, 1413–1426. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Morel, I.; Lescoat, G.; Cogrel, P.; Sergent, O.; Pasdeloup, N.; Brissot, P.; Cillard, P.; Cillard, J. Antioxidant and iron chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem. Pharmacol. 1993, 45, 13–19. [Google Scholar] [CrossRef]
- Nakao, M.; Takio, S.; Ono, K. Alkyl peroxyl radical-scavenging activity of catechins. Phytochemistry 1998, 49, 2379–2382. [Google Scholar] [CrossRef]
- Nanjo, F.; Goto, K.; Seto, R.; Suzuki, M.; Sakai, M.; Hara, Y. Scavenging effects of tea catechins and their derivativeson 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic. Biol. Med. 1996, 21, 895–902. [Google Scholar] [CrossRef]
- Yilmaz, Y. Novel uses of catechins in foods. Trends Food Sci. Technol. 2006, 17, 64–71. [Google Scholar] [CrossRef]
- Terao, J.; Piskuli, M.; Yao, Q. Protective effects of epicatechin, epicatechin gallate and quercetin on lipid peroxidation in phospholipid bilayers. Arch. Biochem. Biop. 1994, 308, 278–284. [Google Scholar] [CrossRef]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Rice-Evans, C.A. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure–activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar] [CrossRef]
- Sanchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res. Inter. 1999, 32, 407–412. [Google Scholar] [CrossRef]
- Cirico, T.L.; Omaye, S.T. Additive or synergetic effects of phenolic compounds on human low density lipoprotein oxidation. Food Chem. Toxicol. 2006, 44, 510–516. [Google Scholar] [CrossRef]
- Graham, H.D. Stabilization of the Prussian blue color in the determination of polyphenols. J. Agric. Food Chem. 1992, 40, 801–805. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid content in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–599. [Google Scholar] [CrossRef]
- Braca, A.; Tommasi, N.D.; Bari, L.D.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia terapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ruan, Z.P.; Zhang, L.L.; Lin, Y.M. Evaluation of the Antioxidant Activity of Syzygium cumini Leaves. Molecules 2008, 13, 2545-2556. https://doi.org/10.3390/molecules13102545
Ruan ZP, Zhang LL, Lin YM. Evaluation of the Antioxidant Activity of Syzygium cumini Leaves. Molecules. 2008; 13(10):2545-2556. https://doi.org/10.3390/molecules13102545
Chicago/Turabian StyleRuan, Zhi Ping, Liang Liang Zhang, and Yi Ming Lin. 2008. "Evaluation of the Antioxidant Activity of Syzygium cumini Leaves" Molecules 13, no. 10: 2545-2556. https://doi.org/10.3390/molecules13102545