Diastereoselective Synthesis of Cyclopentanediols by InCl3/Al Mediated Intramolecular Pinacol Coupling Reaction in Aqueous Media
Abstract
:Introduction
Results and Discussion
Screening of reaction temperature
Investigation of the scope of the InCl3/Al catalyzed intramolecular pinacol coupling
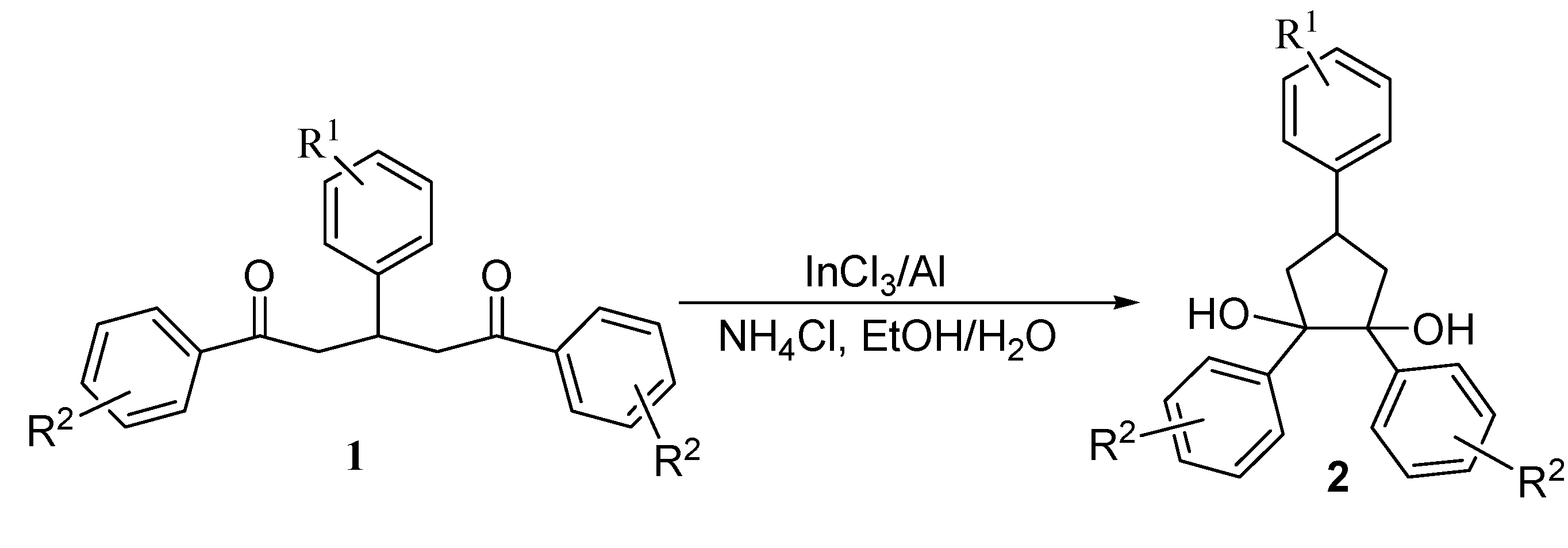
| Entry | Diketone | Product | Yield (%)b | d. e. (%)c | |
|---|---|---|---|---|---|
| R1 | R2 | ||||
| 1 | H | H | 2a | 65 | > 99 |
| 2 | H | 4-CH3 | 2b | 78 | > 99 |
| 3 | H | 4-CH3O | 2c | 75 | 58 |
| 4 | H | 4-Cl | 2d | 62 | > 99 |
| 5 | 4-CH3 | H | 2e | 80 | > 99 |
| 6 | 4-Cl | H | 2f | 83 | > 99 |
| 7 | 4-CH3O | H | 2g | 88 | > 99 |
| 8 | 2-Cl | H | 2h | 61 | 44 |
| 9 | 4-CH3 | 4-CH3 | 2i | 90 | > 99 |
| 10 | 4-F | H | 2j | 79 | > 99 |
| 11 | 4-N(CH3)2 | H | - | trace | - |
| 12 |  | - | - | - | |
| 13d | |||||
| 14e |  | nr | |||
Experimental
General
General procedure of the intramolecular pinacol coupling reaction
Acknowledgements
References
- Kahn, B. E.; Rieke, R. D. Carbonyl coupling reactions using transition metals, lanthanides, and actinides. Chem. Rev. 1988, 88, 733–745. [Google Scholar] [CrossRef]
- Fittig, R. Ueber einige metamorphosen des acetones de essigisäure. Justus Leibigs Ann. Chem. 1859, 110, 13–45. [Google Scholar]
- McMurry, J. E.; Rico, J. G.; Shih, Y. N. Synthesis and stereochemistry of sarcophytol B: An anticancer cembranoid. Tetrahedron Lett. 1989, 30, 1173–1176. [Google Scholar] [CrossRef]
- McMurry, J. E.; Dushin, R. G. Total synthesis of (+-)-isolobophytolide and (+-)-crassin by tatanium-induced carbonyl coupling. J. Am. Chem. Soc. 1990, 112, 6942–6949. [Google Scholar] [CrossRef]
- Li, T.-Y.; Cui, W.; Liu, J.-G.; Zhao, J.-Z.; Wang, Z.-M. A highly dl-stereoselective pinacolization of aromatic aldehydes mediated by TiCl4-Zn. Chem. Commun. 2000, 139–140. [Google Scholar]
- Handy, S. T.; Omune, D. A. Chelation effect on the pathway between intramolecular hydrodimerization and pinacol coupling. Org. Lett. 2005, 7, 1553–1555. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hattori, R.; Itoh, K. Highly trans-selective intramolecular pinacol coupling of diols catalyzed bulky Cp2TiPh. Chem. Commun. 1999, 825–826. [Google Scholar] [CrossRef]
- Ueda, T.; Kanomata, N.; Machida, H. Synthesis of planar-chiral paracyclophanes via samarium (II)-catalyzed intramolecular pinacol coupling. Org. Lett. 2005, 7, 2365–2368. [Google Scholar] [CrossRef]
- Molander, G. A.; Kenny, C. Intramolecular reductive coupling reactions promoted by samarium diiodide. J. Am. Chem. Soc. 1989, 111, 8236–8246. [Google Scholar] [CrossRef]
- Svatos, A.; Boland, W. Reductive pinacol coupling reactions of aromatic carbonyl compounds catalytic in chromium(II). Synlett. 1998, 549. [Google Scholar] [CrossRef]
- Nair, V.; Ros, S.; Jayan, C. N.; Rath, N. P. Indium/indium trichloride mediated pinacol cross-coupling reaction of aldehydes and chalcones in aqueous media: a facile stereoselective synthesis of substituted but-3-ene-1,2-diols. Tetrahedron Lett. 2002, 43, 8967–8969. [Google Scholar] [CrossRef]
- Xu, X.; Hirao, T. Vanadium-catalyzed pinacol coupling reaction in water. J. Org. Chem. 2005, 70, 8594–8596. [Google Scholar] [CrossRef]
- Buchammagari, H.; Toda, Y.; Hirano, M.; Hososno, H.; Takeuchi, D.; Osakada, K. Room temperature-stable electride as a synthetic organic reagent: application to pinacol coupling reaction in aqueous media. Org. Lett. 2007, 9, 4287–4289. [Google Scholar] [CrossRef]
- Corey, E. J.; Danheiser, R. L.; Chandrasekaran, S. New reagent for the intermolecular and intramolecular pinacolic coupling of ketones and aldehydes. J. Org. Chem. 1976, 41, 260–265. [Google Scholar]
- Hays, D. S.; Fu, G. C. Metal hydride mediated intramolecular pinacol couplings of dialdehydes and ketoaldehydes. J. Am. Chem. Soc. 1995, 117, 7283–7284. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hattori, R.; Itoh, K. Highly trans-selective intramolecular pinacol coupling of dials catalyzed by bulky Cp2TiPh. Chem. Commun. 1999, 825–826. [Google Scholar] [CrossRef]
- Li, C.-J.; Chan, T.-H. Organic syntheses using indium-mediated and catalyzed reactions in aqueous media. Tetrahedron 1999, 55, 11149–11176. [Google Scholar] [CrossRef]
- Auge, J.; Lubin-Germain, N.; Uziel, J. Recent advances in indium-promoted organic reactions. Synthesis 2007, 1739–1764. [Google Scholar]
- Wang, C.-Y.; Su, H.; Yang, D.-Y. Regio- and stereoselective dimerization of terminal alkynes to enynes in InCl3-NaBH4 system. Synlett. 2004, 561–563. [Google Scholar]
- Wang, C.-Y.; Yan, L.; Zheng, Z.-G.; Yang, D.-Y.; Pan, Y.-J. Synthesis of (E)-alkenes via hydroindation of C=C in InCl3-NaBH4 system. Tetrahedron 2006, 62, 7712–7717. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Wan, J.-P.; Zheng, Z.-G.; Pan, Y.-J. A new InCl3-catalyzed reduction of anthrones and anthraquinones by using aluminum powder in aqueous media. Tetrahedron 2007, 63, 5071–5075. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Pan, Y.-J.; Wu, A.-X. InCl3/Al mediated pinacol coupling reactions of aldehydes and ketones in aqueous media. Tetrahedron 2007, 63, 429–434. [Google Scholar] [CrossRef]
- Hirsch, S. S.; Bailey, W. J. Base-catalyzed alkylation of cyclopentadiene rings with alcohols and amines. J. Org. Chem. 1978, 43, 4090. [Google Scholar] [CrossRef]
- Yanagisawa, A.; Takahashi, H.; Arai, T. One-pot synthesis of 1,5-diketones catalyzed by barium isopropoxide. Tetrahedron 2007, 63, 8581–8585. [Google Scholar] [CrossRef]
- Rong, L.-C.; Li, X.-Y.; Wang, H.-Y.; Shi, D.-Q.; Tu, S.-J. Solvent-free Michael addition reaction of cyclohexanone with chalcone. Chin. J. Org. Chem. 2007, 27, 1292–1295. [Google Scholar]
- Sample Availability: Samples of compounds 2a-2j are available from the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, Y.; Wan, J.; Wang, C.; Sun, C. Diastereoselective Synthesis of Cyclopentanediols by InCl3/Al Mediated Intramolecular Pinacol Coupling Reaction in Aqueous Media. Molecules 2008, 13, 2652-2658. https://doi.org/10.3390/molecules13102652
Chen Y, Wan J, Wang C, Sun C. Diastereoselective Synthesis of Cyclopentanediols by InCl3/Al Mediated Intramolecular Pinacol Coupling Reaction in Aqueous Media. Molecules. 2008; 13(10):2652-2658. https://doi.org/10.3390/molecules13102652
Chicago/Turabian StyleChen, Yunhua, Jieping Wan, Chunyan Wang, and Cuirong Sun. 2008. "Diastereoselective Synthesis of Cyclopentanediols by InCl3/Al Mediated Intramolecular Pinacol Coupling Reaction in Aqueous Media" Molecules 13, no. 10: 2652-2658. https://doi.org/10.3390/molecules13102652





