Stable Spirocyclic Meisenheimer Complexes
Abstract
:Introduction
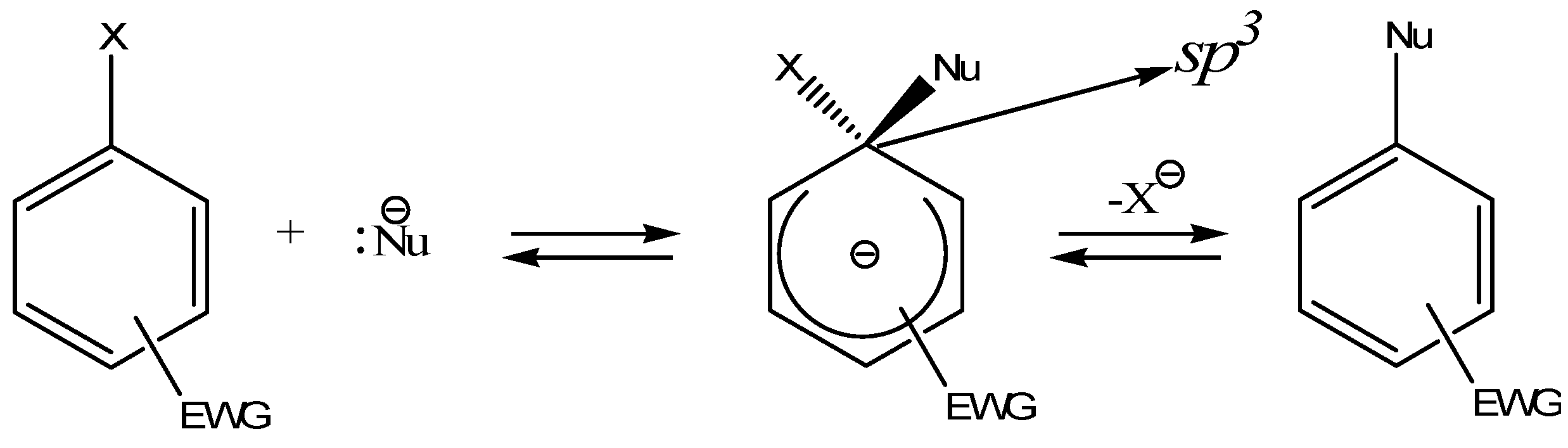
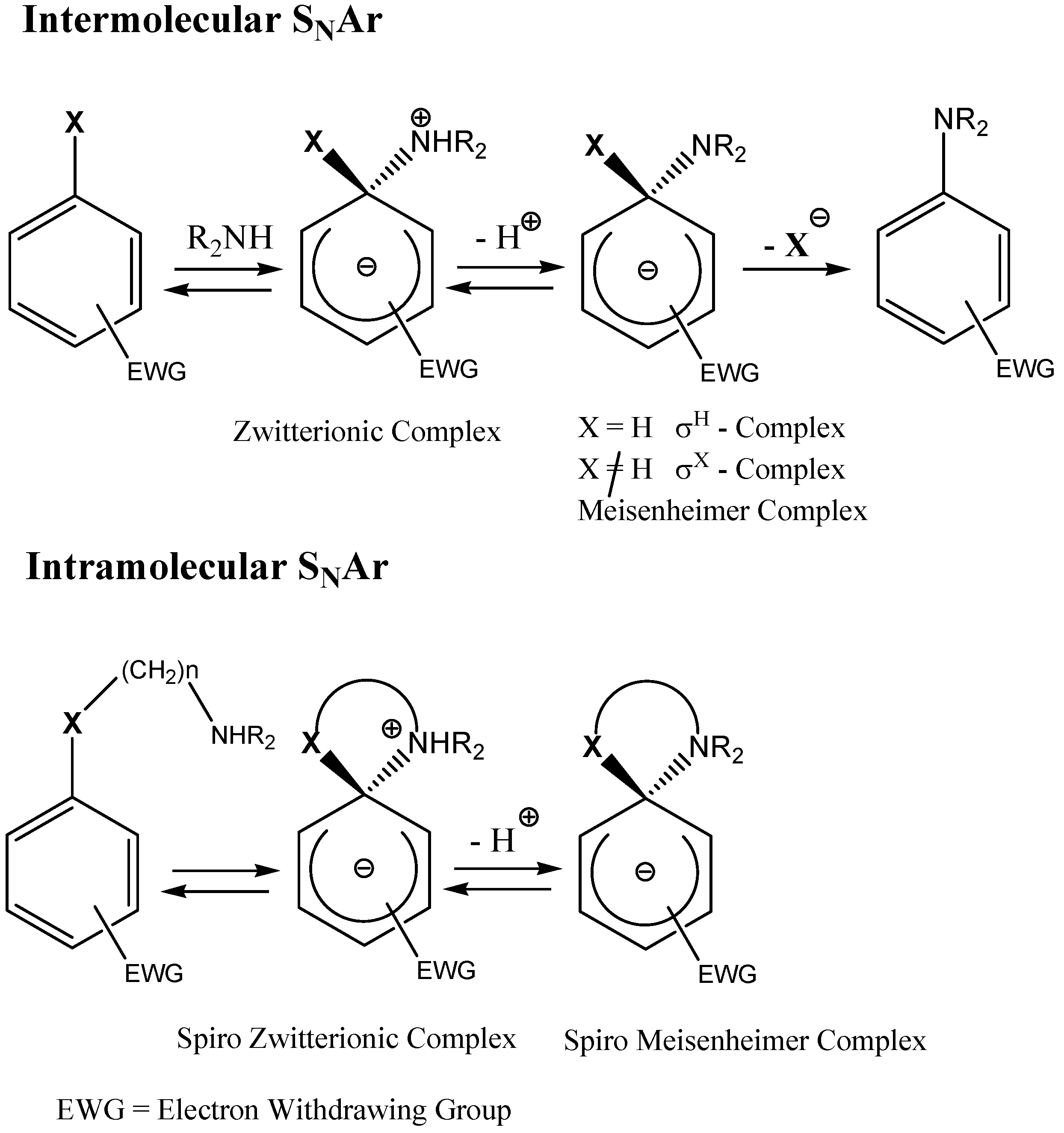
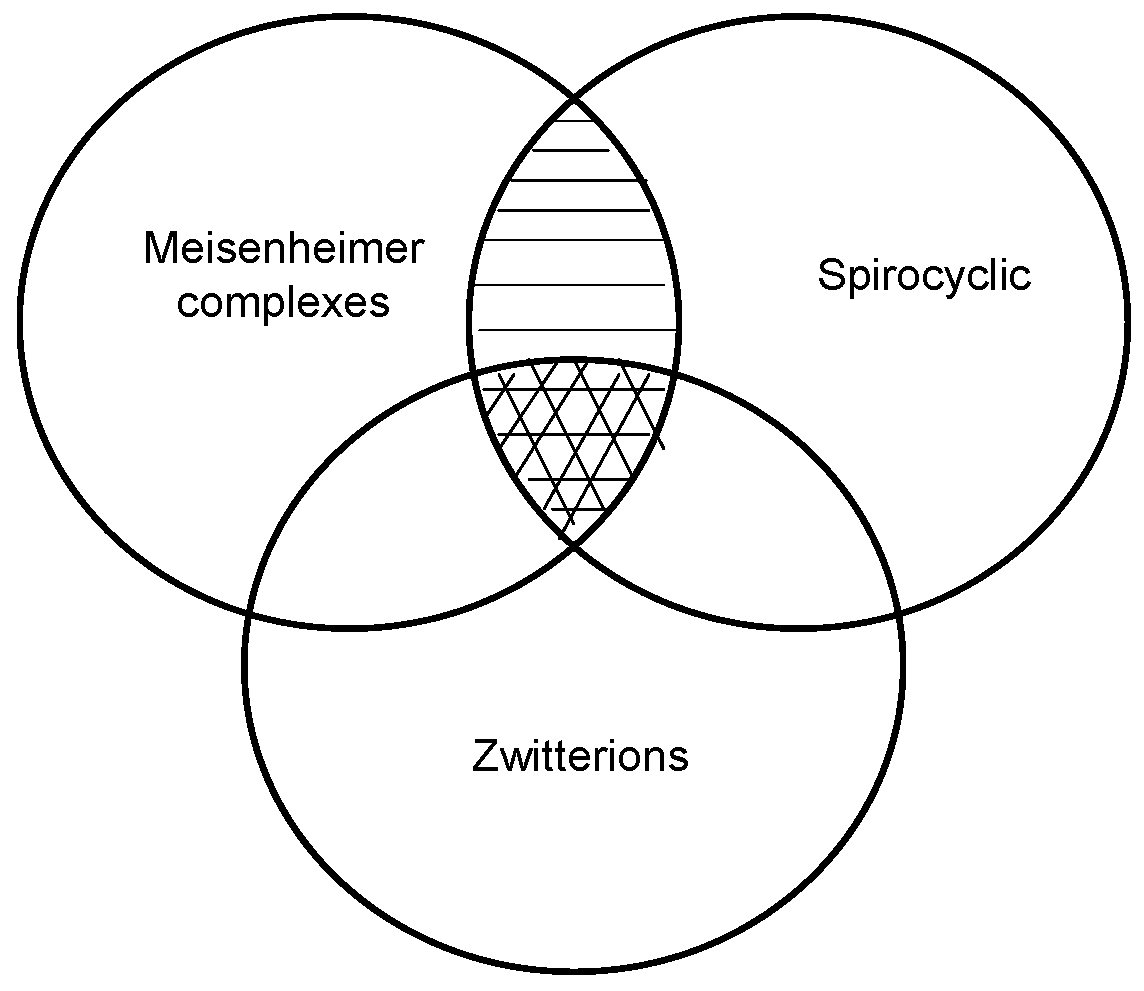
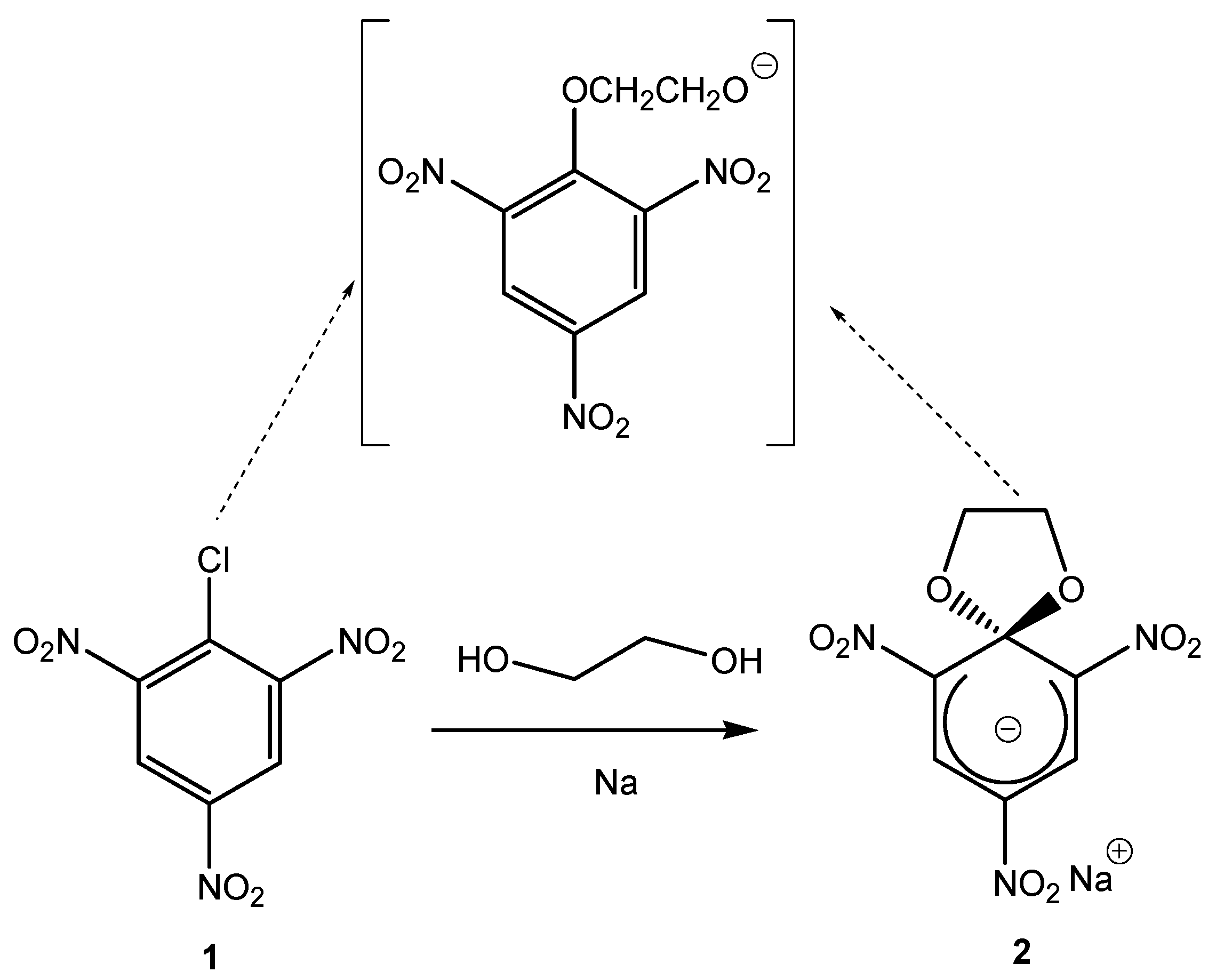
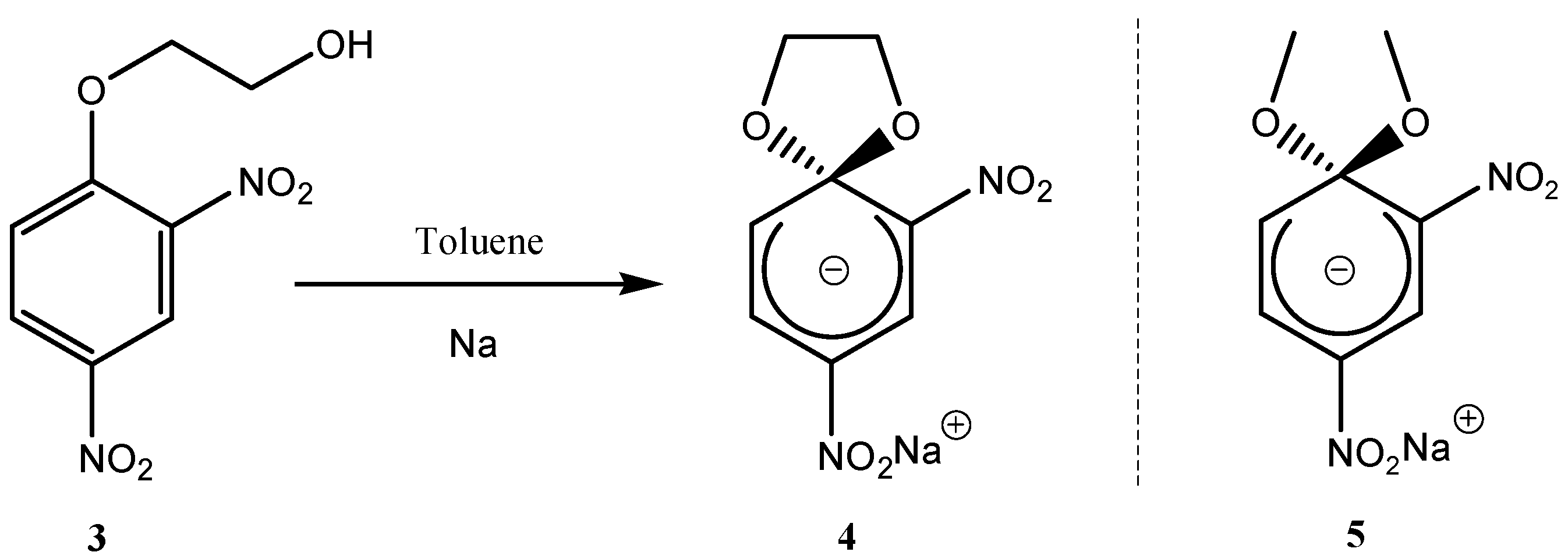
2. a. Spirocyclic Meisenheimer Complexes (SMCs)
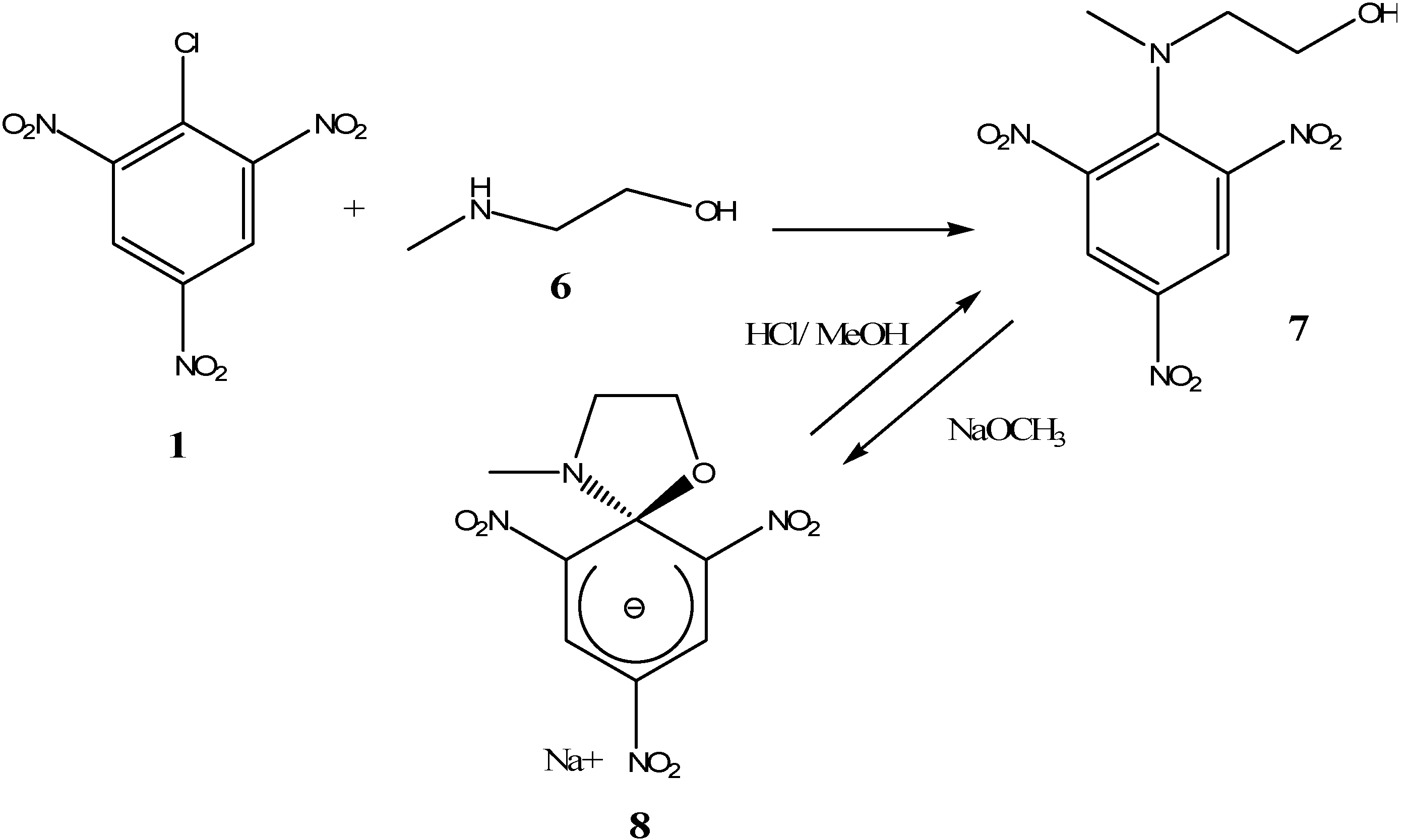


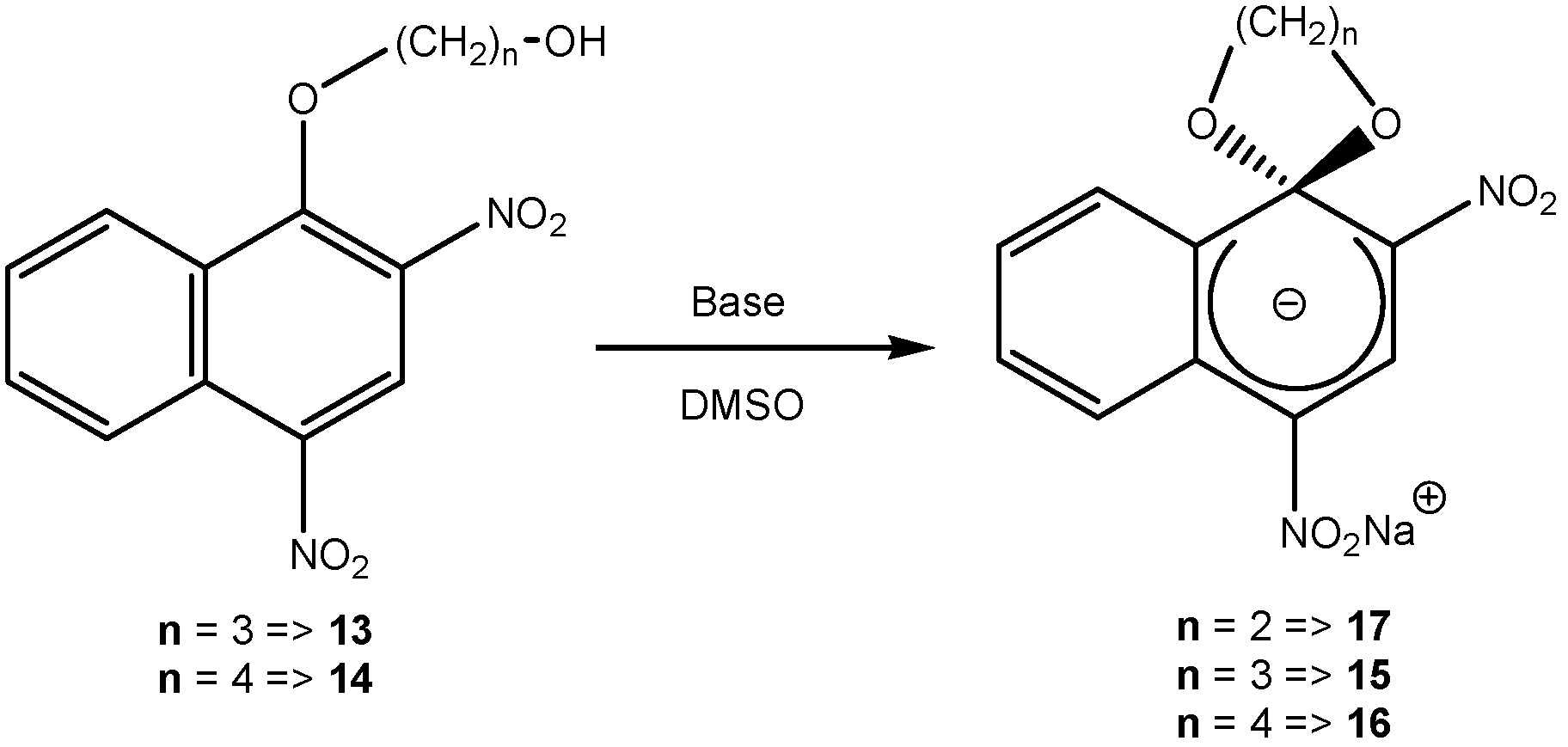

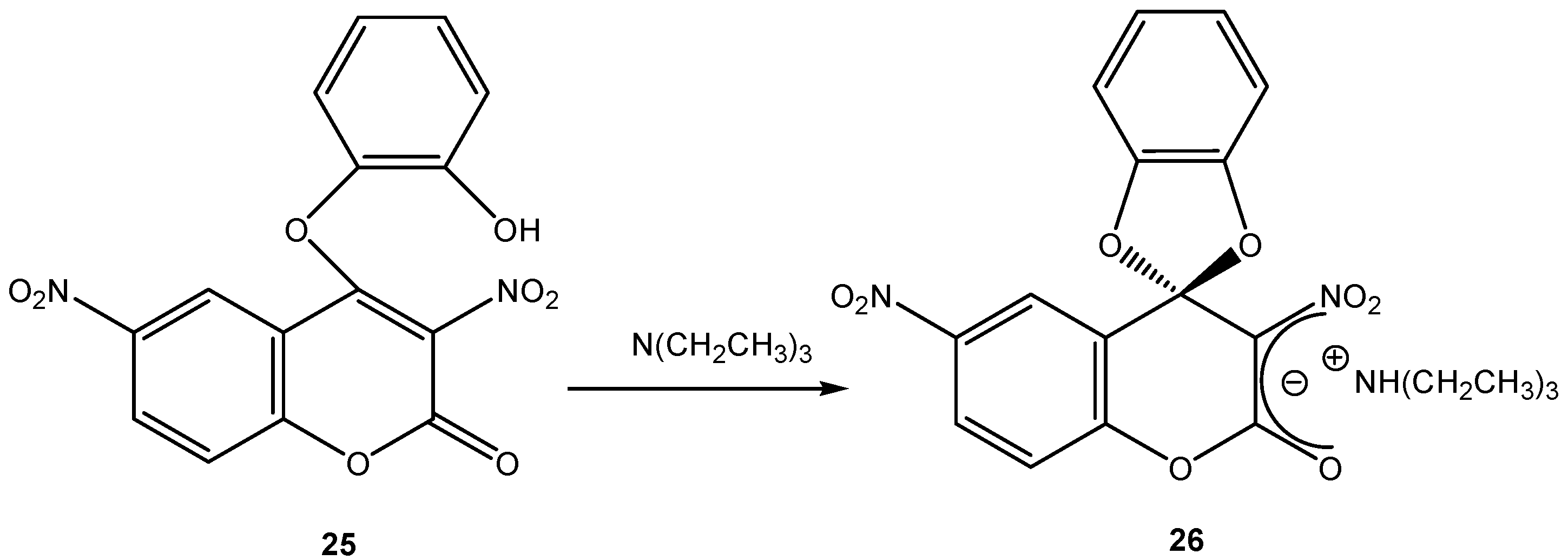
2. b. Russian contributions to SMCs
| SMC | Reactants | Conditions | Reference |
|---|---|---|---|
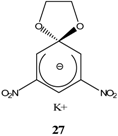 | 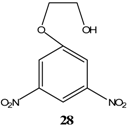 | KOCH3 / MeOH | [26] |
 |  | KOCH3 / MeOH | [27] |
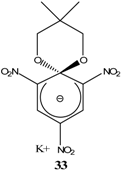
 |  | KOC(CH3)3/ t-butanol | [28] |
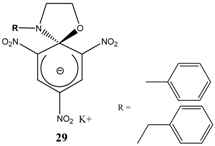
 | 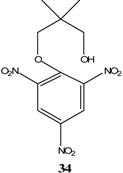 | KOC(CH3)3/ t-butanol | [29] |
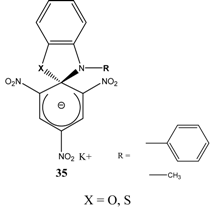 | 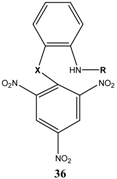 | KOC(CH3)3/ t-butanol | [30] |
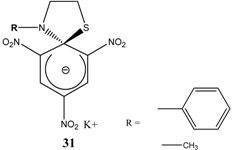
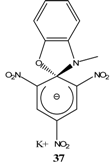 |  | KOC(CH3)3/ t-butanol | [30, 31] |
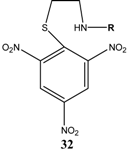
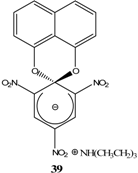 |  | Et3N/ CH2Cl2 | [32] |
 |  | Et3N/ CH2Cl2 | [37] |
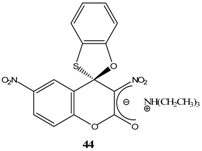 |  | Et3N/ CH2Cl2 | [48] |
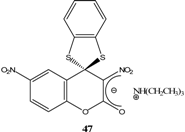 | 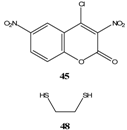 | Et3N/ CH2Cl2 | [49] |
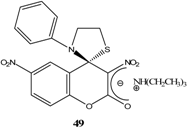 |  | Et3N/ CH2Cl2 | [53] |
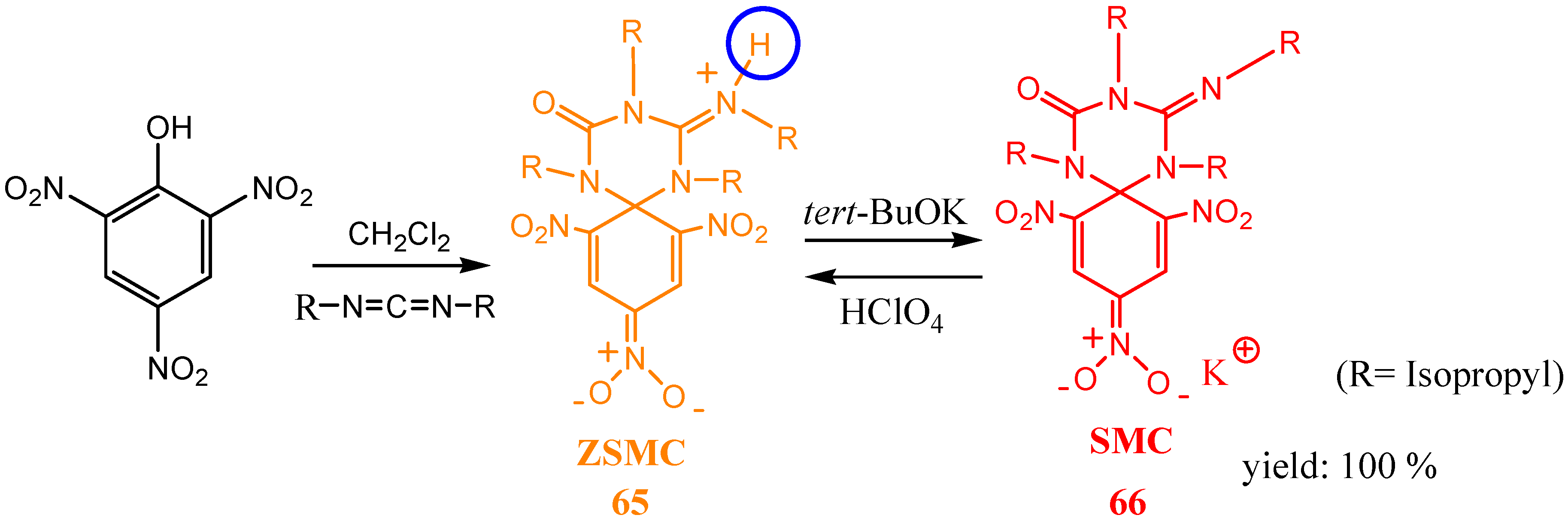
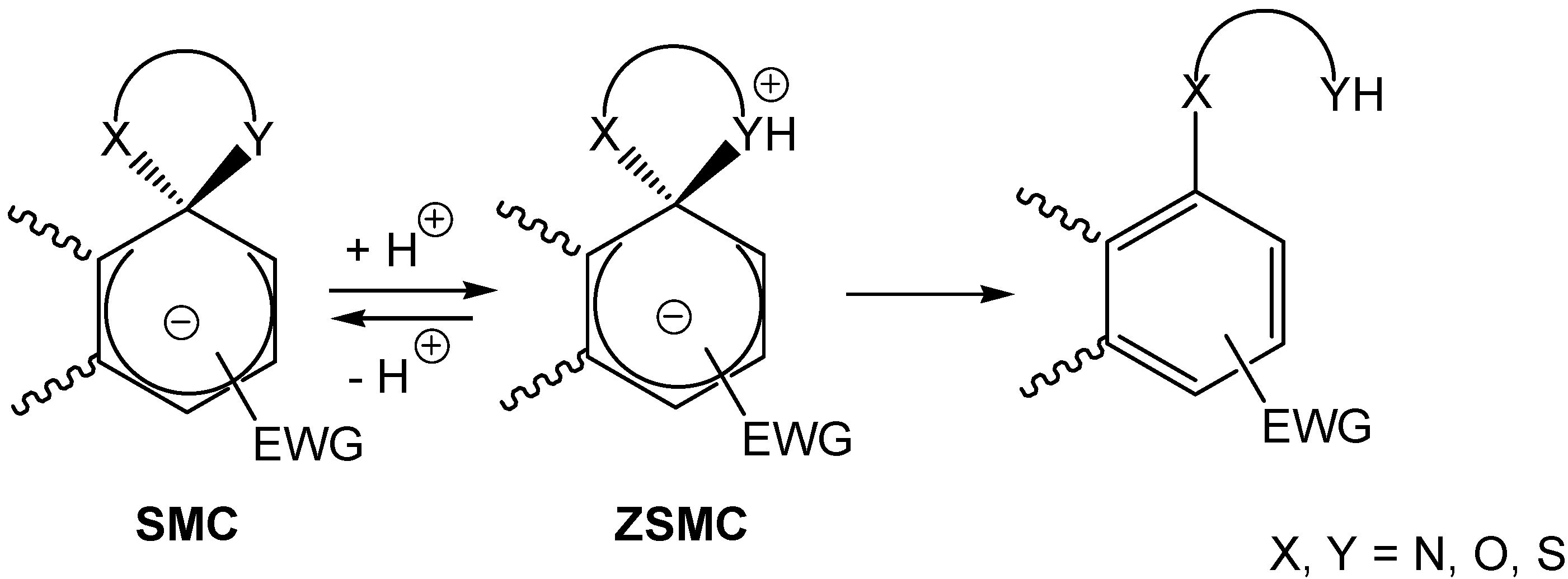
3. Zwitterionic Spirocyclic Meisenheimer Complexes (ZSMCs)
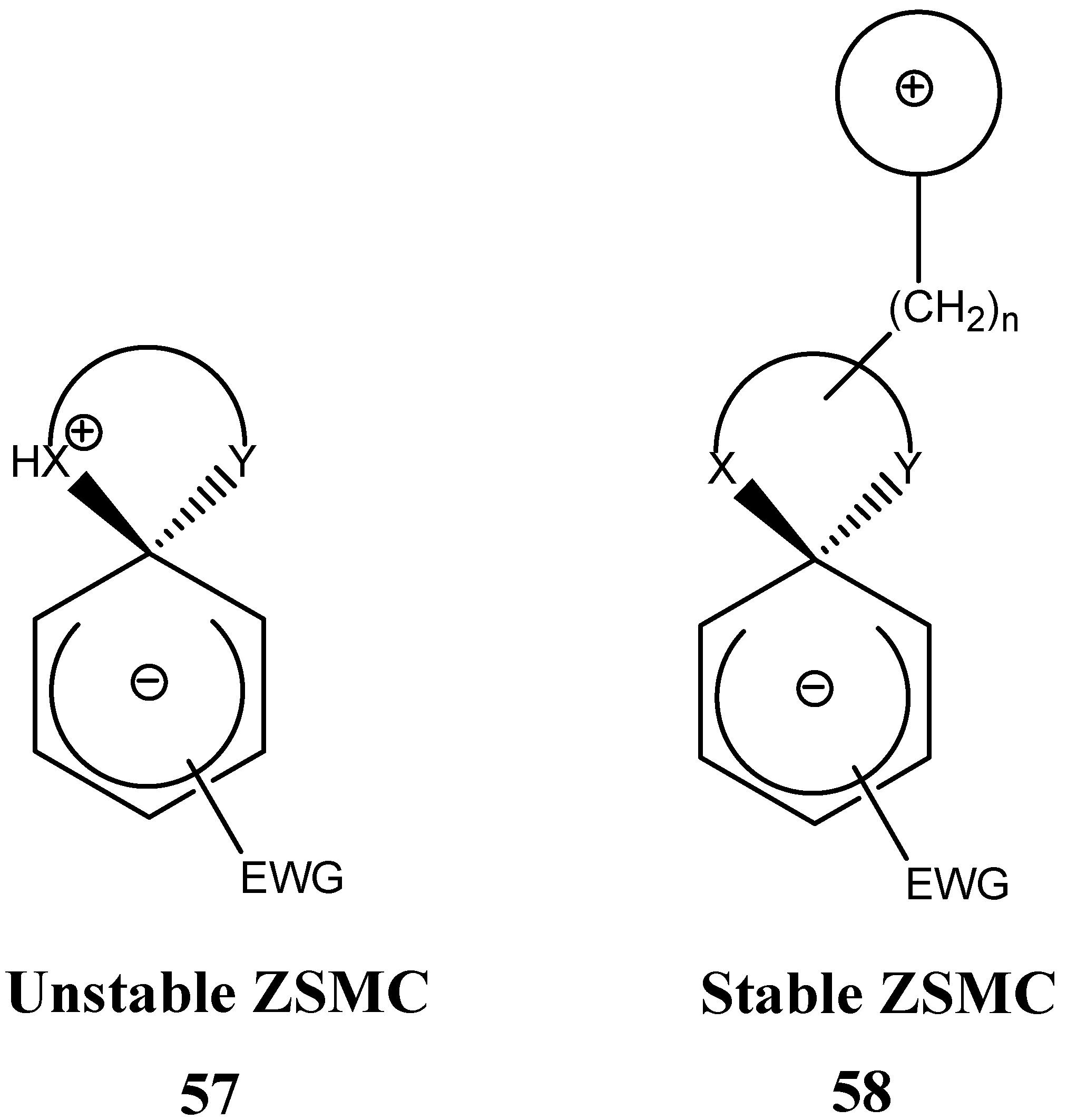
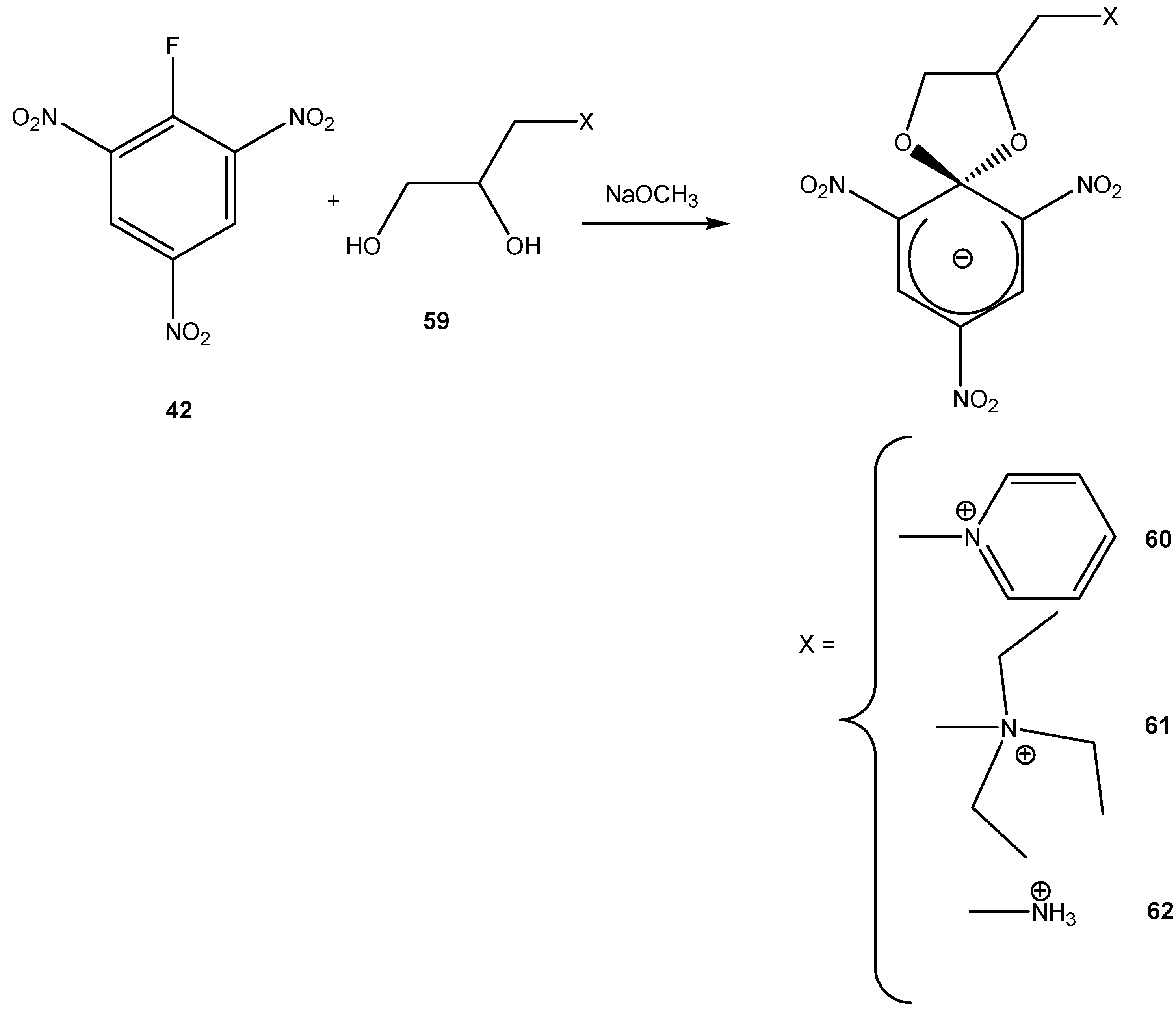
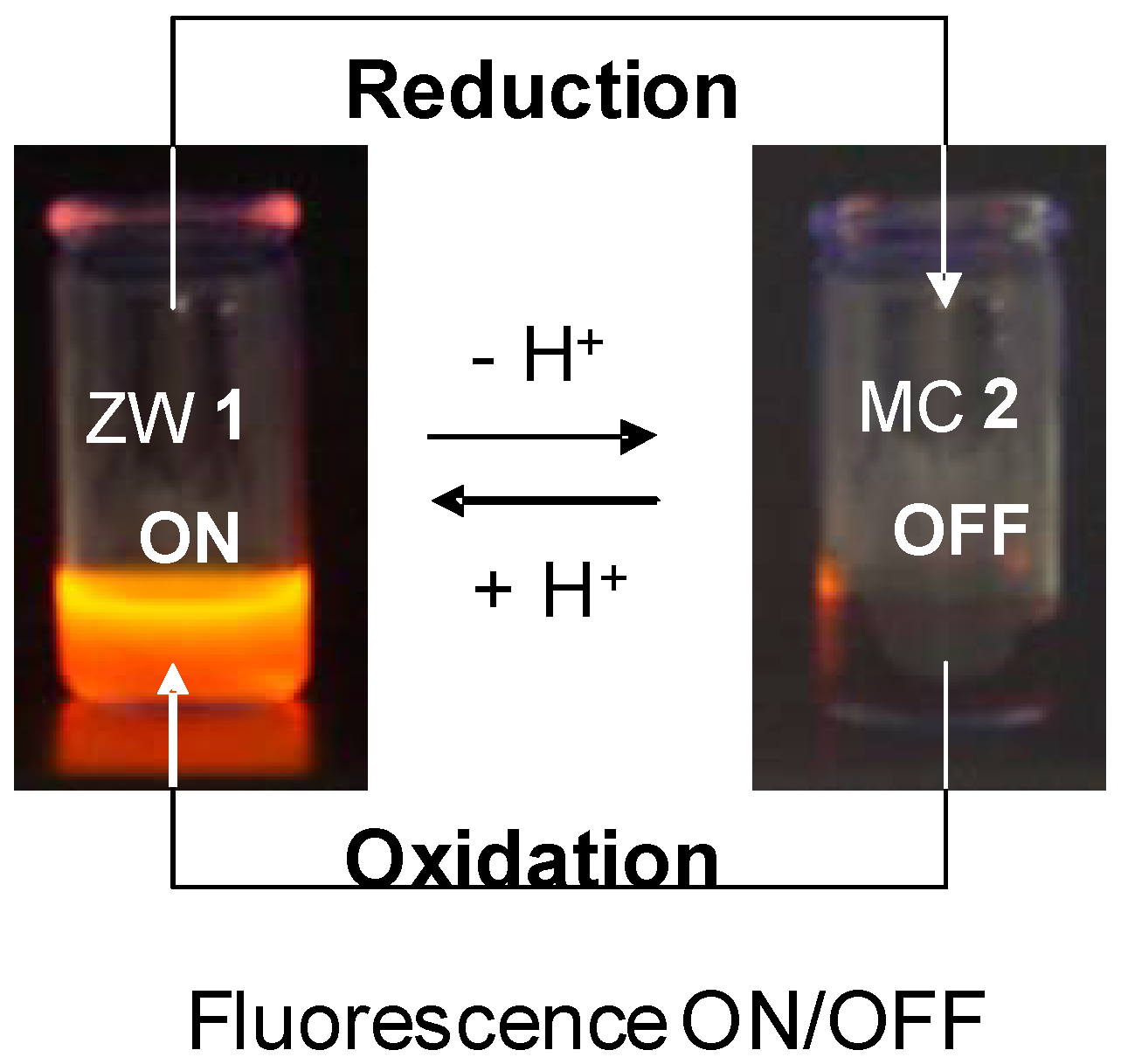
4. Fluorescent ZSMCs

5. Conclusions
Acknowledgements
References and Notes
- Bunnett, J. F.; Zahler, R. E. Aromatic Nucleophilic Substitution Reactions. Chem. Rev. 1951, 49, 273–412. [Google Scholar] [CrossRef]
- Jackson, C. J.; Gazzolo, F. H. Certain coloured substances derived from nitro-compounds. Amer. Chem. J. 1900, 23, 376. [Google Scholar]
- Meisenheimer, J. Reactions of aromatic nitro structures. Justus Liebigs Ann. Chem. 1902, 205. [Google Scholar] [CrossRef]
- Foster, R.; Fyfe, C. A. Meisenheimer and related compounds. Rev. Pure Appl. Chem. 1966, 16, 61. [Google Scholar]
- Buncel, E.; Norris, A. R.; Russell, K. E. The interaction of aromatic nitro compounds with bases. Quart. Rev. Chem. Soc. 1968, 22, 123. [Google Scholar] [CrossRef]
- Buck, P. Reactions of Aromatic Nitro Compounds with Bases. Angew. Chem., Int. Ed. 1969, 8, 120. [Google Scholar] [CrossRef]
- Crampton, M. R. Meisenheimer Complexes. Advan. Phys. Org. Chem. 1969, 7, 211. [Google Scholar]
- Artamkina, G. A.; Egorov, M. P.; Beletskaya, I. P. Some aspects of anionic sigma-complexes. Chem. Rev. 1982, 82, 427–459. [Google Scholar] [CrossRef]
- Strauss, M. J. Anionic sigma complexes. Chem. Rev. 1970, 70, 667–712. [Google Scholar] [CrossRef]
- Morris, J. W. Spiro Meisenheimer compounds. Rec. Trav. Chim. Pays-Bas 1965, 84, 516–20. [Google Scholar]
- Griffin, C. E.; Fendier, E. J.; Byrne, W. E.; Fendler, J. H. Intermediates in nucleophilic aromatic substitution. Part II. Spiro meisenheimer complexes derived from 1-(β-hydroxyethoxy)-2,4-dinitroarenes. Tetrahedron Lett. 1967, 8, 4473–6. [Google Scholar] [CrossRef]
- Fendler, E. J.; Fendler, J. H.; Byrne, W. E.; Griffin, C. E. Intermediates in nucleophilic aromatic substitution. IV. Structures and stabilities of spiro Meisenheimer complexes of dinitro-substituted arenes. J. Org. Chem. 1968, 33, 4141–5. [Google Scholar] [CrossRef]
- Crampton, M. R. The stabilities of Meisenheimer complexes. Part VI. Spiro-complexes. J. Chem. Soc., Perkin 2 1973, 15, 2157–62. [Google Scholar] [CrossRef]
- Sekiguchi, S.; Shiojima, T. Aromatic Nulceophilic Substitution. I. Preparation of Spiro Meisenheimer Complexes of Arenes. Bull. Chem. Soc. Japan 1973, 46, 693–4. [Google Scholar] [CrossRef] [Green Version]
- Sekiguchi, S.; Itagaki, T.; Hirose, T.; Matsui, K. Aromatic nucleophilic substitution-II: Intermediates in the reactions of 2,4-dinitro- or 2,4,5-trinitro-1-naphthyl ethyl ether with secondary amines and preparation of a spiro meisenheimer complex. Tetrahedron 1973, 29, 3527–31. [Google Scholar] [CrossRef]
- Farina, E.; Veracini, C. A.; Pietra, F. A stable thio-analogue of a Meisenheimer complex via a spiroannelation route: requirements for annelating chain length. J. Chem. Soc., Chem. Commun. 1974, 16, 672–3. [Google Scholar] [CrossRef]
- Crampton, M. R.; Willison, M. J. The stabilities of Meisenheimer complexes. Part XIII. Kinetic and equilibrium data for spiro-complex formation from 1-(2-mercaptoethylthio)- and 1-(2-hydroxyethylthio)-2,4,6-trinitrobenzene in water. J. Chem. Soc., Perkin 2 1976, 901–6. [Google Scholar] [CrossRef]
- Crampton, M. R.; Willison, M. J. The stabilities of Meisenheimer complexes. Part XI. The effects of ring-size of spiro-complex formation. J. Chem. Soc., Perkin 2 1976, 155–60. [Google Scholar]
- Bernasconi, C. F.; Gandler, J. R. Intermediates in nucleophilic aromatic substitution. 17. Kinetics of spiro Meisenheimer complexes. Effect of ring size. J. Org. Chem. 1977, 42, 3387–93. [Google Scholar] [CrossRef]
- Bernasconi, C. F.; Fairchild, D. E. Intermediates in nucleophilic aromatic substitution. Part 23. Kinetics of spiro meisenheimer complex formation from 3,6-dimethylcatechol 2,4,6-trinitrophenyl ether. Competition between a trapping and a preassociation mechanism. J. Am. Chem. Soc. 1988, 110, 5498–505. [Google Scholar] [CrossRef]
- Bernasconi, C. F.; Howard, K. A. Intermediates in nucleophilic aromatic substitution. 22. Kinetics of acid-catalyzed breakdown of spiro Meisenheimer complexes. J. Am. Chem. Soc. 1983, 105, 4690–7. [Google Scholar] [CrossRef]
- Bernasconi, C. F.; Howard, K. A. Intermediates in nucleophilic aromatic substitution. 21. Stereoelectronic and conformational effects in Meisenheimer complexes. Intrinsic reactivities of spiro vs. 1,1-dimethoxy and 1-methoxy-1-phenoxy complexes. J. Am. Chem. Soc. 1982, 104, 7248–57. [Google Scholar] [CrossRef]
- Ah-Kow, G.; Terrier, F. Spiro Meisenheimer complexes from 7-(2-hydroxyethoxy)-4-nitrobenzofurazan and 7-(2-hydroxyethoxy)-4-nitrobenzofuroxan. A kinetic study in aqueous solution. J. Org. Chem. 1978, 43, 3578–84. [Google Scholar] [CrossRef]
- Noruis, W. P.; Spear, R. J.; Read, R. W. Explosive Meisenheimer Complexes Formed by Addition of Nucleophilic Reagents to 4,6-Dinitrobenzofurazan 1-Oxide. Aust. J. Chem. 1983, 36, 297–309. [Google Scholar] [CrossRef]
- 25 Drozd, V. N.; Knyazev, V. N.; Nam, N. L.; Yufit, D. S.; Struchkov, Y. T.; Stankevich, I. V.; Chistyakov, A. L.; Lezina, V. P.; Mozhaeva, T. Ya.; Savel'ev, V. L. Anionic spirocyclization of 3-nitro-4-(2-hydroxyphenoxy)-2H-1-benzopyran-2-one: formation of stabilized Meisenheimer type salts. Tetrahedron 1992, 48, 469–80. [Google Scholar] [CrossRef]
- Knyazev, V. N.; Drozd, V. N.; Klimov, A. A. Spirocyclic Meisenheimer complexes. V. Intramolecular Meisenheimer 1,2-complex from 1-(β-hydroxyethoxy)-3,5-dinitrobenzene. Zhur. Org. Khim. 1976, 12, 2387–92. [Google Scholar]
- Drozd, V. N.; Knyazev, V. N.; Minov, V. M. Spirocyclic Meisenheimer complexes. VII. Spirocyclic Meisenheimer complexes with the oxazolidine ring. Zhur. Org. Khim. 1977, 13, 396–402. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N.; Minov, V. M.; Akimova, N. P. Spirocyclic Meisenheimer complexes. VIII. Reversible Smiles rearrangement during the conversion of trinitrocyclohexadienate spiro complexes with thiazolidine rings to 1,4-benzothiazines. Zhur. Org. Khim. 1977, 13, 1255–62. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N.; Minov, V. M. Spirocyclic Meisenheimer complexes. IX. Intramolecular nucleophilic cyclization of 1-(γ-hydroxypropylthio)-2,4,6-trinitrobenzene and 1-(γ-hydroxy-β,β-dimethylpropoxy)-2,4,6-trinitrobenzene. Zhur. Org. Khim. 1978, 14, 105–10. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N.; Mozhaeva, T. Ya. Spirocyclic Meisenheimer complexes. Trinitrocyclohexadienate spiro complexes with benzothiazolidine and benzoxazolidine rings. Zhur. Org. Khim. 1979, 15, 1107. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N.; Mozhaeva, T. Ya. Spirocyclic Meisenheimer complexes. XIII. Intramolecular nucleophilic substitution of the nitro group in the cyclization of some o-hydroxyphenylpicramides to phenoxazines. Zhur. Org. Khim. 1980, 16, 876–82. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N.; Mozhaeva, T. Ya. Spirocyclic Meisenheimer complexes. Stable trinitrocyclohexadienate spiro complex with a naphtho[1,8-de]-m-dioxin ring. Zhur. Org. Khim. 1980, 16, 2012–13. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N.; Mozhaeva, T. Ya. Spirocyclic Meisenheimer complexes. XV. Formation of 2,3-dihydro-1,4-benzothiazines from the intramolecular nucleophilic cyclization of 1-(β-N-R-aminoethylthio)-2,4- and -2,6-dinitrobenzenes. Zhur. Org. Khim. 1981, 17, 2376–83. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N. Spirocyclic Meisenheimer complexes and problems of intramolecular aromatic nucleophilic substitution. Ser. Khim. Nauk 1983, 4, 43–50. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N. Spirocyclic Meisenheimer complexes. XVIII. Intramolecular 1,2-σ-complexes from 2-(3,5-dinitrophenylthio)ethanol and its S,S-dioxide. Zhur. Org. Khim. 1985, 21, 2590–4. [Google Scholar]
- Knyazev, V. N.; Lipovtsev, V. N.; Belostotskaya, I. S.; Vol'eva, V. B.; Drozd, V. N.; Ershov, V. V. Spirocyclization of Meisenheimer complexes. XIX. Steric stabilization of trinitrocyclohexadienate Meisenheimercomplexes containing a 1,3-benzodioxole ring by tert-butyl groups. Zhur. Org. Khim. 1986, 22, 1951–5. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N.; Lipovtsev, V. N.; Kurapov, P. B. Spirocyclic Meisenheimer complexes. XX. Steric and stereoelectronic effects of 4- and 5-alkyl groups in the 1,3-dioxolane ring on the stability of spirocyclic Meisenheimer complexes. Zhur. Org. Khim. 1987, 23, 1283–91. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N.; Lipovtsev, V. N.; Patalakha, N. S. Spirocyclic Meisenheimer complexes. XXII. Effect of the 4-substituent of the 1,3-dioxolane ring in trinitrocyclohexadienate spiro Meisenheimer complexes on their relative stability and regioselectivity of their decomposition in acidic media. Zhur. Org. Khim. 1988, 24, 2183–93. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N.; Lipovtsev, V. N.; Kurapov, P. B.; Yufit, D. S.; Struchkov, Yu. T. Spirocyclic Meisenheimer complexes. XXI. Stereoisomeric 2',4',6'-trinitrocyclohexadionate spiro Meisenheimer complexes containing a 4,5-dimethoxycarbonyl-1,3-dioxolane ring. Zhur. Org. Khim. 1988, 24, 2174–82. [Google Scholar]
- Knyazev, V. N. Spirocyclic Meisenheimer complexes. XXIII. Reversible double Smiles rearrangement in the 3-(methylpicrylamino)-1,2-propanediol - 1-(methylamino)-3-picryloxy-2-propanol system with the intermediacy of two tautomeric spiro Meisenheimer complexes. Zhur. Org. Khim. 1989, 25, 2176–81. [Google Scholar]
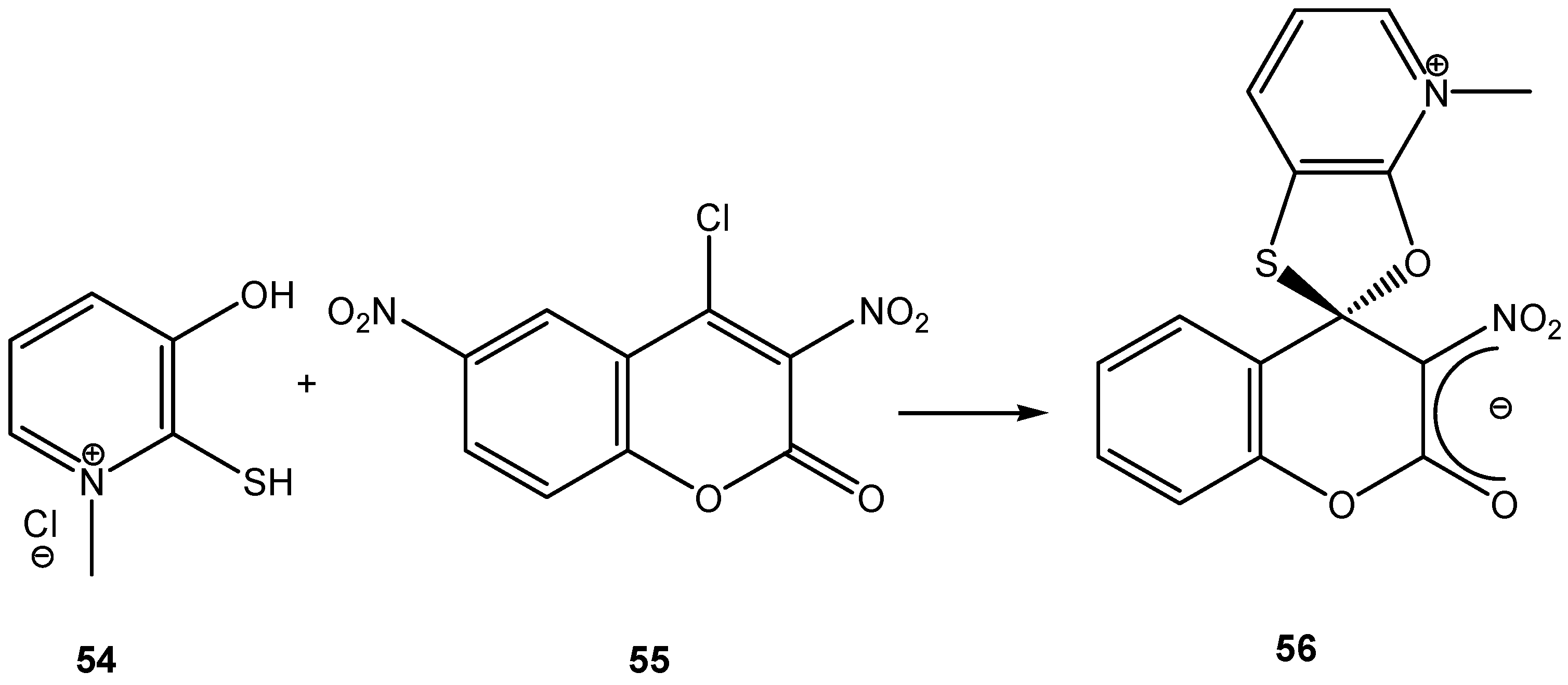
- Drozd, V. N.; Knyazev, V. N.; Mozhaeva, T. Ya.; Savel'ev, V. L.; Lezina, V. P. Spirocyclic Meisenheimer complexes. XXIV. Formation of spiro Meisenheimer complexes with 1,3-dithiolane and 1,3-oxathiolane rings in a 3-nitrocoumarin system. Zhur. Org. Khim. 1991, 27, 175–84. [Google Scholar]
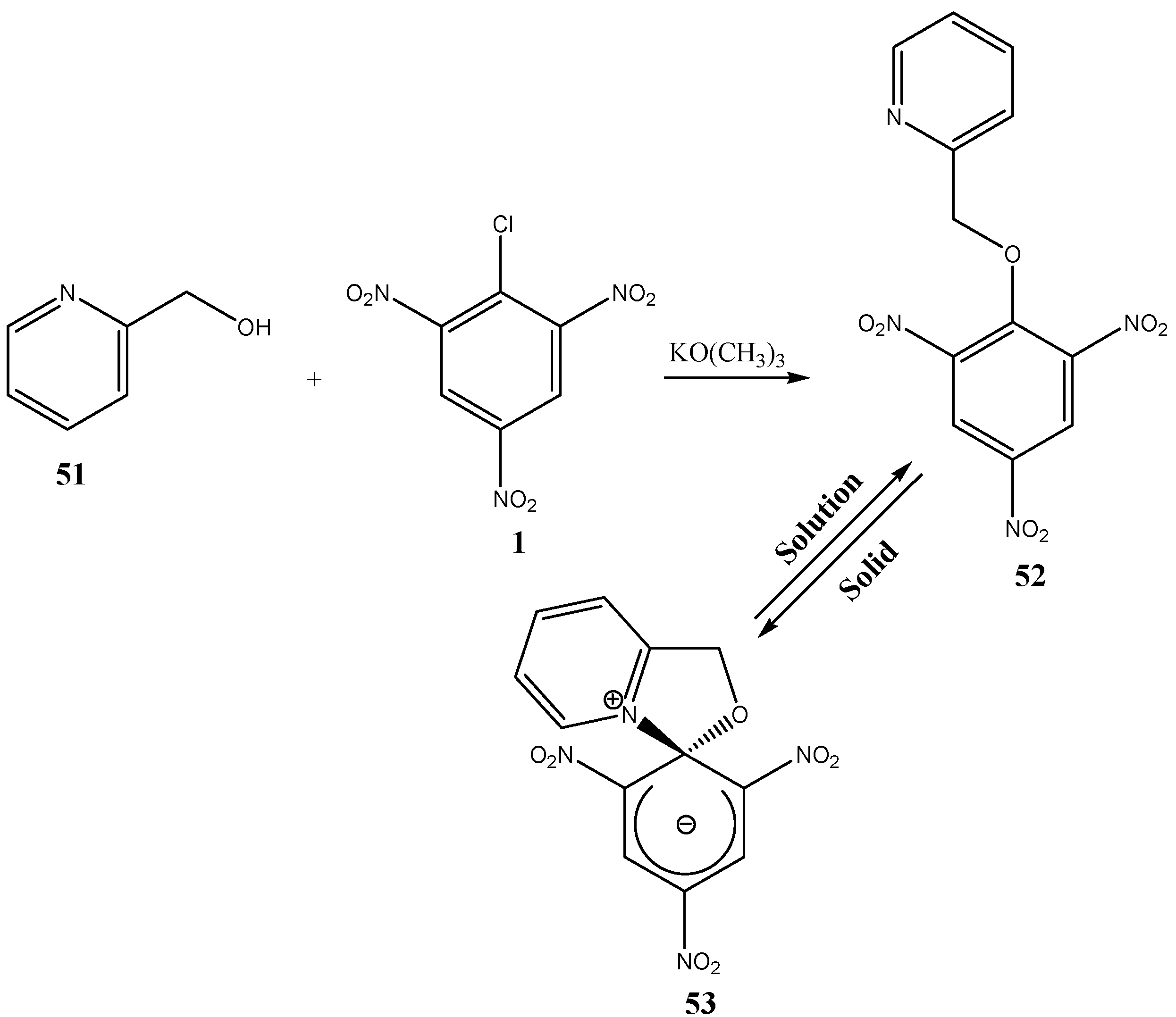
- Knyazev, V. N.; Drozd, V. N.; Patalakha, N. S.; Yufit, D. S.; Struchkov, Yu. T. Spirocyclic Meisenheimer complexes. XXVI. Structure and tautomerism of picryl derivatives of 2-(hydroxymethyl)pyridine and 8-hydroxyquinoline. Zhur. Org. Khim. 1991, 27, 192–200. [Google Scholar]
- Mozhaeva, T. Ya.; Lezina, V. P.; Savel'ev, V. L.; Knyazev, V. N.; Drozd, V. N. Spirocyclic Meisenheimer complexes. XXV. Effect of ring size on intramolecular nucleophilic cyclization processes during reaction of dithiols with 3-nitro-4-chlorocoumarins. Zhur. Org. Khim. 1991, 27, 185–91. [Google Scholar]
- Knyazev, V. N.; Mozhaeva, T. Ya.; Lezina, V. P.; Savel'ev, V. L.; Drozd, V. N. Spirocyclic Meisenheimer complexes. XXVII. Formation of anionic spiro Meisenheimer-type complexes with a 1,3-dioxolane ring in the 3-nitrocoumarin system. New route to 4-alkoxy-3-nitrocoumarins. Zhur. Org. Khim. 1991, 27, 1727–33. [Google Scholar]
- Mozhaeva, T. Ya.; Lezina, V. P.; Savel'ev, V. L.; Knyazev, V. N.; Drozd, V. N. Spirocyclic Meisenheimer complexes. XXVIII. Ratio of open-chain and cyclic structures in the 3-nitro-4-γ-hydroxypropoxycoumarin-anionic spiro complex system. Zhur. Org. Khim. 1991, 27, 1733–8. [Google Scholar]
- Khilkova, N. L.; Knyazev, V. N.; Patalokha, N. S.; Drozd, V. N. Spirocyclic Meisenheimer complexes. XXXII. Anionic spiro-σ-complexes with an oxazolidine ring in the 5,7-dinitroquinoline system. Simple and double Smiles rearrangements. Zhur. Org. Khim. 1992, 28, 1732–41. [Google Scholar]
- Mozhaeva, T. Ya.; Lezina, V. P.; Savel'ev, V. L.; Nam, N. L.; Knyazev, V. N.; Drozd, V. N. Spirocyclic Meisenheimer complexes. XXX. Formation of anionic spiro σ complexes from 6-substituted 4-[(2-hydroxyphenyl)thio]-3-nitro-2H-1-benzopyran-2-ones and intramolecular ortho-cyclization accompanied by a Smiles rearrangement. Zhur. Org. Khim. 1992, 28, 1489–95. [Google Scholar]
- Drozd, V. N.; Nam, N. L.; Knyazev, V. N.; Yufit, D. S.; Struchkov, Yu. T.; Mozhaeva, T. Ya.; Lezina, V. P.; Savel'ev, V. L. Spirocyclic Meisenheimer complexes. XXXI. Reaction of 4-chloro-3-nitro-2H-1-benzopyran-2-ones with 1,3-propane- and 1,4-butanedithiols. Effect of chain length on the structure of the condensation products and their reactivity. Zhur. Org. Khim. 1992, 28, 1496–507. [Google Scholar]
- Mozhaeva, T. Ya.; Nam, N. L.; Lezina, V. P.; Savel'eva, V. L.; Knyazev, V. N.; Drozd, V. N. Spirocyclic Meisenheimer complexes. XXXIII. Formation of anionic spiro complexes with oxazolidine and benzoxazoline rings in a 3-nitro-2H-1-benzopyran-2-one system. Zhur. Org. Khim. 1992, 28), 2127–33. [Google Scholar]
- Drozd, V. N.; Knyazev, V. N.; Nam, N. L.; Lezina, V. P.; Mozhaeva, T. Ya.; Savel'ev, V. L. Spirocyclic Meisenheimer complexes. XXXV. Possibility of anionic spiro-σ-complexes in the 3-nitro-2(1H)-quinolinone system. Zhur. Org. Khim. 1993, 29, 782–8. [Google Scholar]
- Drozd, V. N.; Knyazev, V. N.; Khilkova, N. L.; Yufit, D. S.; Struchkov, Yu. T.; Stankevich, I. V.; Chistyakov, A. L. Spirocyclic Meisenheimer complexes. XXXIV. Anionic spiro-σ-complexes with 1,3-dithiolane and 1,3-oxathiolane rings in the 5,7-dinitroquinoline system. Study of structure and charge distribution. Zhur. Org. Khim. 1993, 29, 770–81. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N. Spirocyclic Meisenheimer complexes. XXXVI. Possibility of formation of zwitterionic spiro-σ-complexes from polynitroaromatic derivatives of 3-hydroxy-2-pyridone, 8-hydroxyquinoline N-oxide, hydroxynaphtho- and -anthraquinones. Zhur. Org. Khim. 1994, 30, 94–100. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N. Spirocyclic Meisenheimer Complexes. XXXVIII. Formation of anionic spiro complexes with a 1,3-thiazolidine ring in the 3-nitro-2H-1-benzopyran-2-one system. Zhur. Org. Khim. 1996, 32, 777–780. [Google Scholar]
- Strauss, M. J.; Johanson, R. G. Isolation of a stable zwitterionic Meisenheimer complex. Chem. Ind. 1969, 8, 242–3. [Google Scholar]
- Bernasconi, C. F. Intermediates in nucleophilic aromatic substitution. III. Visible absorption spectra of the acid and basic form of the 1,3,5-trinitrobenzene-piperidine Meisenheimer complex in 10% dioxane-90% water. J. Org. Chem. 1970, 35, 1214–16. [Google Scholar] [CrossRef]
- Bernasconi, C. F.; Gehriger, C. L.; De Rossi, R. H. Intermediates in nucleophilic aromatic substitution. 16. Toward a complete characterization of the mechanism of nucleophilic aromatic substitution by an amine. Kinetics of spiro Meisenheimer complexes derived from N-methylethanolamine. J. Am. Chem. Soc. 1976, 98, 8451–9. [Google Scholar] [CrossRef]
- Bernasconi, C. F.; Muller, M. C.; Schmid, P. Intermediates in nucleophilic aromatic substitution. 20. Rate-limiting proton transfer in the formation of Meisenheimer complexes between 1,3,5-trinitrobenzene and amines. The effect of dimethyl sulfoxide on proton-transfer rates. Relative leaving-group abilities of amines and alkoxide ions. J. Org. Chem. 1979, 44, 3189–96. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N. Spirocyclic Meisenheimer complexes. XXXVI. Possibility of formation of zwitterionic spiro-σ-complexes from polynitroaromatic derivatives of 3-hydroxy-2-pyridone, 8-hydroxyquinoline N-oxide, hydroxynaphtho- and -anthraquinones. Zhur. Org. Khim. 1994, 30, 94–100. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N. Spirocyclic Meisenheimer complexes. XXXVII. Polynitro aromatic derivatives of 3-hydroxy-1-methylpyridine-2(1H)-thione. Zhur. Org. Khim. 1995, 31, 121–6. [Google Scholar]
- Knyazev, V. N.; Drozd, V. N. Spirocyclic Meisenheimer complexes. XXXIX. Stable zwitterionic Meisenheimer complexes with a positive charge localized on the group CH2X+ in position 4 of a 1,3-dioxolane ring spiro-fused with a 2,4,6-trinitrocyclohexadienide system. Zhur. Org. Khim. 1996, 32(9), 1394–1399. [Google Scholar]
- Borbulevych, O. Y..; Shishkin, O. V.; Knyazev, V. N. A zwitterionic Meisenheimer complex of 2,4,6-trinitrobenzene. Acta Crystallogr. C: Cryst. Struct. Commun. 1999, C55, 1704–1706. [Google Scholar] [CrossRef]
- Vowinkel, E. Reactions of phenols with dicyclohexylcarbodiimide. Chem. Ber. 1963, 96, 1702. [Google Scholar] [CrossRef]
- Vowinkel, E. Mechanism of the formation of aryl alkyl ethers by the carbodiimide method. Chem. Ber. 1966, 99, 42. [Google Scholar] [CrossRef]
- Bartos, J. Condensation products from dicyclohexylcarbodiimide and dibenzylcarbodiimide with picric acid. Bull. Soc. Chim. France 1965, 12, 3694. [Google Scholar]
- Hawtrey, A. O. The addition of 2,4,6-trinitrophenol to N,N-dicyclohexyl carbodiimide. Tetrahedron Lett. 1966, 49, 6103. [Google Scholar] [CrossRef]
- Bartos, J. F. CODEN: FRXXAK FR. 1968; 1520382, 19680412.
- a) It has been recently established that the compound formed is 64; these results will be published shortly; b) Al-Kaysi, R. O.; Gallardo, I.; Guirado, G. unpublished results. 2008.
- Batelaan, P. H.; Heerma, W.; Tadema, G. Proc. K. Ned. Acad. Wet. Ser. B. 1969, 72, 132.
- Al-Kaysi, R. O.; Guirado, G.; Valente, E. J. Synthesis and characterization of a new fluorescent zwitterionic spirocyclic Meisenheimer complex of 1,3,5-trinitrobenzene. Eur. J. Org. Chem. 2004, 16, 3408–3411. [Google Scholar] [CrossRef]
- Al-Kaysi, R. O.; Creed, D.; Valente, E. J. Meisenheimer complex from picric acid and diisopropylcarbodiimide. J. Chem. Crystallogr. 2004, 34, 685–692. [Google Scholar] [CrossRef]
- Al-Kaysi, R. O.; Bourdelande, J. L.; Gallardo, I.; Guirado, G.; Hernando, J. Investigation of an acid-base and redox molecular switch: from bulk to the single-molecule level. Chem.-Eur. J. 2007, 13, 7066–7074. [Google Scholar] [CrossRef]
- Gallardo, I.; Guirado, G. Electrochemical mechanism of spiro and zwitterionic Meisenheimer compounds: A potential fluorescence molecular switching system. Electrochem. Commun. 2007, 9, 173–179. [Google Scholar] [CrossRef]
- Al-Kaysi, R. O.; Mueller, A. M.; Ahn, T. S.; Lee, S.; Bardeen, C. J. Effects of Sonication on the Size and Crystallinity of Stable Zwitterionic Organic Nanoparticles Formed by Reprecipitation in Water. Langmuir 2005, 21, 7990–7994. [Google Scholar] [CrossRef]
- Al-Kaysi, R. O.; Ahn, T. S.; Mueller, A. M.; Bardeen, C. J. The photophysical properties of chromophores at high (100 mM and above) concentrations in polymers and as neat solids. Phys. Chem. Chem. Phys. 2006, 8, 3453–3459. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds (63-65) are available from the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Kaysi, R.O.; Gallardo, I.; Guirado, G. Stable Spirocyclic Meisenheimer Complexes. Molecules 2008, 13, 1282-1302. https://doi.org/10.3390/molecules13061282
Al-Kaysi RO, Gallardo I, Guirado G. Stable Spirocyclic Meisenheimer Complexes. Molecules. 2008; 13(6):1282-1302. https://doi.org/10.3390/molecules13061282
Chicago/Turabian StyleAl-Kaysi, Rabih O., Iluminada Gallardo, and Gonzalo Guirado. 2008. "Stable Spirocyclic Meisenheimer Complexes" Molecules 13, no. 6: 1282-1302. https://doi.org/10.3390/molecules13061282