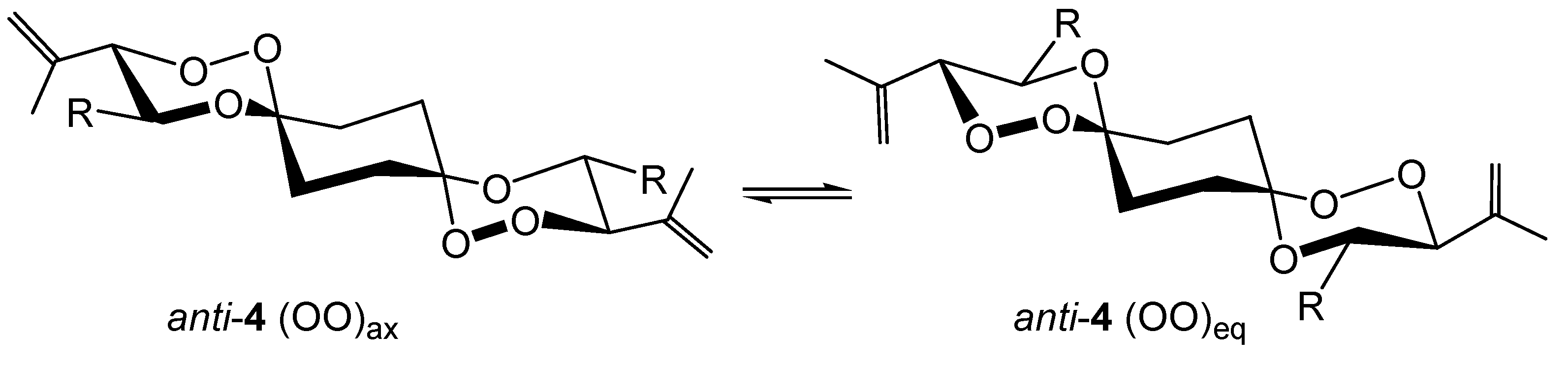Results and Discussion
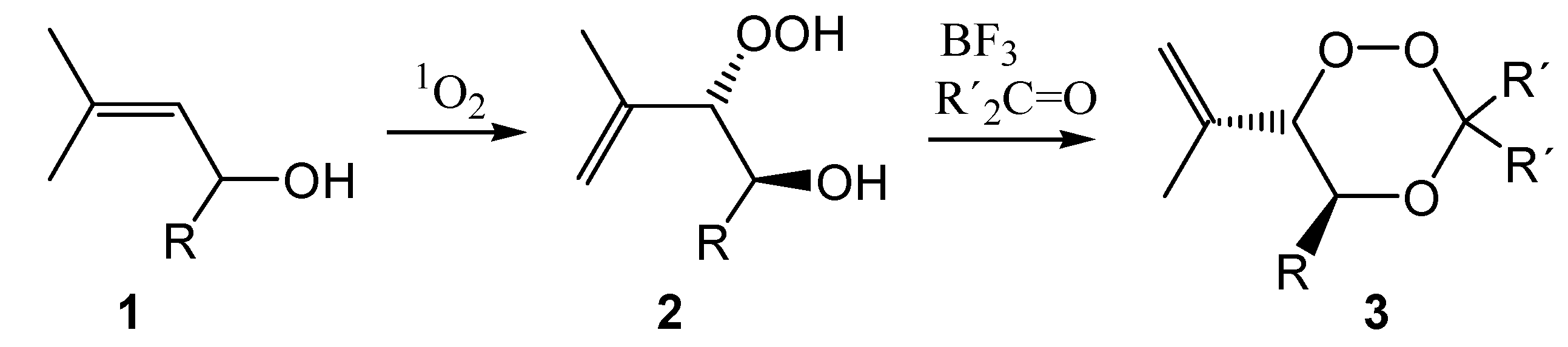
The synthesis of the allylic hydroperoxides
2 from the allylic alcohols
1 proceeded according to literature procedures published by us for solid state photooxygenation [
15]. This reaction protocol is advantageous because of the sensitizer-free isolation of the product from the polymer network. The conversion of allylic hydroperoxides into 1,2,4-trioxanes
3 requires Lewis-acid catalysis in the presence of the target carbonyl compound. We have carefully optimized the reaction conditions and in most cases applied the carbonyl compound in excess in order to drive the reaction to completion. In cases where this approach was not possible (e.g. carbonyl compounds with high molecular weight or non-separable substrates) equimolar amounts were used, which drastically reduced the yields of the trioxane products because of Lewis-acid catalyzed hydroperoxide cleavage.
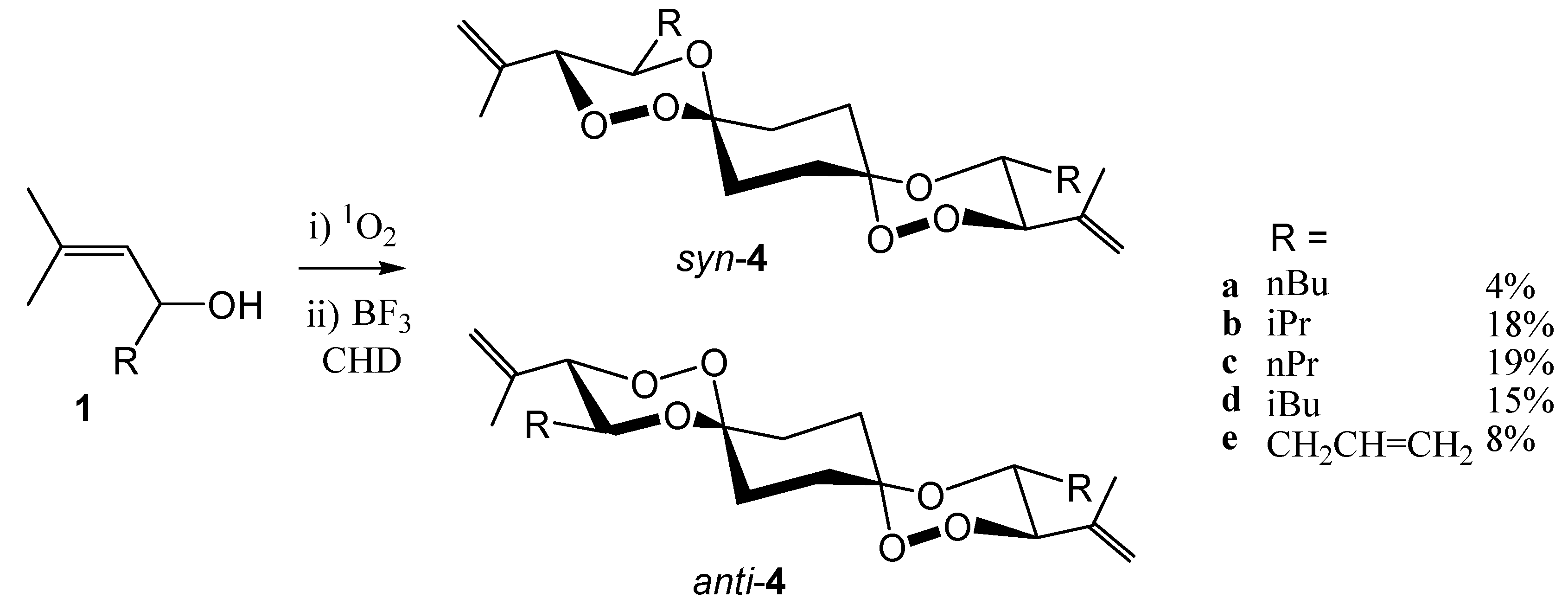
In order to condense allylic hydroperoxides
2 with bifunctional compounds, obviously the hydroperoxides
2 have to be applied in excess. This did, however, additionally increase the competition by the Lewis-acid catalyzed cleavage of the substrate [
9c]. The isolated yields of
syn/
anti-mixtures of the bis-spiroanellated trioxanes
4 from the reaction with cyclohexane-1,4-dione were consequently low (
Scheme 3).
Only products deriving from the
threo-diastereoisomeric substrates
2 were detected (albeit
threo/
erythro mixtures (≈ 8:1) were applied). In the largely preferred low-energy conformation, all substituents at the two trioxane rings are in equatorial the position, simplifying the otherwise complex symmetry of dispiranes [
16]. While the basic structures (i.e. the tricyclic ring system
9 –
vide infra - without substituents) of the
syn-isomers are of C
1-symmetry, the
anti-isomers have inversion symmetry. The two
anti-isomers
4a and
4c were analyzed by X-ray structure analyses. From space-filling models based on several X-ray structures it becomes apparent qualitatively that both dispirane isomers have rod-like core structures decorated with substituents at both ends. In the
syn spiro compounds the peroxo-bridges are unshielded and exposed to the one side of the dispirane skeleton, whereas in the
anti-isomers, the 1,2,4-trioxane structures are shielded by the alkyl substituents at the trioxane rings. We are currently studying the consequences on the antimalarial activities of these structural features.
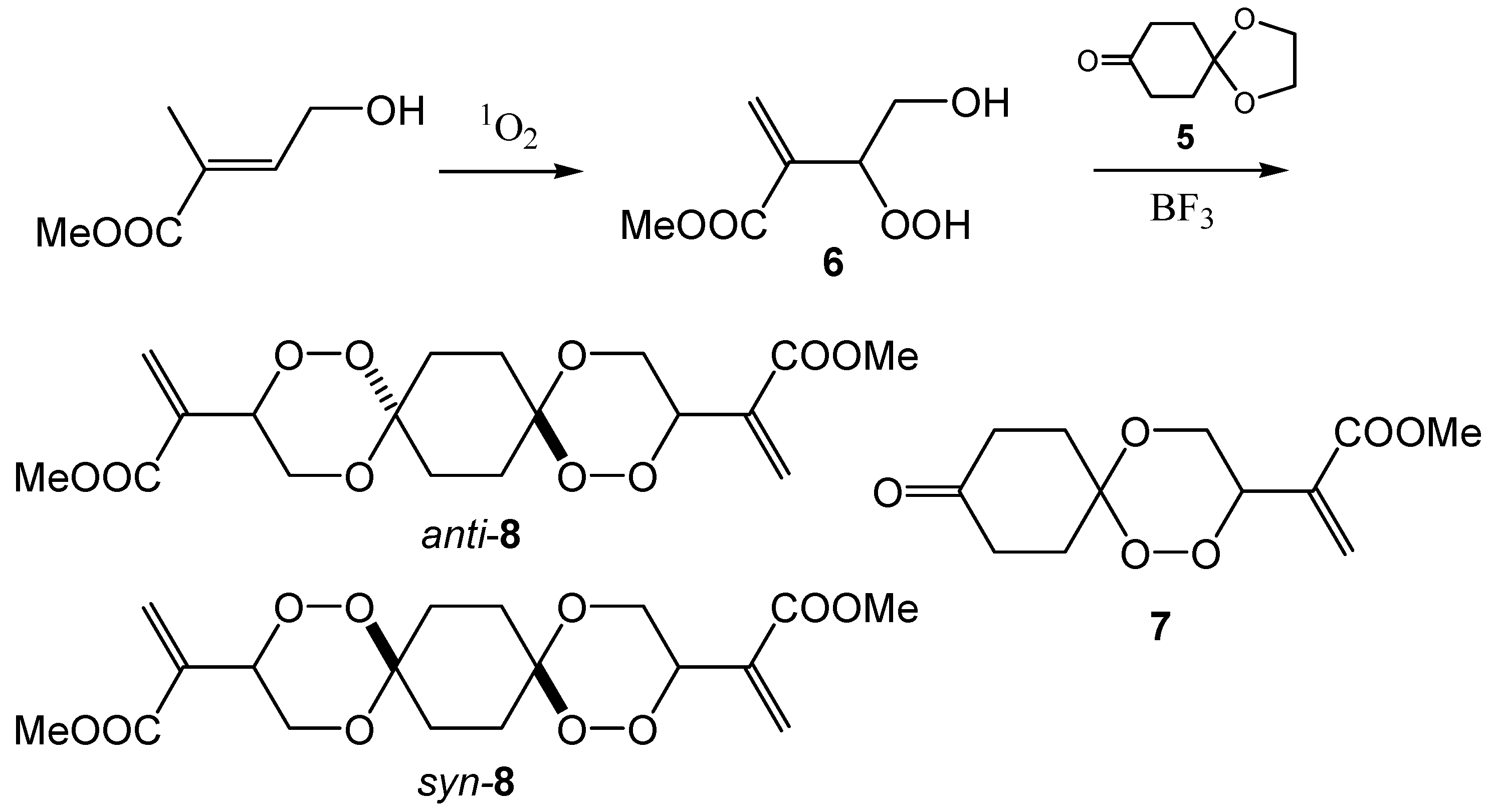
Another substrate which we investigated as a peroxyacetalization substrate was the hydroperoxide
6 derived from the methyl ester of γ-hydroxy tiglic acid [
17]. This substrate is a reluctant singlet oxygen acceptor and prolonged irradiation times are necessary. First, we studied the peroxyacetalization of
6 using equimolar amounts of
6 and the monoprotected cyclohexane-1,4-dione
5. In this case, a mixture of the trioxaspiro[5.5]undecane
7 and the hexaoxadispiro[5.2.5.2]hexadecanes
8 was isolated. The relative proportion of the dispiro product was improved by lowering the amount of the ketone
5 and the yield for
8 could be improved to 20%.
Structures of 1,2,5,10,11,14-hexaoxadispiro[5.2.5.2]hexadecanes
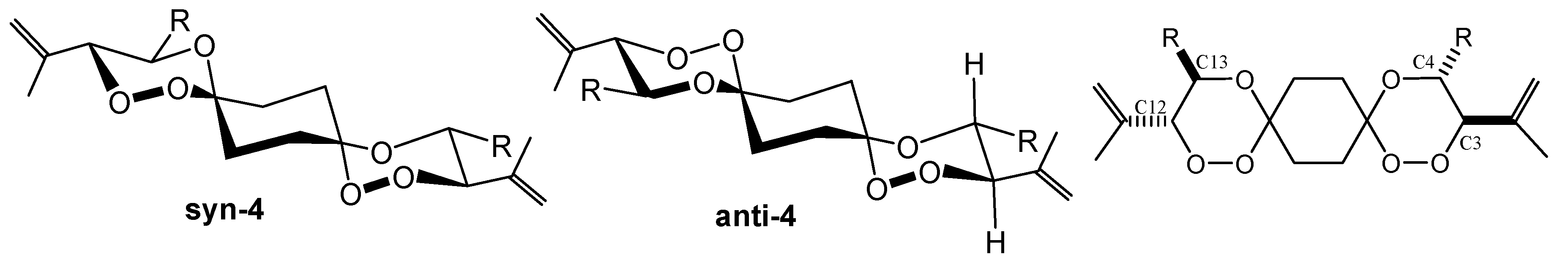
In all cases, mixtures of syn- and anti-dispiro[5.2.5.2]hexadecanes were isolated from the threo-diastereoisomeric hydroperoxides 2. Diastereoisomers originating from the erythro hydroperoxides and mixed products combing threo with erythro compounds were formed but could not be isolated in pure forms and were separated from the major products during chromatographic work-up. The X-ray structure analysis of several of these compounds revealed that in all cases all-equatorial conformers exist in the solid state. In liquid phase, analysis of the 3JHH coupling pattern (3JHH = 9.7 – 10.2 Hz) confirmed that this is also true for isotropic conditions.
The preferred bis-equatorial alignment of the substituents at the stereogenic centers (the former hydroperoxy- and hydroxyl-bearing carbon atoms) restricts the conformational mobility of the 1,2,4-trioxane rings that decorate the central cyclohexane. Only two diastereoisomeric forms were detected in the NMR spectra.
We assume, however, that
syn-
4 as well as
anti-
4 exist in two diastereoisomeric forms as well with respect to the relative configuration of the two trioxane ring systems, i.e.
syn-
4 is a mixture of (3R*, 4R*, 12R*, 13R*) and (3R*, 4R*, 12S*, 13S*) diastereoisomers. Due to the minimal structural differences, these isomers could, however, not be differentiated by spectroscopic methods. Whereas the spirotricyclic ring structures of
syn-
4 or
syn-
8 are in a degenerate conformer equilibration with respect to the central cyclohexane,
anti-
4 or
anti-
8 can exist in two energetically different conformers, namely the
anti(OO)
ax and
anti(OO)
eq isomers (
Scheme 6).
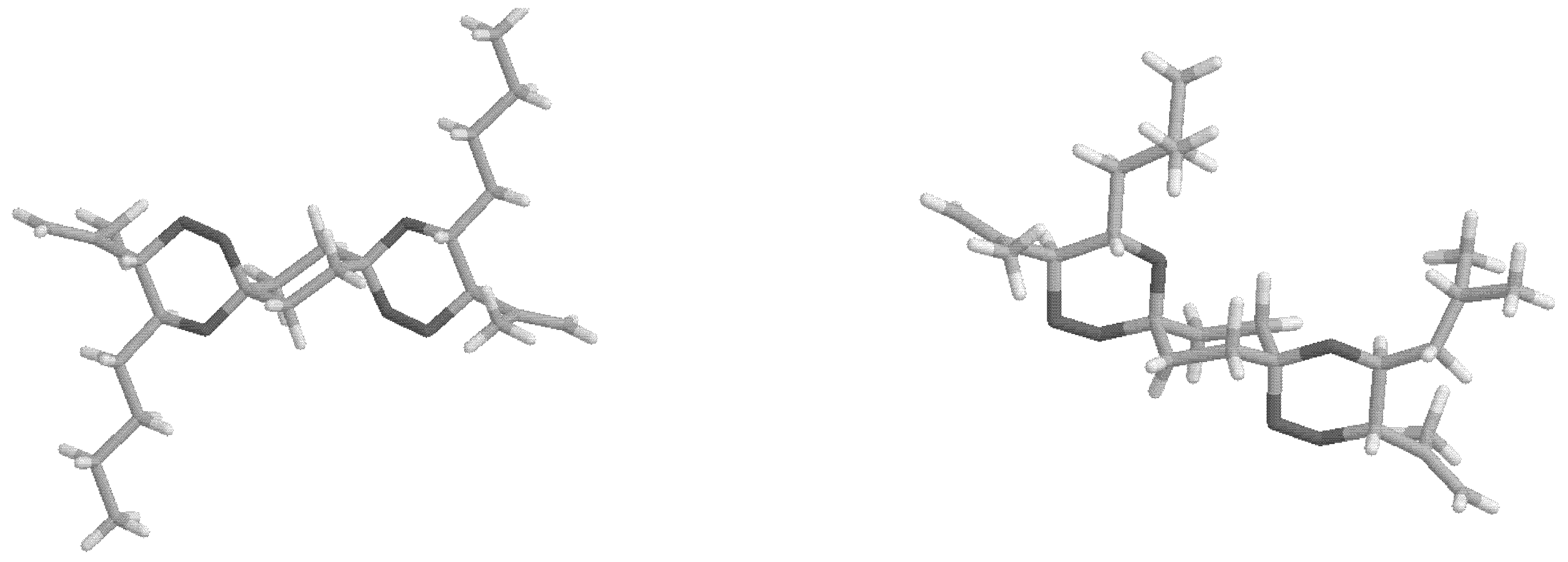
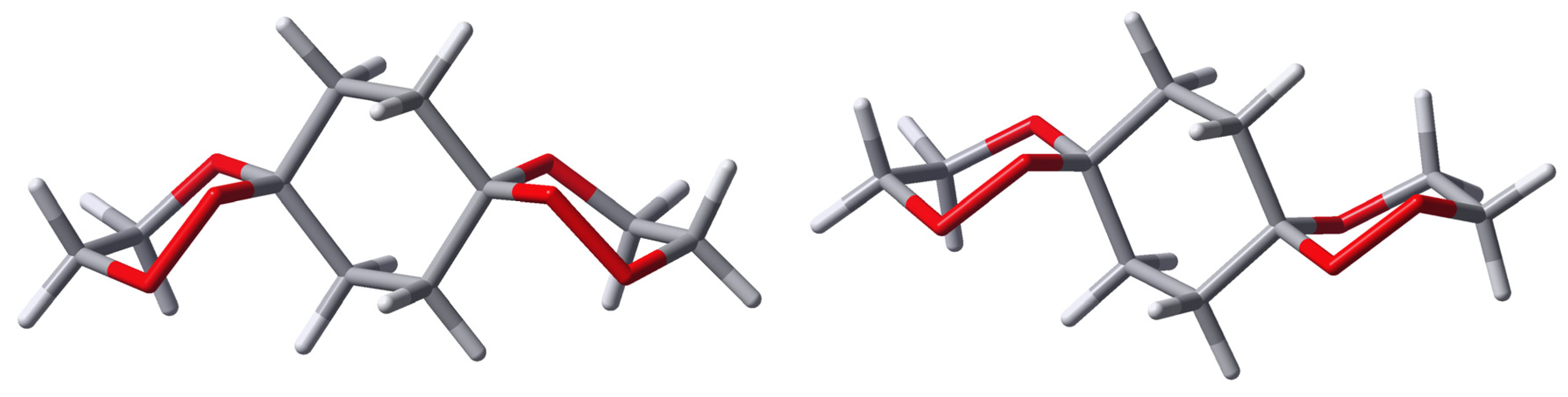
From the X-ray structure analyses of two of the
bis-spiro compounds
anti-
4a (
Figure 1) and compound
anti-
8 (
Figure 2), it appears that the
anti(OO)
ax is preferred in the crystal.
Figure 1.
X-ray structure of anti-4a and syn-4d.
Figure 1.
X-ray structure of anti-4a and syn-4d.
The C-O bond lengths to the spiro carbon atoms are: in anti-4a C-OOax=1.425 Å and C-Oeq=1.432 Å, in syn-4d C-OOax=1.432 Å, C-OOeq=1.392 Å and C-Oax=1.433 Å, C-Oeq=1.419 Å, in anti-8 C-OOax=1.436 Å and C-Oeq=1.437 Å.
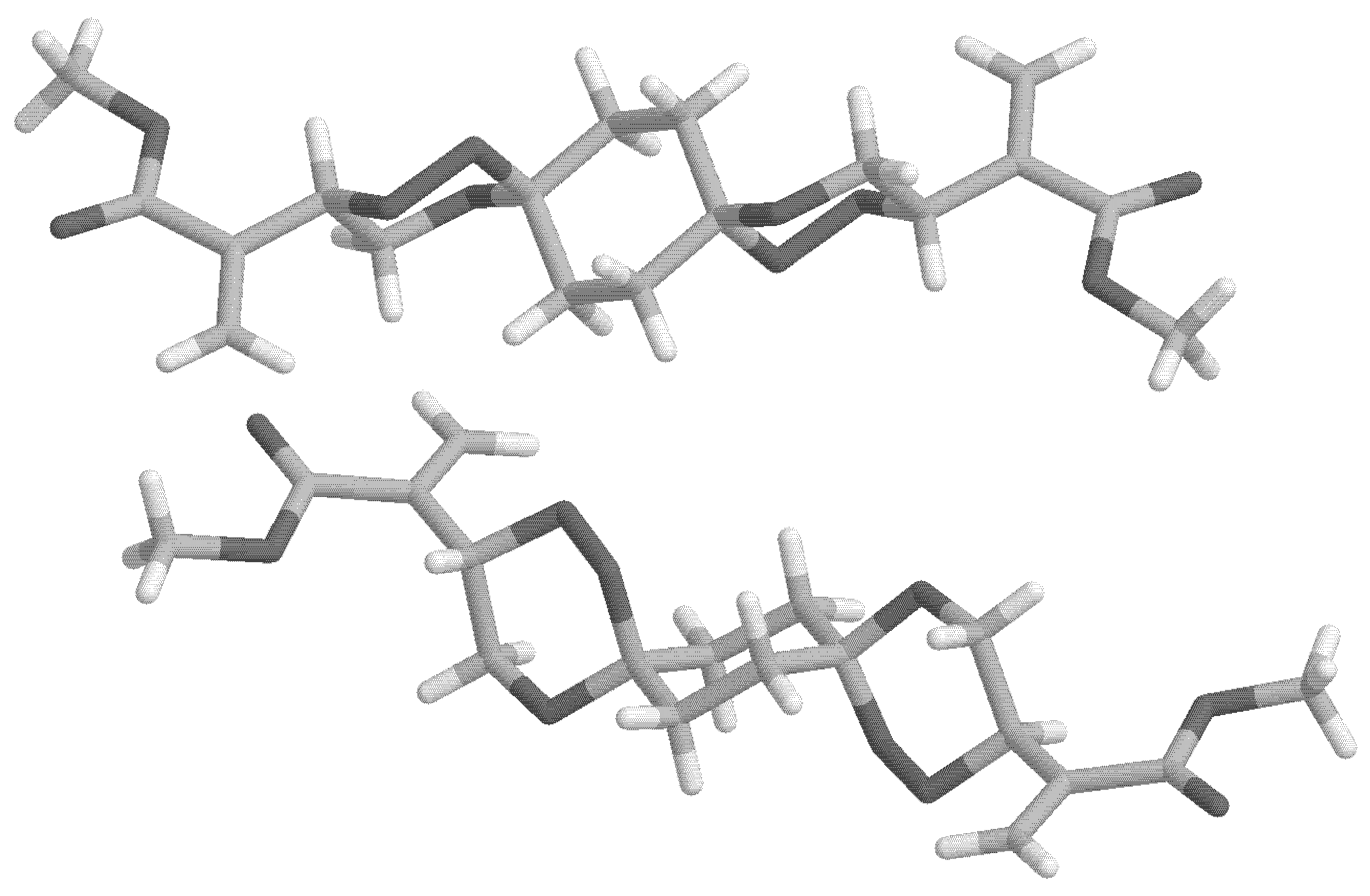
Figure 2.
X-ray structure of anti-8 (two chair views).
Figure 2.
X-ray structure of anti-8 (two chair views).
Theoretical Considerations
Beyond the synthetic approach to and chemical features of the new bis-spirofused compounds their structural aspects are of special interest. While the spirotricyclic ring structures of
syn-
4 are in a degenerate conformer equilibration with respect to the central ring (as shown in
Scheme 6),
anti-
4 can exist in two energetically different conformers, namely the
anti-
4(OO)
ax and
anti-
4(OO)
eq isomers. The X-ray structures in
Figure 1 and
Figure 2 show that the peroxo moieties in each case of an
anti-structure are situated
axial with respect to the central cyclohexane ring. In the only exception
syn-
4d (
Figure 1) one of the peroxo groups is forced into the equatorial position due to structural reasons as long as the central ring is in the chair conformation.
The axial preference of the peroxo groups in 1,2,5-trioxaspiro[5.5]undecane derivatives also holds for most of the few examples of other compounds which can be found in the Cambridge Structural Database (cf.
Table 1).
Table 1.
Conformational situationa of the peroxo groups in molecular structures containing a 1,2,5-trioxa-spiro[5.5]undecane substructure.
Table 1.
Conformational situationa of the peroxo groups in molecular structures containing a 1,2,5-trioxa-spiro[5.5]undecane substructure.
| CSD Structure Code | Sterochemical arrangement of the Peroxo group | Ref. |
|---|
| AWUDUR / AWUFAZb | Axial | 18 |
| AWUFED / AWUFIHb | axial / equatorialc | 18 |
| FIXBAQ | Axial | 9c |
| JOJCOA / JOJCUGb | axial / equatorial | 19 |
We were interested in investigating whether the predominance of the (OO)ax arrangement allows an educated guess that this molecular arrangement might be preferred due to a stereoelectronic principle. To elucidate this topic in more detail we have started a theoretical study on the basis of density functional theory (DFT) calculations.
All calculations have been performed employing the Becke three parameter hybrid functional [
20] with the correlation functional of Lee, Yang and Parr [
21] (B3LYP) in combination with the 6-311G(d) basis set as implemented in the Gaussian 03 [
22] program under tight convergence criteria and employing an ultrafine integration grid (99,590). The ground state character of each stationary point has been proven by frequency calculations confirming the absence of any negative Eigenvalues in the Hessian matrix. The absolute energies as well as the zero point correction energies (ZPE) have been calculated and compiled in
Table 2.
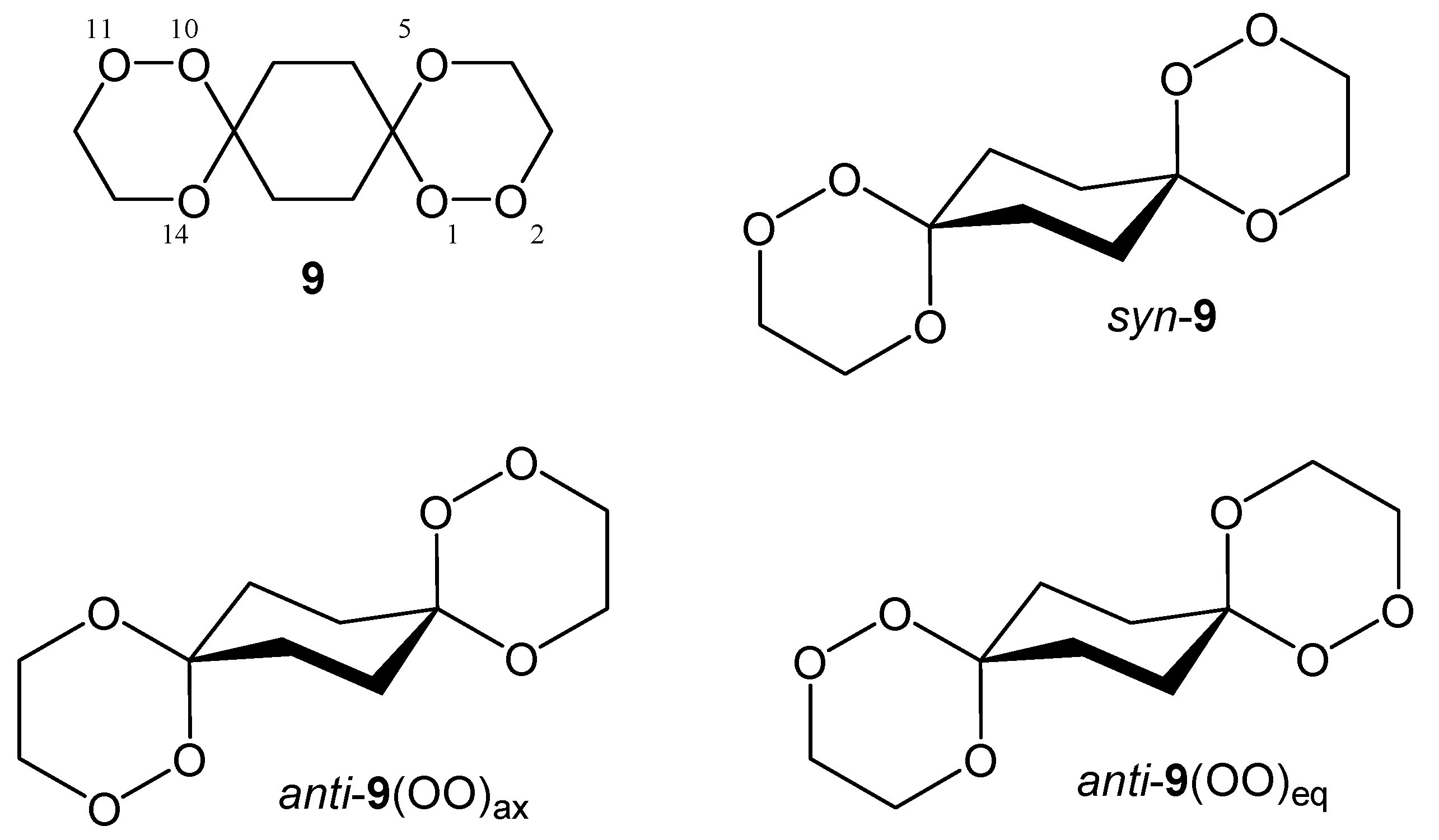
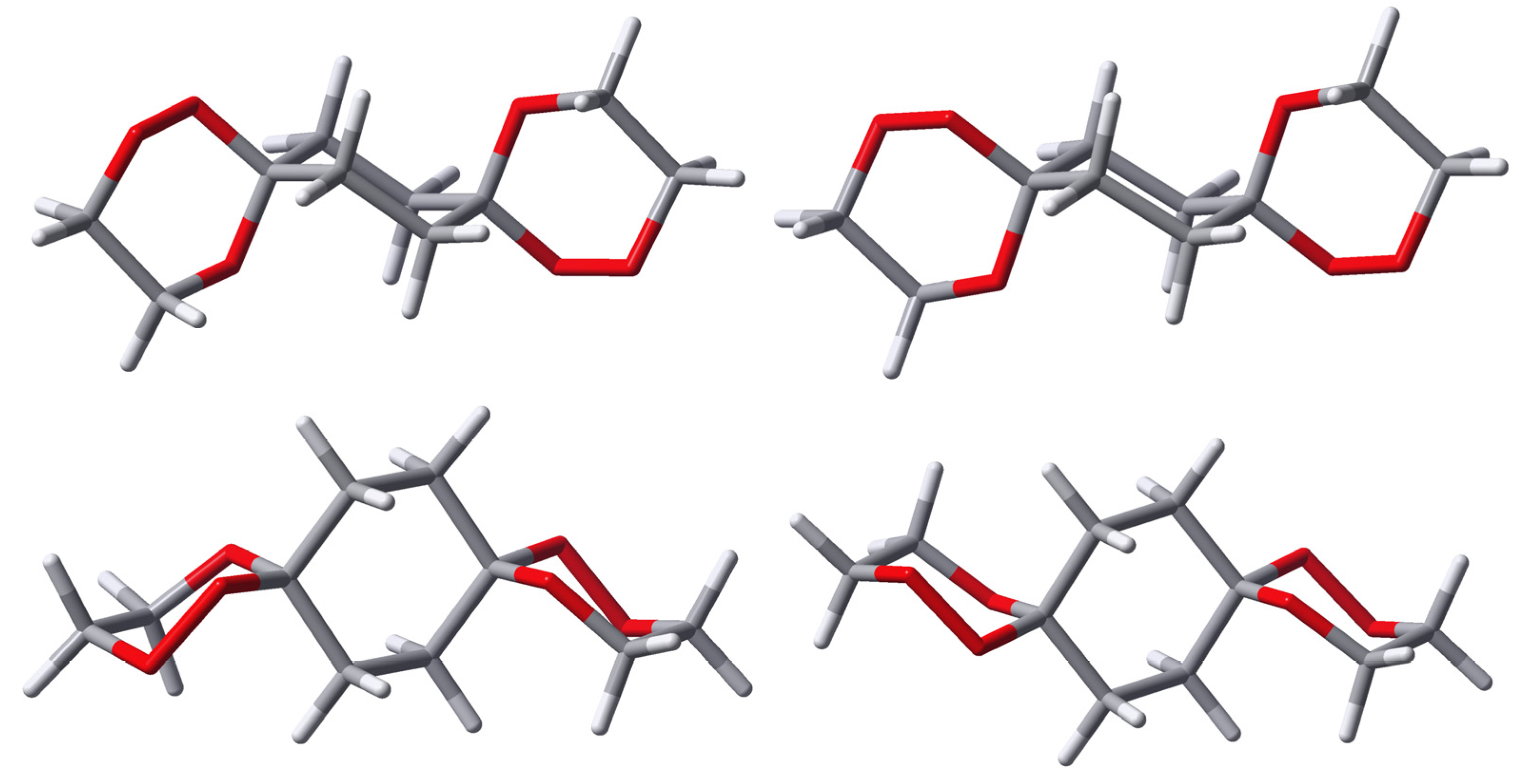
To concentrate on the energetic effects of the (OO)ax- vs. (OO)eq-alignment in the spirotricyclic ring, i.e. to exclude the significant energetic contribution of the conformational arrangement of the peripheral substituents in 4 and 8, we have calculated and will discuss in the following the common partial structure of all the presented peroxo diacetals 4 and 8, namely 1,2,5,10,11,14-hexaoxadispiro[5.2.5.2]hexadecane (9). For all structures we restrict our considerations on the energetically preferred chair conformation of all the rings, which, of course, is slightly distorted in the case of the trioxane substructures.
As already mentioned,
syn-
9 exists in a degenerate conformational situation with respect to its central cyclohexane ring. Due to the
syn-arrangement of the peroxo groups, one of them is always located in an axial position while the other is oriented equatorial. This remains so even if the middle ring inverts its chair conformation. Nevertheless, two distinct structures are possible for
syn-
9 with respect to the relative arrangement of the outer trioxane rings (cf.
Figure 3). Both structures are of C
1-symmetry but in one of them the outer trioxane rings are flipped to the same side of the molecule (equilateral) while in the other they are directed to the opposite sides of the molecule (antilateral), the latter being the energetically favoured.
Figure 3.
Calculated structures of two distinct conformers (left: equilateral, right: antilateral) of syn-9.
Figure 3.
Calculated structures of two distinct conformers (left: equilateral, right: antilateral) of syn-9.
Due to the structural arrangement of the peroxo groups
anti-
9 (cf.
Figure 4) their orientation is always the same on both sides, i.e. both are located axial or both are located equatorial, respectively. Each of these middle ring conformers again split up into two distinct conformers with respect to the outer trioxane rings. Here, the equilateral structures possess C
2-symmetry, while in the antilateral structures are of C
i-symmetry. The energies calculated for the various different structures of
9 are compiled in
Table 2.
Table 2.
Symmetry and energiesa calculated for the model structures 9.
Table 2.
Symmetry and energiesa calculated for the model structures 9.
| Structure | | Sym. | Eabs. (Hartree) | EZP (Hartree) | Eabs.+EZP (Hartree) | Erel. (kJ/mol) |
|---|
| syn-9 | antilateral | C1 | -842.04548417 | 0.267329 | -841.77815517 | 0.00 |
| syn-9 | equilateral | C1 | -842.04542546 | 0.267338 | -841.77808746 | 0.18 |
| Anti-9 (OO)ax | antilateral | Ci | -842.04603154 | 0.267327 | -841.77870454 | 0.00 |
| Anti-9 (OO)ax | equilateral | C2 | -842.04599155 | 0.267295 | -841.77869655 | 0.02 |
| Anti-9 (OO)eq | antilateral | Ci | -842.04512847 | 0.267395 | -841.77773347 | 2.55 |
| Anti-9 (OO)eq | equilateral | C2 | -842.04511732 | 0.267367 | -841.77775032 | 2.51 |
Figure 4.
Calculated structures of anti-9(OO)ax, left: equilateral (C2), right: antilateral (Ci). Each conformer is shown from two perspectives (upper and lower part).
Figure 4.
Calculated structures of anti-9(OO)ax, left: equilateral (C2), right: antilateral (Ci). Each conformer is shown from two perspectives (upper and lower part).
Figure 5.
Calculated structures of anti-9(OO)eq, left: equilateral (C2), right: antilateral (Ci). Each conformer is shown from two perspectives (upper and lower part).
Figure 5.
Calculated structures of anti-9(OO)eq, left: equilateral (C2), right: antilateral (Ci). Each conformer is shown from two perspectives (upper and lower part).
As can be seen from the relative energies listed in
Table 2, the energetic discrimination between the antilateral and the equilateral arrangement of the outer trioxane rings is negligible. The energetic difference between the (OO)
ax and the (OO)
eq conformer is still quite small with the axial situation being preferred by about 2.5 kJ/mol. Without putting an overemphasis on this relatively small energy difference (about one-third of a typical anomeric effect) it might be the decisive factor driving the conformational equilibrium towards the (OO)
ax arrangement during crystallization – probably supported by intermolecular interactions and packing forces which are not considered in our calculations – and it would not be the first case where nature fells decisions on such small energetic disparities. The origin of the energetic preference of the (OO)
ax situation in 1,2,5-trioxa-spiro[5.5]undecane derivatives is the subject of current investigations in our research groups.
Experimental
General
IR: Infrared spectra were obtained using Perkin-Elmer 1600 series FTIR spectrometer and are given in cm-1 units. Solid samples are measured as CsI or KBr discs while liquids are measured as neat between two NaCl plates; 1H-NMR: The 1H-NMR spectra were recorded on Bruker AC 300, Bruker DPX 300 spectrometers operating at 300 MHz or on Bruker DRX 500 spectrometer instrument operating at 500 MHz. Chemical shifts are reported as δ in ppm and the coupling constant, J, in Hz units. In all spectra solvent peaks were used as internal standard. Solvent used are CDCl3 (δ = 7.24 ppm), DMSO-d6 (δ = 2.49 ppm), acetone-d6 (δ = 2.04 ppm), and MeOH-d4 (δ = 3.35, 4.78 ppm). Splitting patterns are designated as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad; 13C-NMR: The 13C-NMR spectra were recorded either on a Bruker AC 300 spectrometer instrument operating at 75 MHz or on Bruker DRX 500 spectrometer instrument operating at 125 MHz; HRMS: High resolution mass spectra were recorded on Finnigan MAT 900 spectrometer and are measured for the molecular ion peak (M+); Combustion analysis: CHN-combustion analyses were measured using Elementar Vario EL Instrument; X-ray analysis: All X-ray measurements were Nonius KappaCCD diffractometer (2Θmax = 54°, MoKα radiation, λ = 0.71073 Å), graphite monochromator, ϕ/ω-scans. The structures were solved using direct methods (SHELXS-97, SHELXL-97).
General procedure for photooxygenation of allylic alcohols 1
Commercially available (Aldrich) polystyrene-divinylbenzene copolymer beads (2.5 g) are distributed over a Petri dish (19 cm diameter) and swollen with CH2Cl2 (20 mL). The substrate (ca. 10 mmol) and the nonpolar sensitizer (TPP – tetraphenylporphyrin - or TTP – tetratolylporphyrin - ca. 3-6 mg) in ethyl acetate (20 mL) are subsequently added and the excess solvent is evaporated by leaving the Petri dish in a well ventilated hood. The Petri dish is then covered with a glass plate and the sandy solid is irradiated with halogen lamp or sodium street lamp. The polymer beads are subsequently rinsed with ethanol (3 x 30 mL) and filtered (the beads are kept for regeneration and reuse). The solvent is evaporated under reduced pressure (caution, water bath temperature should not exceed 30 °C).
General procedure for the peroxyacetalization reaction used for the synthesis of spiro-bistrioxanes 4
To a stirred solution of the β-hydroxy hydroperoxide 2 and 0.5 equivalents of cyclohexane-1,4-dione in dry CH2Cl2 (100 mL) was added at room temperature a catalytic amount of boron trifluoride etherate (ca. 0.2 mL) and the mixture was further stirred for about 12 h (overnight) at the same temperature. The reaction mixture was partitioned between CH2Cl2 and saturated NaHCO3 solution and the phases were separated. The aqueous phase was extracted with CH2Cl2 (3 x 30 mL) and the combined organic phases were washed with brine, water, dried over Na2SO4. Solvent evaporation (Caution: water bath temperature should not exceed 30 °C!), followed by chromatographic purification afforded the spiro-bistrioxanes as pure products.
4,13-Dibutyl-3,12-diisopropenyl-1,2,5,10,11,14-hexaoxadispiro[5.2.5.2]hexadecane (syn-4a) and 4,13-dibutyl-3,12-diisopropenyl-1,2,5,10,11,14-hexaoxadispiro-[5.2.5.2]hexadecane (anti-4a)
Following the general procedure, a solution of 3-hydroperoxy-2-methyloct-1-en-4-ol (2a, 1.45 g, 8.33 mmol) and cyclohexane-1,4-dione (0.46 g, 4.11 mmol) in CH2Cl2 was treated with a catalytic amount of BF3xEt2O (0.2 mL). Usual work-up followed by preparative thick-layer chromatography (SiO2, EA/n-hex, 1:10, Rf = 0.53) afforded a 1:1 diastereomeric mixture of the pure 1,2,4-trioxanes syn-4a and anti-4a (0.07 g, 0.17 mmol, 4 %) as oil which crystallizes on standing, m.p. 123-124°C; 1H-NMR: (300 MHz, CDCl3, both diastereomers) δ (ppm) = 0.86 (t, 3H, J = 7.0 Hz, CH3CH2), 1.05-1.72 (m, 8H, CH2), 1.60 (m, 3H, CH3C=), 2.20 (m, 2H, CH2), 3.86 (m, 1H, OCH), 4.25 (d, 1H, J = 9.6 Hz, OOCH), 5.04 (s, 2H, CH2=); 13C-NMR: (75.5 MHz, CDCl3, 1st diastereomer) δ (ppm) = 13.9 (q, CH3CH2), 19.7 (q, CH3C=), 22.5 (t, CH2CH3), 25.2 (t, CH2), 27.0 (t, CH2CH2), 30.4 (t, CH2CH2), 30.8 (t, CH2), 69.8 (d, OCH), 87.7 (d, OOCH), 102.4 (s, OCOO), 118.1 (t, CH2=C), 139.2 (s, C=CH2); additional significant signals of the 2nd diastereomer δ (ppm) = 19.8 (q, CH3C=), 22.5 (t, CH2CH3), 24.9 (t, CH2), 27.1 (t, CH2CH2), 30.5 (t, CH2CH2), 31.2 (t, CH2), 69.8 (d, OCH), 87.7 (d, OOCH), 102.3 (s, OCOO), 118.1 (t, CH2=C), 139.1 (s, C=CH2); IR: (Film) ν (cm-1) = 3083, 2957, 2873, 1648, 1455, 1373, 1258, 1105, 1007, 928, 911; Elemental Analysis: (C24H40O6, M = 424.57) Calcd.: C 67.89 H 9.50; Found: C 67.43 H 9.37
3,12-Diisopropenyl-4,13-diisopropyl-1,2,5,10,11,14-hexa-oxadispiro[5.2.5.2]hexadecane (syn-4b) and 3,12-diisopropenyl-4,13-diisopropyl-1,2,5,10,11,14-hexaoxa-dispiro[5.2.5.2]hexadecane (anti-4b)
Following the general procedure, a solution of 4-hydroperoxy-2,5-dimethylhex-5-en-3-ol (2b, 1.60 g, 10.0 mmol) and cyclohexane-1,4-dione (0.56 g, 5.0 mmol) in CH2Cl2 was treated with a catalytic amount of BF3xEt2O (0.2 mL). Usual work-up followed by preparative thick-layer chromatography (SiO2, EA/n-hex, 1:10, Rf = 0.70) afforded a diastereomeric mixture of the pure 1,2,4-trioxanes syn-4b and anti-4b in a ratio 1:1 (0.36 g, 0.91 mmol, 18 %) as oil which crystallizes on standing into yellow crystals. 1H-NMR: (300 MHz, CDCl3, both diastereomers) δ (ppm) = 0.90 (d, 3H, J = 6.9 Hz, CH3CH), 0.97 (d, 3H, J = 6.8 Hz, CH3CH), 0.79-1.10 (m, CH2), 1.60-1.80 (m, 1H, CH(CH3)2, 1.74 (m, 3H, CH3C=), 3.76 (m, 1H, OCH), 4.45 (d, 1H, J = 9.8 Hz, OOCH), 5.07 (m, 2H, CH2=); 13C-NMR: (75.5 MHz, CDCl3, 1st diastereomer) δ (ppm) = 15.0 (q, CH3CH), 19.6 (q, CH3C=), 19.9 (q, CH3CH), 24.8 (t, CH2), 28.1 (d, CH(CH3)2), 31.1 (t, CH2), 73.4 (d, OCH), 85.7 (d, OOCH), 102.2 (s, OCOO), 118.0 (t, CH2=C), 139.3 (s, C=CH2); additional significant signals of 2nd diastereomer δ (ppm) = 20.0 (q, CH3CH), 25.0 (t, CH2) 30.7 (t, CH2); IR: (Film) ν (cm-1) = 3083, 2965, 1731, 1649, 1469, 1373, 1258, 1118, 919, 825; Elemental Analysis: (C22H36O6, M = 396.25) Calcd.: C 66.64 H 9.15; Found: C 66.26 H 9.24.
3,12-Diisopropenyl-4,13-dipropyl-1,2,5,10,11,14-hexaoxadispiro[5.2.5.2]hexadecane (syn-4c) and 3,12-diisopropenyl-4,13-dipropyl-1,2,5,10,11,14-hexaoxadispiro-[5.2.5.2]hexadecane (anti-4c)
Following the general procedure, a solution of 4-hydroperoxy-2,5-dimethylhex-5-en-3-ol (2c) (1.32 g, 8.25 mmol) and cyclohexane-1,4-dione (0.46 g, 4.11 mmol) in CH2Cl2 was treated with a catalytic amount of BF3xEt2O (0.2 mL). Usual work-up followed by preparative thick-layer chromatography (SiO2, EA/n-hex, 1:10, Rf = 0.64) afforded a diastereomeric mixture of the pure 1,2,4-trioxanes syn-4c and anti-4c in a ratio 1:1 (0.31 g, 0.78 mmol, 19 %) as oil which crystallizes on standing into yellow crystals, m.p. 103-104°C; 1H-NMR: (300 MHz, CDCl3, 1st diastereomer) δ (ppm) = 0.88 (t, 3H, J = 6.9 Hz, CH3CH2), 1.26-1.58 (m, 4H, CH2CH2), 1.67 (m, 2H, CH2), 1.72 (m, 3H, CH3C=), 2.04-2.32 (m, 2H, CH2), 3.89 (m, 1H, OCH), 4.25 (d, 1H, J = 9.7 Hz, OOCH), 5.04 (m, 2H, CH2=C); 13C-NMR: (75.5 MHz, CDCl3) δ (ppm) = 13.8 (q, CH3CH2), 18.1 (t, CH2CH3), 19.7 (q, CH3C=), 25.1 (t, CH2), 30.8 (t, CH2), 32.9 (t, CH2CH2), 69.5 (d, OCH), 87.6 (d, OOCH), 102.3 (s, OCOO), 118.0 (t, CH2=C), 139.1 (s, C=CH2); 2nd diastereomer) δ (ppm) = 13.9 (q, CH3CH2), 18.1 (t, CH2CH3), 19.7 (q, CH3C=), 24.8 (t, CH2), 31.2 (t, CH2), 32.9 (t, CH2CH2), 69.5 (d, OCH), 87.6 (d, OOCH), 102.3 (s, OCOO), 118.0 (t, CH2=C), 139.1 (s, C=CH2); IR: (Film) ν (cm-1) = 3083, 2956, 2873, 1680, 1649, 1454, 1374, 1258, 1105, 1006, 918; Elemental Analysis: (C22H36O6, M = 396.25) Calcd.: C 66.64 H 9.15; Found: C 67.11 H 9.02.
4,13-Diisobutyl-3,12-diisopropenyl-1,2,5,10,11,14-hexaoxadispiro[5.2.5.2]hexadecane (syn-4d) and 4,13-diisobutyl-3,12-diisopropenyl-1,2,5,10,11,14-hexaoxadispiro-[5.2.5.2]hexadecane (anti-4d)
Following the general procedure, a solution of 3-hydroperoxy-2,6-dimethylhept-1-en-4-ol (2d) (1.32 g, 7.59 mmol) and cyclohexane-1,4-dione (0.42 g, 3.75 mmol) in CH2Cl2 was treated with a catalytic amount of BF3xEt2O (0.2 mL). Usual work-up and further purification of the crude product by preparative thick-layer chromatography (SiO2, EA/n-hex, 1:10, Rf = 0.71) afforded a diastereomeric mixture of the 1,2,4-trioxanes syn-4d and anti-4d (0.23 g, 0.54 mmol, 15 %) as oil which crystallizes on standing. 1H-NMR: (300 MHz, CDCl3, both diastereomers) δ (ppm) = 0.81-0.94 (m, 6H, (CH3)2CH), 1.06 (m, 1H, CH2CH), 1.33 (m, 1H, CH2CH), 1.59-1.89 (m, 3H, CH2 and CHCH2), 1.72 (m, 3H, CH3C=), 2.10-2.35 (m, 2H, CH2), 3.97 (m, 1H, OCH), 4.24 (d, 1H, J = 9.5 Hz, OOCH), 5.05 (m, 2H, CH2=C); 13C-NMR: (75.5 MHz, CDCl3, 1st diastereomer) δ (ppm) = 19.7 (q, CH3C=), 21.3 (q, CH3CH), 23.5 (d, CHCH2), 23.7 (q, CH3CH), 25.2 (t, CH2), 31.2 (t, CH2), 39.6 (t, CH2CH), 67.8 (d, OCH), 88.1 (d, OOCH), 102.3 (s, OCOO), 118.2 (t, CH2=C), 139.0 (s, C=CH2); additional signals of 2nd diastereomer δ (ppm) = 19.6 (q, CH3C=), 23.5 (d, CHCH2), 24.7 (t, CH2), 30.7 (t, CH2), 39.6 (t, CH2CH), 88.1 (d, OOCH), 102.3 (s, OCOO), 139.0 (s, C=CH2); Elemental Analysis: (C24H40O6, M = 424.57) Calcd.: C 67.89 H 9.50; Found: C 67.62 H 9.82.
4,13-Diallyl-3,12-diisopropenyl-1,2,5,10,11,14-hexaoxadispiro[5.2 .5.2]hexadecane (syn-4e) and 4,13-diallyl-3,12-diisopropenyl-1,2,5,10,11,14-hexaoxadispiro[5.2.5.2]-hexadecane (anti-4e)
Following the general procedure, a solution of 3-hydroperoxy-2-methylhepta-1,6-dien-4-ol (2e) (1.30 g, 8.23 mmol) and cyclohexane-1,4-dione (0.46 g, 4.11 mmol, 0.5 equiv.) in CH2Cl2 was treated with a catalytic amount of BF3xEt2O (0.2 mL). Usual work-up and further purification of the crude product (0.87 g, 2.22 mmol, 54 %) by preparative thick-layer chromatography (SiO2, EA/n-hex, 1:10, Rf = 0.55) afforded a diastereomeric mixture of the pure 1,2,4-trioxanes syn-4e and anti-4e (0.13 g, 0.33 mmol, 8 %) as viscous colorless oil. 1H-NMR: (300 MHz, CDCl3, 1st diastereomer) δ (ppm) = 1.60-2.39 (m, 6H, 3 x CH2), 1.74 (s, 3H, CH3C=), 3.97 (m, 1H, OCH), 4.31 (d, 1H, J = 9.7 Hz, OOCH), 5.07 (m, 4H, CH2=CH and CH2=C), 5.82 (m, 1H, CH=CH2); 13C-NMR: (75.5 MHz, CDCl3, 1st diastereomer) δ (ppm) = 19.7 (q, CH3C=), 24.8 (t, CH2), 31.1 (t, CH2), 35.3 (t, CH2CH=), 69.5 (d, OCH), 86.9 (d, OOCH), 102.4 (s, OCOO), 117.2 (t, CH2=CH), 118.4 (t, CH2=C), 133.6 (d, CH=CH2), 138.8 (s, C=CH2); additional significant signals from the 2nd diastereomer δ (ppm) = 3.35 (m, 1H, OCH), 4.30 (d, 1H, J = 9.7 Hz, OOCH); 13C-NMR: (75.5 MHz, CDCl3, 2nd diastereomer) δ (ppm) = 19.7 (q, CH3C=), 24.8 (t, CH2), 31.1 (t, CH2), 35.3 (t, CH2CH=), 69.5 (d, OCH), 87.1 (d, OOCH), 102.4 (s, OCOO), 117.1 (t, CH2=CH), 118.4 (t, CH2=C), 133.7 (d, CH=CH2), 138.9 (s, C=CH2); Elemental Analysis: (C22H32O6, M = 392.49) Calcd.: C 67.32 H 8.22; Found: C 66.91 H 8.47.
2-(9-Oxo-1,2,5-trioxaspiro[5.5]undec-3-yl)acrylic acid methylester (7) and 2-[12-(1-methoxy-carbonyl- vinyl)-1,2,5,10,11,14-hexadispiro[5.2.5.2]hexadec-3-yl]acrylic acid methyl ester (syn- and anti-8)
Following the general procedure, a solution of 0.33 g (2.0 mmol) methyl 3-hydroperoxy-4-hydroxy-2-methylene-butanoate (6) and 0.31 g (2.0 mmol) 1,4‑dioxaspiro[4.5]decan-8-one in CH2Cl2 (40 mL) was treated with a catalytic amount of p-TosOH (50 mg). Usual work up afforded a mixture of compound 7 and compound 8 (50:50 anti/syn-diasteroisomeric mixture) in a ratio of 1:1. Following thick-layer chromatography (SiO2, EA/petroleum 1:10, Rf (7)= 0.08, Rf (8)= 0.12) yielded the pure products as colourless oils, which crystallized on standing. A second experiment was performed using 0.5 eq. of 1,4‑dioxaspiro[4.5]decan-8-one (0.2 g, 1.28 mmol ketone and 0.4 g, 2.55 mmol of the peroxide 6). In this case a mixture of 7 and 8 was obtained in a ratio of 25:75. Compound 7 was recrystallized from isopropanol/dichloromethane for X-ray analyses. Compound 7: yield: 0.1 g, 0.39 mmol (20 %), m.p.: 83-85 °C; 1H-NMR: (CDCl3, 300 MHz) δ = 1.98 (m; 2H, CH2), 2.06 (m; 2H, CH2), 2.43 (m; 4H, CH2), 3.77 (s; 3H, CH3), 3.89 (dd; 1H, OCH2), 4.05 (m; 1H, OCH2), 5.21 (dd, J1 = 9.5 Hz, J2 = 2.8 Hz; 1H, OOCH), 5.88 (s; 1H, CH2 olef.), 6.43 (s; 1H, CH2 olef.); 13C-NMR: (CDCl3, 75 MHz) δ = 33.8 (t; 1C, CH2), 36.4 (t; 1C, CH2), 36.5 (t; 1C, CH2), 38.1 (t; 1C, CH2), 52.3 (q; 1C, CH3), 64.6 (t; 1C, OCH2), 77.1 (d; 1C, OOCH), 101.2 (s; 1C, Cq), 128.8 (d; 1C, CH2 olef.), 135.1 (s; 1C, Cq olef.), 164.8 (s; 1C, COOCH3), 209.7 (s; 1C, CO); MS: (EI, 70 eV) m/z (%) = 256 (M+; < 1), 224 (C12H16O4+; 5), 112 (C6H8O2+; 70), 57 (C3H5O+; 50), 56 (C3H4O+·; 93), 55 (C3H3O+; 100); IR: (ATR) (cm-1) ν = 2957, 2893, 1716, 1631, 1439, 1269, 1115, 1089, 1032, 969, 954, 921, 911. Compound 8: yield: 0.1 g, 0.25 mmol (10 %); 1H-NMR: (CDCl3, 300 MHz; 1st diastereomer) δ = 1.70 (m; 4H, CH2), 1.82 (m; 4H, CH2), 3.75 (s; 6H, CH3), 3.81 (dd; 2H, OCH2), 4.05 (m; 2H, OCH2), 5.13 (dd; 2H, OOCH), 5.86 (s; 2H, CH2 olef.), 6.40 (s; 2H, CH2 olef.); 13C-NMR: (CDCl3, 75 MHz; 1st diastereomer) δ = 30.6 (t; 4C, CH2), 52.2 (q; 2C, CH3), 64.3 (t; 2C, OCH2), 76.9 (d; 2C, OOCH), 102.0 (s; 2C, Cq), 128.5 (d; 2C, CH2 olef.), 135.3 (s; 2C, Cq olef.), 164.9 (s; 2C, CO); additional significant signals of the 2nd diastereomer δ = 30.8 (t; 4C, CH2), 64.4 (t; 2C, CH2), 102.1 (s; 2C, Cq), 135.4 (s; 2C, Cq olef.); MS: (EI, 70 eV) m/z (%) = 112 (C6H8O2; 100), 57 (C3H5O; 20), 56 (C3H4O; 33), 55 (C3H3O; 52); IR: (ATR) ν (cm-1) = 2956, 2853, 1725, 1634, 1438, 1273, 1250, 1199, 1161, 1116, 967, 954, 920, 814.