Differential Cytotoxicity of MEX: a Component of Neem Oil Whose Action Is Exerted at the Cell Membrane Level
Abstract
:Introduction
Results and Discussion
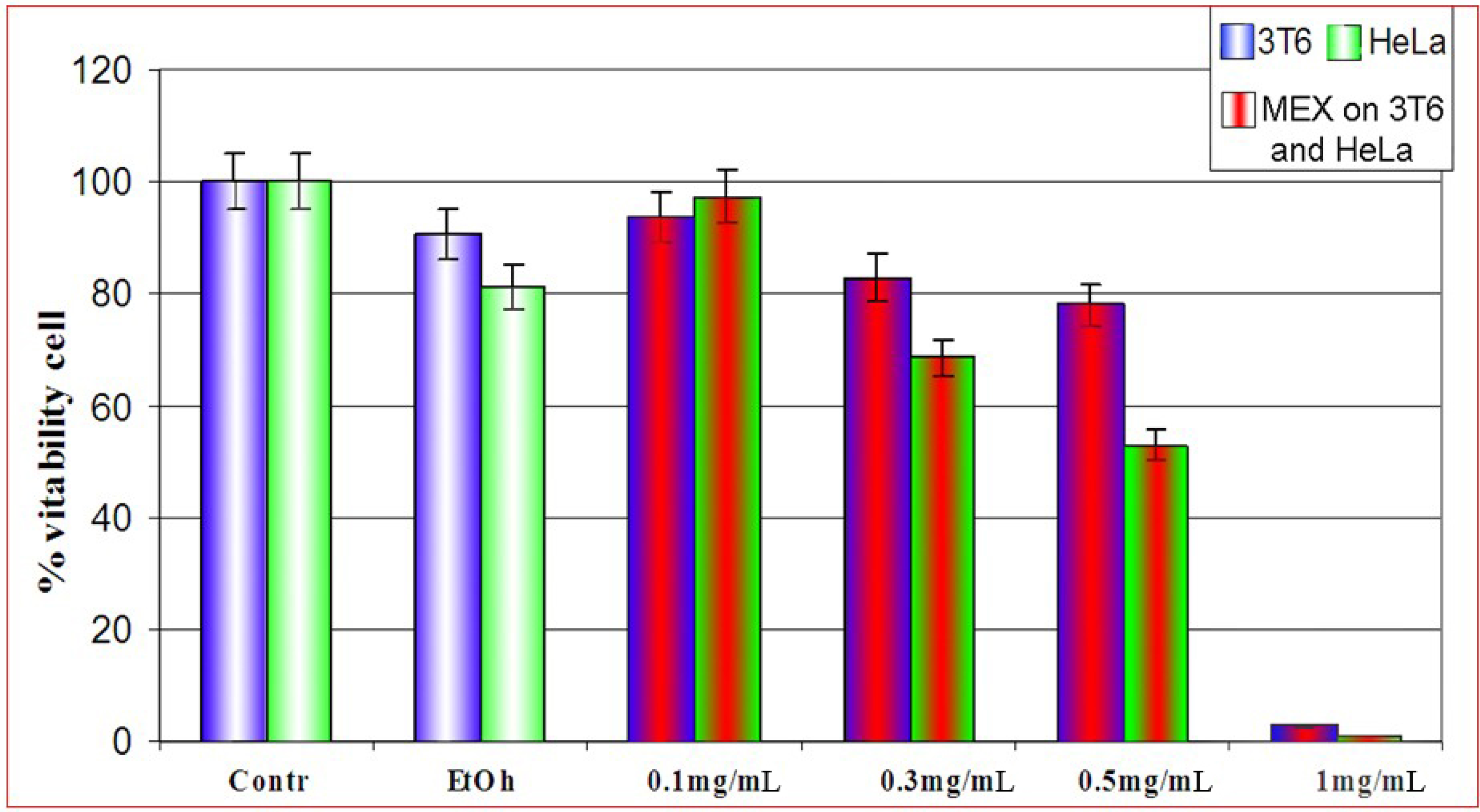
Effect of MEX on the cell viability: differential sensitivity of 3T6 and HeLa cells
| Sample | Vital | Dead | Sample | Vital | Dead |
| 1 | 72±2 | 6±1 | 1 | 75±3 | 7±1 |
| 2 | 74±2 | 7±1 | 2 | 88±4 | 10±1 |
| 3 | 81±3 | 9±2 | 3 | 100±2 | 14±3 |
| Average cell mortality 8.7% | Average cell mortality 9.1% | ||||
| Sample | Vital | Dead | Sample | Vital | Dead |
| 1 | 56±2 | 12±1 | 1 | 36±3 | 20±2 |
| 2 | 60±1 | 15±1 | 2 | 54±2 | 42±1 |
| 3 | 48±2 | 12±2 | 3 | 57±2 | 39±1 |
| Average cell mortality 19.2% | Average cell mortality 40% | ||||
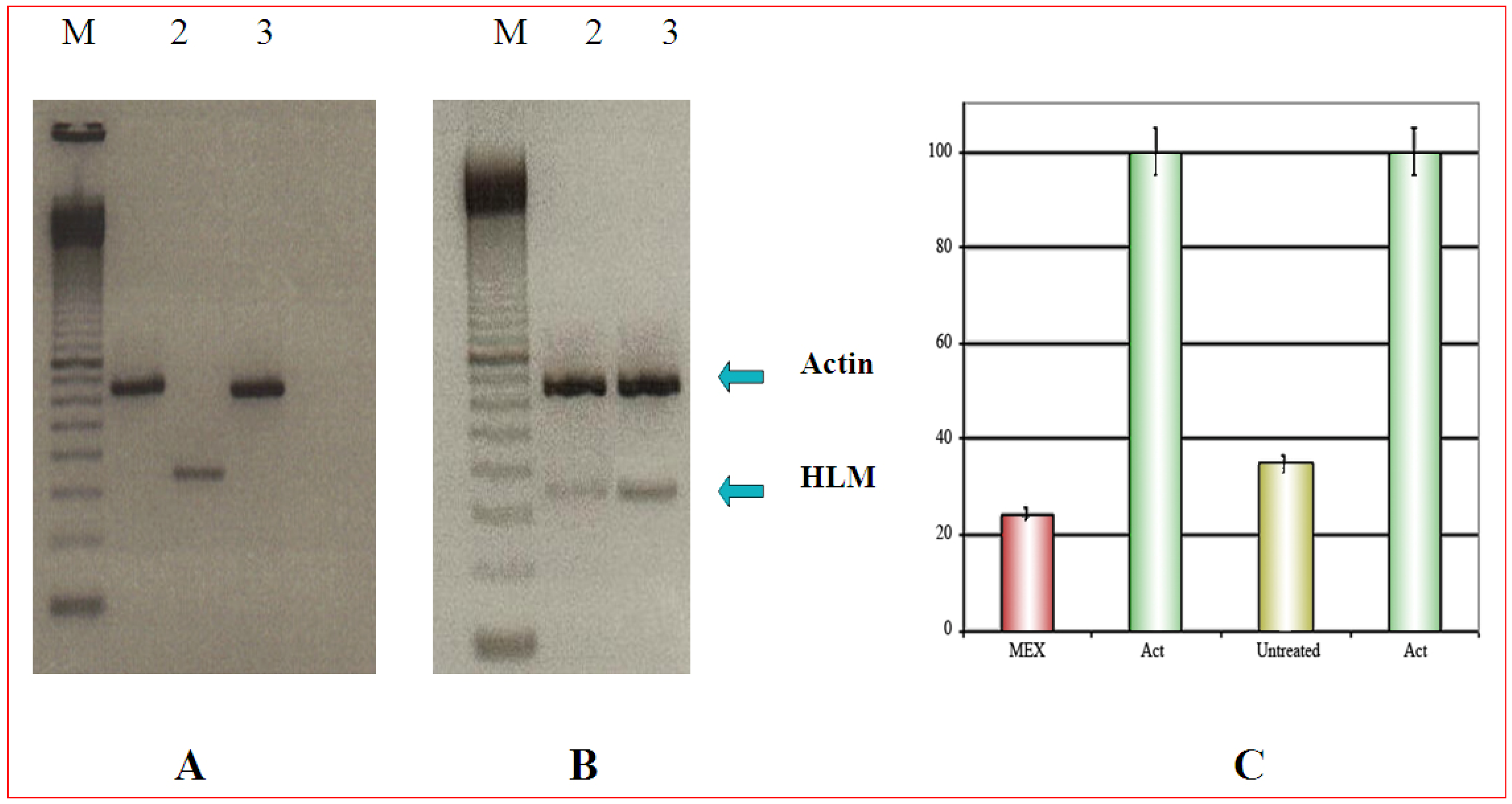
Selective cytotoxicity can be demonstrated by PCR
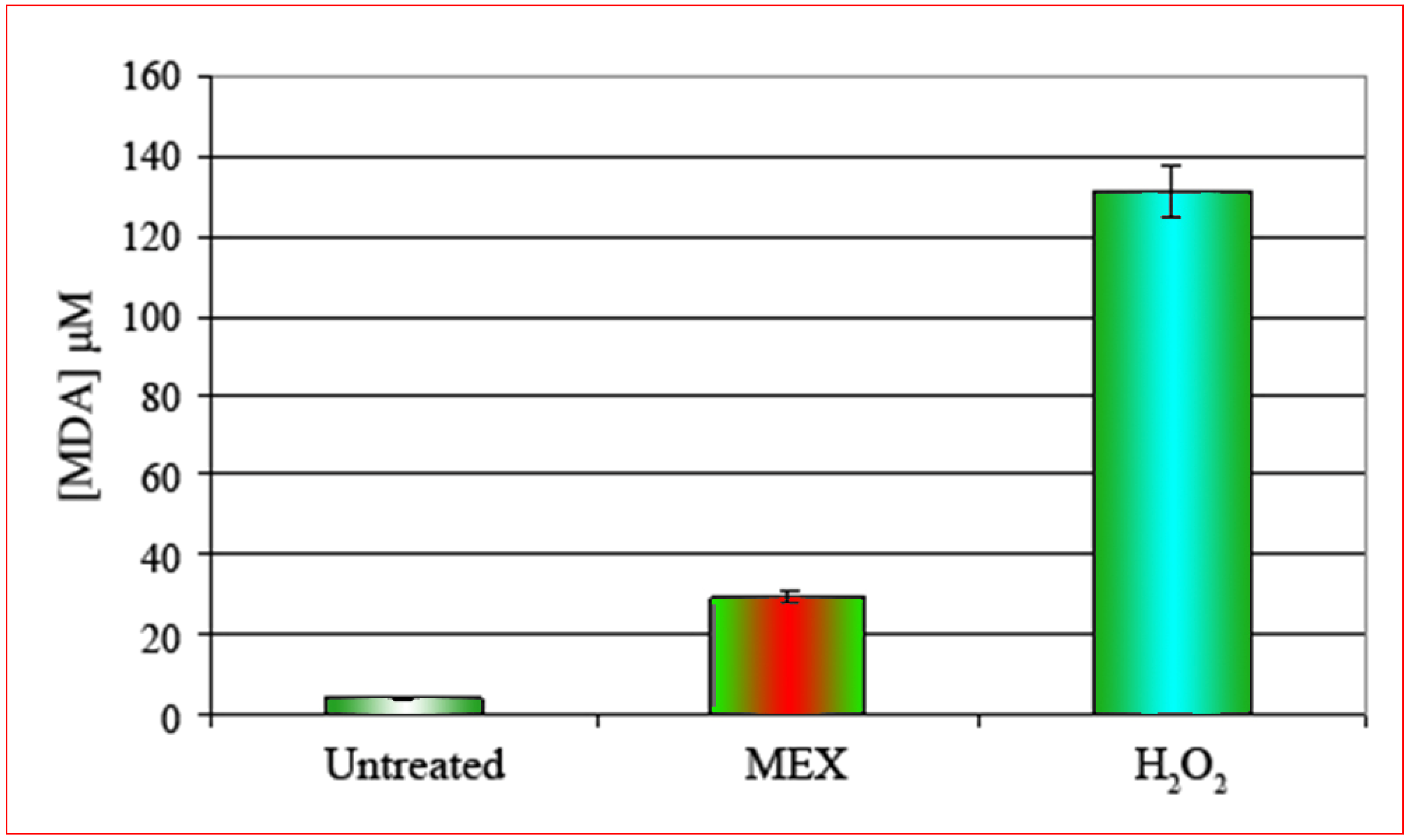
The cell membrane is the main target of MEX and the oxidative damage is abolished by the presence of antioxidants.

Conclusions
Experimental
- Act fw: 5’ CGGGGTCTTTGTCTGAGC 3’, Act rv: 5’ CACGATTGGGGATAAAGGAA 3’
- HLM fw: 5’GTGCACTTGGAGGAAACCAT3’, HLM rv: 5’ACTCGCCTCTTGACTTTGGA3’
Acknowledgements
References
- Subapriya, R.; Nagini, S. Medicinal properties of neem leaves: a review. Curr. Med. Chem. Anticancer Agent 2005, 5, 149–156. [Google Scholar] [CrossRef]
- Subapriya, R.; Nagini, S. Ethanolic neem leaf extract protects against N-methyl-N'-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in Wistar rats. Asian Pac. J. Cancer Prev. 2003, 4, 215–223. [Google Scholar]
- Schmutterer, H. The neem tree and other meliaceous plants; Neem Foundation: Mumbai, India, 2002. [Google Scholar]
- Di Ilio, V.; Pasquariello, N.; van der Esch, S.A.; Cristofaro, M.; Scarsella, G.; Risuleo, G. Cytotoxic and antiproliferative effects induced by a non terpenoid polar extract of A. indica seeds on 3T6 murine fibroblasts in culture. Mol. Cell. Biochem. 2006, 287, 69–77. [Google Scholar] [CrossRef]
- Iacoangeli, A.; Melucci-Vigo, G.; Risuleo, G. Mechanism of the inhibition of murine polyomavirus DNA replication induced by the ionophore monensin. Biochimie 2000, 82, 35–39. [Google Scholar] [CrossRef]
- Campanella, L.; Delfini, M.; Ercole, P.; Iacoangeli, A.; Risuleo, G. Molecular characterization and action of usnic acid: a drug that inhibits proliferation of mouse polyomavirus in vitro and its main target is RNA transcription. Biochimie 2002, 84, 329–334. [Google Scholar] [CrossRef]
- Bonincontro, A.; Di Ilio, V.; Pedata, O.; Risuleo, G. Dielectric properties of the plasma membrane of cultured murine fibroblasts treated with a nonterpenoid extract of Azadirachta indica seeds. J. Membr. Biol. 2007, 215, 75–79. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular grow and survival: application to proliferation and cytotoxixity assay. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Chancerelle, Y.; Kergonou, J.F. Immunologic relevance of malonic dialdehyde. Ann. Pharm. Fr. 1995, 53, 241–250. [Google Scholar]
- Petit, E.; Divoux, D.; Chancerelle, Y.; Kergonou, J.F.; Nouvelot, A. Immunological approach to investigating membrane cell damages induced by lipoperoxidative stress. Application to far UV-irradiated erythrocytes. Biol. Trace Elem. Res. 1995, 47, 17–27. [Google Scholar] [CrossRef]
- Shimizu, S.; Umezawa, K.; Takada, M.; Arber, N.; Imoto, M. Induction of hydrogen peroxide production and Bax expression by caspase-3(-like) proteases in tyrosine kinase inhibitor-induced apoptosis in human small cell lung carcinoma cells. Exp. Cell. Res. 1998, 10, 197–203. [Google Scholar]
- Yang, H.W.; Hwang, K.J.; Kwon, H.C.; Kim, H.S.; Choi, K.W.; Oh, K.S. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum. Reprod. 1998, 13, 998–1002. [Google Scholar] [CrossRef]
- Yamamoto, H.; Ozaki, T.; Nakanishi, M.; Kikuchi, H.; Yoshida, K.; Horie, H.; Kuwano, H; Nakagawara, A. Oxidative stress induces p53-dependent apoptosis in hepatoblastoma cell through its nuclear translocation. Genes Cells 2007, 12, 461–471. [Google Scholar] [CrossRef]
- Bonincontro, A.; Iacoangeli, A.; Melucci-Vigo, G.; Risuleo, G. Apoptosis dependent decrease of the intramembrane ion traffic in cultured mousefibroblasts shown by conductivity dispersion. Biosci. Rep. 1997, 17, 547–556. [Google Scholar] [CrossRef]
- Cristofanilli, M; Pescosolido, N.; Risuleo, G.; Scarsella, G. A murine cell culture model for post-trabeculectomy anfibrotic treatment:Induction of apoptosis by Cyclosporin. Acta Ophthalmol. Scand. 2001, 79, 309–312. [Google Scholar] [CrossRef]
- Calandrella, N.; Scarsella, G.; Pescosolido, N.; Risuleo, G. Degenerative and apoptotic events at retinal and optic nerve level after experimental induction of ocular hypertension. Mol. Cell. Biochem. 2007, 301, 155–163. [Google Scholar] [CrossRef]
- Bakhshi, J.; Weinstein, L.; Poksay, K.S.; Nishinaga, B.; Bredesen, D.E.; Rao, R.V. Coupling endoplasmic reticulum stress to the cell death program in mouse melanoma cells: effect of curcumin. Apoptosis 2008, 13, 904–914. [Google Scholar] [CrossRef]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef]
- Fournier, M.V.; Guimaraes da Costa, F.; Paschoal, M.E.; Ronco, L.V.; Carvalho, M.G.; Pardee, A.B. Identification of a gene encoding a human oxysterol-binding protein-homologue: a potential general molecular marker for blood dissemination of solid tumors. Cancer Res. 1999, 59, 3748–3753. [Google Scholar]
- Draper, H.H.; Hadley, M. A review of recent studies on the metabolism of exogenous and endogenous malondialdehyde. Xenobiotica 1990, 20, 901–907. [Google Scholar] [CrossRef]
- Cazzola, R.; Russo-Volpe, S.; Cervato, G.; Cestaro, B. Biochemical assessments of oxidative stress, erythrocyte membrane fluidity and antioxidant status in professional soccer players and sedentary controls. Eur. J. Clin. Invest. 2003, 33, 924–930. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldialdehyde and related aldehydes. Free Rad. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Montilla-López, P.; Muñoz-Agueda, M.C.; Feijóo López, M.; Muñoz-Castañeda, J.R.; Bujalance-Arenas, I.; Túnez-Fiñana, I. Comparison of melatonin versus vitamin C on oxidative stress and antioxidant enzyme activity in Alzheimer's disease induced by okadaic acid in neuroblastoma cells. Eur. J. Pharmacol. 2002, 451, 237–243. [Google Scholar] [CrossRef]
- Nair, U.; Bartsch, H.; Nair, J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Rad. Biol. Med. 2007, 43, 1109–1120. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the methanolic extract MEX are available from the authors (GR or FR).
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ricci, F.; Berardi, V.; Risuleo, G. Differential Cytotoxicity of MEX: a Component of Neem Oil Whose Action Is Exerted at the Cell Membrane Level. Molecules 2009, 14, 122-132. https://doi.org/10.3390/molecules14010122
Ricci F, Berardi V, Risuleo G. Differential Cytotoxicity of MEX: a Component of Neem Oil Whose Action Is Exerted at the Cell Membrane Level. Molecules. 2009; 14(1):122-132. https://doi.org/10.3390/molecules14010122
Chicago/Turabian StyleRicci, Francesca, Valerio Berardi, and Gianfranco Risuleo. 2009. "Differential Cytotoxicity of MEX: a Component of Neem Oil Whose Action Is Exerted at the Cell Membrane Level" Molecules 14, no. 1: 122-132. https://doi.org/10.3390/molecules14010122




