Synthesis of Lipoamino Acids and Their Activity against Cerebral Ischemic Injury
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical synthesis
2.2. Biological activity
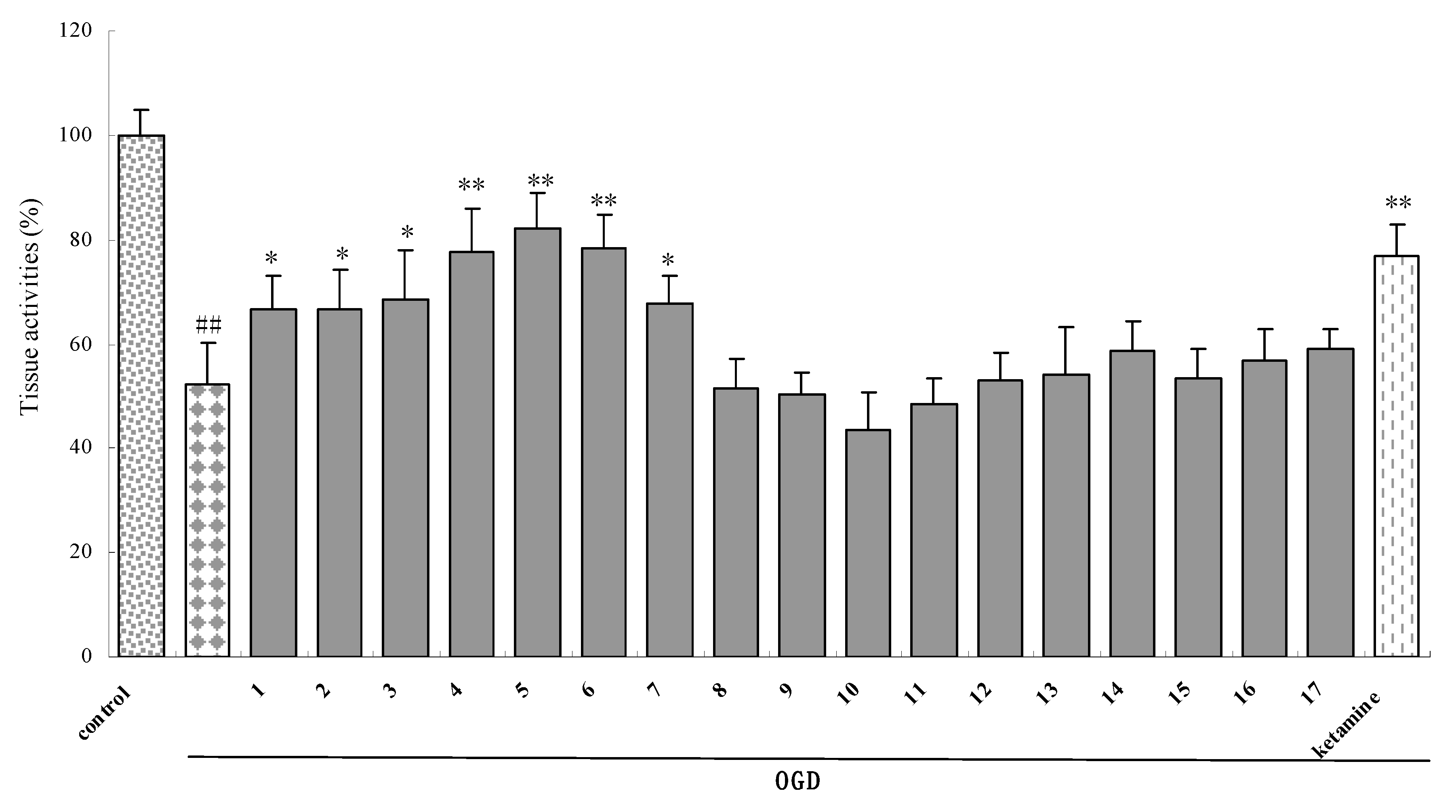
2.2.1. Effect of the synthesized compounds on OGD-insulted rat brain slices
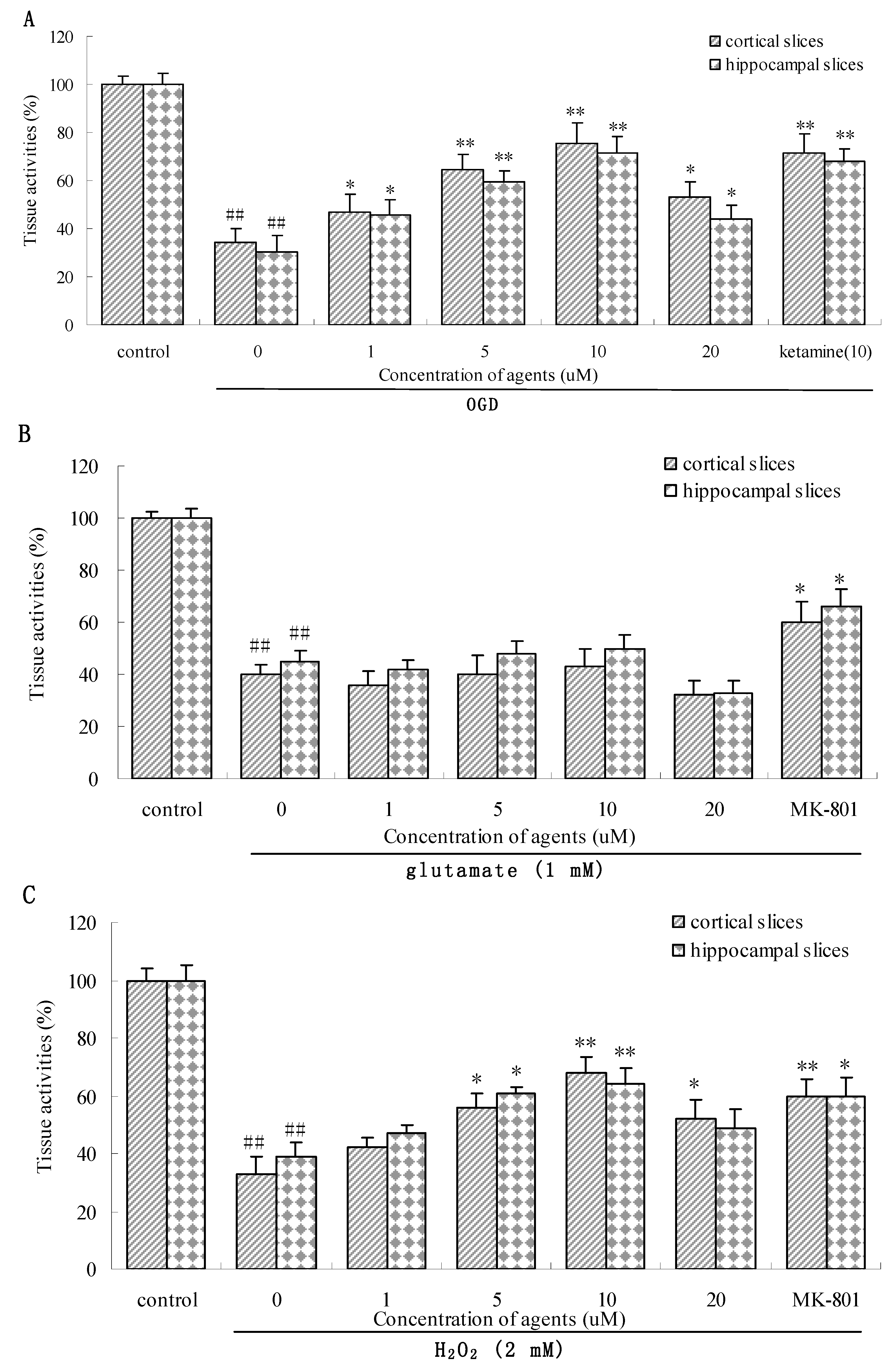
2.2.2. Effect of 5 on three types of insulted brain slices
3. Experimental
3.1. General
3.2. Preparation of N-stearoylamino acids and their derivatives
3.2.1. Synthesis and spectroscopic data of N-stearoylamino acids 1-11
3.2.2. Synthesis and spectroscopic data of N-stearoylamino acid methyl esters 12-14
3.2.3. Synthesis and spectroscopic data of N-palmitoyl-l-tyrosine (15)
3.2.4. Synthesis and spectroscopic data of N-lauroyl-l-tyrosine (16)
3.2.5. Synthesis and spectroscopic data of N-stearoyl-l-tyrosinol (17)
3.3. Neuroprotective effects of the target compounds on rat brain slices in vitro injury models
4. Conclusions
Acknowledgments
References and Notes
- Harukuni, I.; Bhardwaj, A. Mechanisms of brain injury after global cerebral ischemia. Neurol. Clin. 2006, 24, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.Y.; Farooqui, A.A. Iron, neuroinflammation, and Alzheimer’s disease. J. Alzheimers Dis. 2005, 8, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Mosley, R.L.; Benner, E.J.; Kadiu, I.; Thomas, M.; Boska, M.D.; Hasan, K.; Laurie, C.; Gendelman, H.E. Neuroinflammation, oxidative stress and the pathogenesis of Parkinson’s disease. Clin. Neurosci. Res. 2006, 6, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Ossa, N.; Dávalos, A. Neuroprotection in cerebral infarction: the opportunity of new studies. Cerebrovasc. Dis. 2007, 24, 153–156. [Google Scholar] [CrossRef] [PubMed]
- LinksPoewe, W. The need for neuroprotective therapies in Parkinson’s disease: A clinical perspective. Neurology 2006, 66, 2–9. [Google Scholar]
- Golde, T.E. The therapeutic importance of understanding mechanisms of neuronal cell death in neurodegenerative disease. Mol. Neurodegener. 2009, 4, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, W.; Liao, G.H.; Bi, X.N.; Baudry, M. Neuroprotection against neonatal hypoxia/ischemia-induced cerebral cell death by prevention of calpain-mediated mGluR1alpha truncation. Exp. Neurol. 2009, 218, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Abe, K. Neuroprotective therapy for ischemic stroke with free radical scavenger and gene-stem cell therapy. Rinsho Shinkeigaku 2008, 48, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Bye, N.; Habgood, M.D.; Callaway, J.K.; Malakooti, N.; Potter, A.; Kossmann, T.; Morganti-Kossmann, M.C. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp. Neurol. 2007, 204, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, M.D. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology 2008, 55, 363–389. [Google Scholar] [CrossRef] [PubMed]
- Galve-Roperh, I.; Aguado, T.; Rueda, D.; Velasco, G.; Guzmán, M. Endocannabinoids: A new family of lipid mediators involved in the regulation of neural cell development. Curr. Pharm. Des. 2006, 12, 2319–2325. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Mazzola, C.; Drago, F. Endocannabinoids and neurodegenerative diseases. Pharmacol. Res. 2007, 56, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Bahr, B.A.; Karanian, D.A.; Makanji, S.S.; Makriyannis, A. Targeting the endocannabinoid system in treating brain disorders. Expert. Opin. Investig. Drugs 2006, 15, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S. The elmiric acids: Biologically active anandamide analogs. Neuropharmacology 2008, 55, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S.H.; Adams, J.K.; Bradshaw, H.B.; Fraioli, C.; Rossetti, R.G.; Salmonsen, R.A.; Shaw, J.W.; Walker, J.M.; Zipkin, R.E.; Zurier, R.B. Potential anti-inflammatory actions of the elmiric (lipoamino) acids. Bioorg. Med. Chem. 2007, 15, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Bisogno, T.; Petros, T.J.; Chang, S.Y.; Zavitsanos, P.A.; Zipkin, R.E.; Sivakumar, R.; Coop, A.; Maeda, D.Y.; De Petrocellis, L.; Burstein, S.; Di Marzo, V.; Walker, J.M. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J. Biol. Chem. 2001, 276, 42639–42644. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Li, G.M.; Nie, B.M.; Lu, Y.; Yin, M. Neuroprotective effect of the stearic acid against oxidative stress via phosphatidylinositol 3-kinase pathway. Chem. Biol. Interact. 2006, 160, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, S.; Vallee, B.L. Superactivation of thermolysin by acylation with amino acid N-hydroxysuccinimide esters. Biochem. 1975, 14, 2410–2419. [Google Scholar] [CrossRef]
- Mckennon, M.J.; Meyers, A.I.; Drauz, K.; Schwarm, M. A convenient reduction of amino acids and their derivatives. J. Org. Chem. 1993, 58, 3568–3571. [Google Scholar] [CrossRef]
- Bhaskar, J.V.; Periasamy, M. Selective reduction of carboxylic acids into alcohols using NaBH4 and I2. J. Org. Chem. 1991, 56, 5964–5965. [Google Scholar]
- Xue, Q.S.; Yu, B.W.; Wang, Z.J.; Chen, H.Z. Effects of ketamine, midazolam, thiopental, and propofol on brain ischemia injury in rat cerebral cortical slices. Acta. Pharmacol. Sin. 2004, 25, 115–120. [Google Scholar] [PubMed]
- Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [CrossRef] [PubMed]
- Mathew, K.S.; McLaughlin, D.P.; Ziabari, L.H.; Toner, C.C.; Street, P.C.; Hisgrove, E.; Bezzina, E.L.; Stamford, J.A. Rapid quantification of ischemic injury cerebroprotection in brain slices using densitometric assessment of 2,3,5-triphenyltetrazolium chloride staining. J. Neurosci. Methods 2000, 102, 43–51. [Google Scholar] [CrossRef]
- Tureyen, K.; Vemuganti, R.; Sailor, K.A.; Dempsey, R.J. Infarct volume quantification in mouse focal cerebral ischemia: A comparison of triphenyltetrazolium chloride and cresyl violet staining techniques. J. Neurosci. Methods 2004, 139, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Toner, C.C.; Miline, A.J.; Blatchford, K.L.; McLaughlin, D.P.; Stamford, J.A. An assessment of the cerebroprotective potential of volatile anaesthetics using two independent methods in an in vitro model of cerebral ischemia. Brain Res. 2002, 958, 390–398. [Google Scholar] [CrossRef]
- Bickler, P.E.; Hansen, B.M. Hypoxia-tolerant neonatal CA1 neurons: relationship of survival to evoked glutamate release and glutamate receptor-mediated calcium changes in hippocampal slices. Brain Res. Dev. Brain Res. 1998, 106, 57–69. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Kan, M.Y.; Yang, Z.H.; Ding, W.L.; Yi, J.; Chen, H.Z.; Lu, Y. Neuroprotective effects of N-stearoyltyrosine on transient global cerebral ischemia in gerbils. Brain Res. 2009, 1287, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.W.; Zimmerman, J.E.; Callahan, F.M. The use of esters of N-hydroxysuccinimide in peptide synthesis. J. Am. Chem. Soc. 1964, 86, 1839–1842. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds 1–17 are available from the authors. |



| Chemical Name | R |
|---|---|
| 1. N-stearoyl-L-phenylalanine | 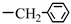 |
| 2. N-stearoyl-DL-phenylalanine |  |
| 3. N-stearoyl-L-proline |  |
| 4. N-stearoyl-L-tyrosine | 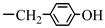 |
| 5. N-stearoyl-L-serine | —CH2OH |
| 6. N-stearoyl-L-threonine | 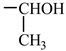 |
| 7. N-stearoyl-L-tryptophan |  |
| 8. N-stearoyl-L-leucine | —CH(CH3)2 |
| 9. N-stearoyl-L-cysteine | —CH2SH |
| 10. N-stearoyl-L-histidine | 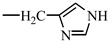 |
| 11. N-stearoyl-L-lysine | —(CH2)4NH2 |
| 12. N-stearoylglycine methyl ester | —H |
| 13. N-stearoyl-L-glutamic acid dimethyl ester | —(CH2)2COOCH3 |
| 14. N-stearoyl-L-phenylalanine methyl ester | 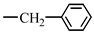 |
| 15. N--palmitoyl-L-tyrosine | —COOH, n = 14 |
| 16. N- lauroyl-L-tyrosine | —COOH, n = 10 |
| 17. N-stearoyl-L-tyrosinol | —CH2OH, n = 16 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yao, L.-Y.; Lin, Q.; Niu, Y.-Y.; Deng, K.-M.; Zhang, J.-H.; Lu, Y. Synthesis of Lipoamino Acids and Their Activity against Cerebral Ischemic Injury. Molecules 2009, 14, 4051-4064. https://doi.org/10.3390/molecules14104051
Yao L-Y, Lin Q, Niu Y-Y, Deng K-M, Zhang J-H, Lu Y. Synthesis of Lipoamino Acids and Their Activity against Cerebral Ischemic Injury. Molecules. 2009; 14(10):4051-4064. https://doi.org/10.3390/molecules14104051
Chicago/Turabian StyleYao, Li-Yun, Qi Lin, Yin-Yao Niu, Ke-Min Deng, Jian-Hua Zhang, and Yang Lu. 2009. "Synthesis of Lipoamino Acids and Their Activity against Cerebral Ischemic Injury" Molecules 14, no. 10: 4051-4064. https://doi.org/10.3390/molecules14104051




