Rhodanineacetic Acid Derivatives as Potential Drugs: Preparation, Hydrophobic Properties and Antifungal Activity of (5-Arylalkylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)acetic Acids
Abstract
:1. Introduction
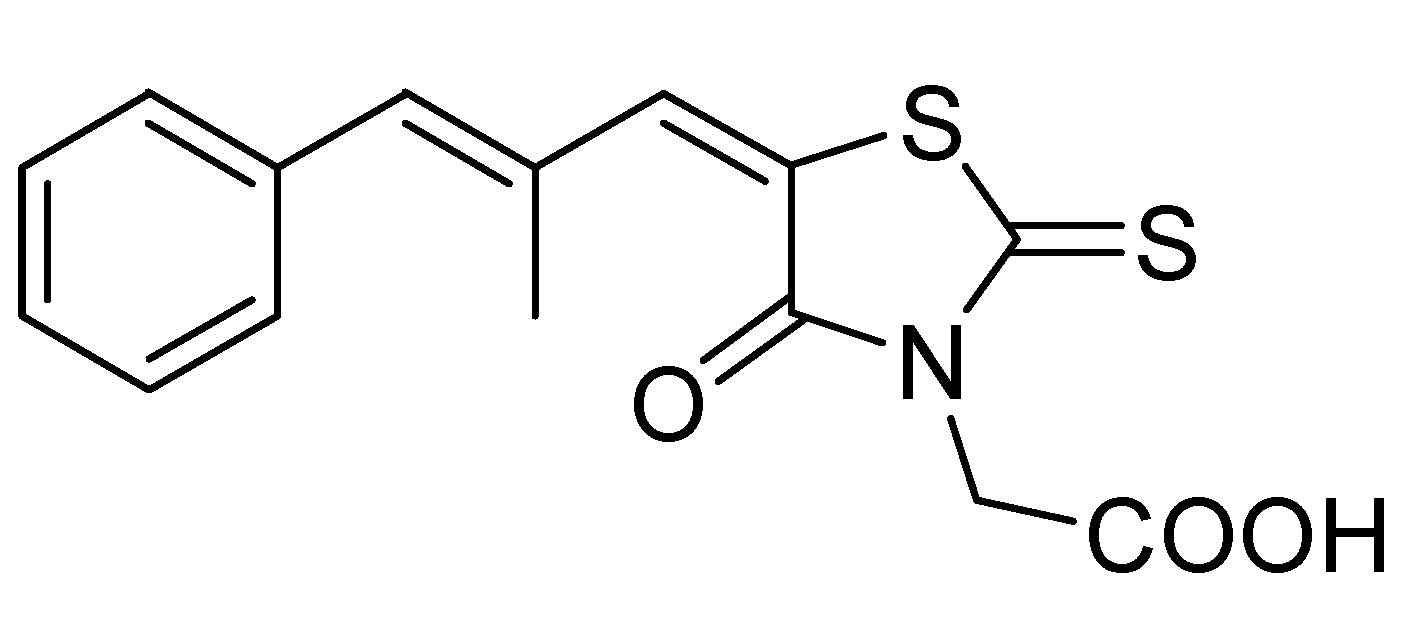
2. Results and Discussion
2.1. Chemistry

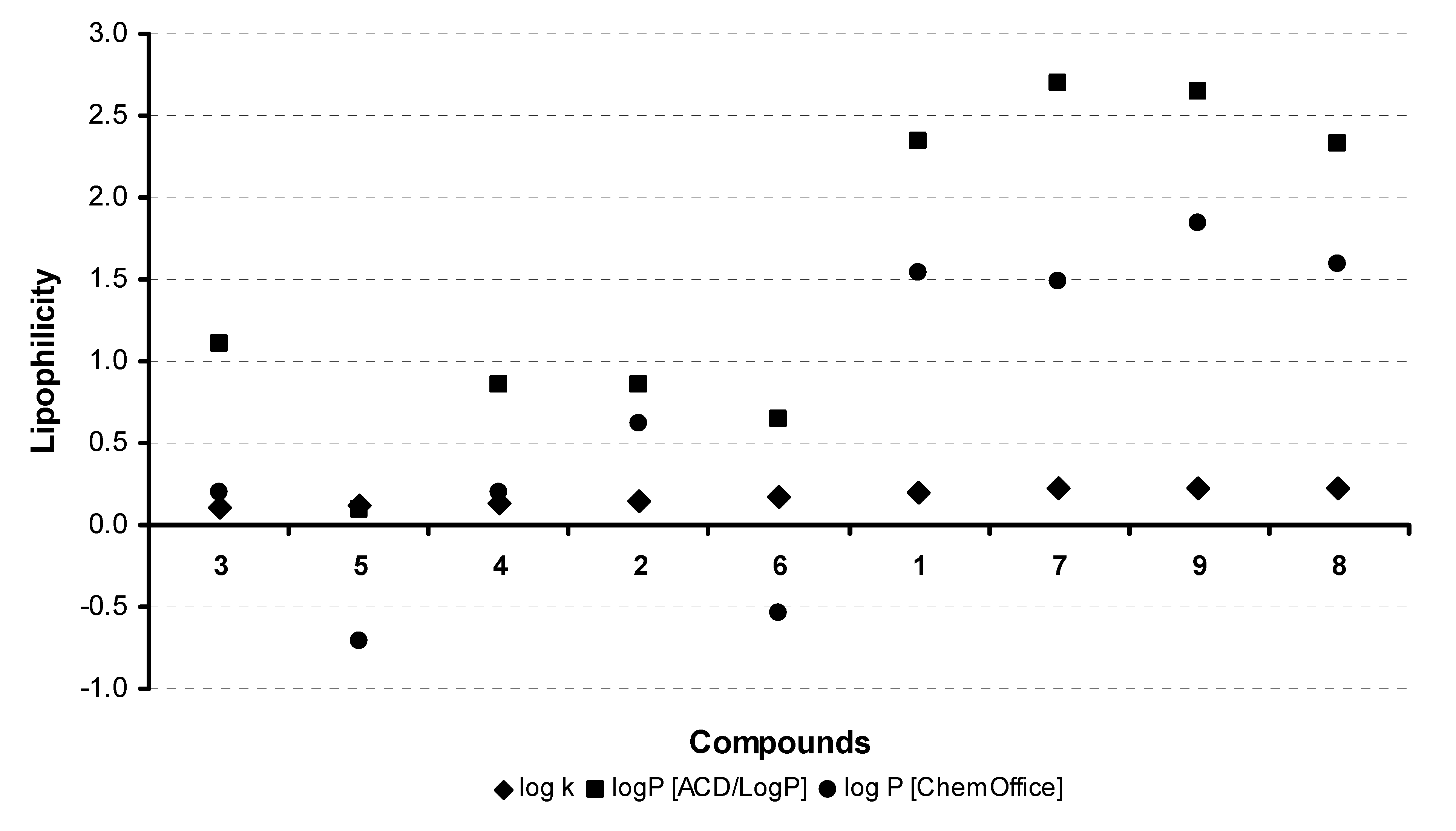
2.2. Lipophilicity
 | ||||||
| Comp. | R | log k | log P | Z | E | ND |
| 1 | 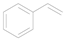 | 0.2013 | 2.34 ± 0.81a | 7.41b | 6.80b | 7.88c7.81d |
| 1.54b | ||||||
| 2 | 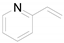 | 0.1399 | 0.85 ± 0.82a | 7.62b | 7.01b | - |
| 0.62b | ||||||
| 3 |  | 0.1116 | 1.10 ± 0.82a | 7.41b | 6.80b | 7.95c |
| 0.20b | ||||||
| 4 | 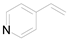 | 0.1342 | 0.85 ± 0.82a | 7.39b | 6.78b | 7.87c |
| 0.20b | ||||||
| 5 | 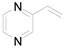 | 0.1165 | 0.09 ± 0.82a | 7.41b | 6.80b | - |
| -0.71b | ||||||
| 6 | 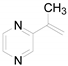 | 0.1734 | 0.65 ± 0.84a | - | - | - |
| -0.54b | ||||||
| 7 | 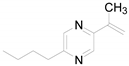 | 0.2270 | 2.70 ± 0.84a | - | - | - |
| 1.49b | ||||||
| 8 | 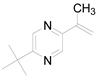 | 0.2301 | 2.33 ± 0.84 | - | - | - |
| a1.59b | ||||||
| 9 | 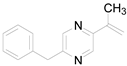 | 0.2276 | 2.64 ± 0.84a | - | - | - |
| 1.84b | ||||||
2.3. In vitro antifungal activity
| Strain | MIC/IC80 [μmol/L] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | FLU | ||
| CA | 24h | >500 | 500 | >500 | >500 | >250 | >500 | >500 | >500 | 500 | 1.09 |
| 48h | >500 | 500 | >500 | >500 | >250 | >500 | >500 | >500 | >500 | 2.17 | |
| CT | 24h | >500 | 0.98 | 500 | >500 | >250 | 500 | 500 | >500 | >500 | 2,72 |
| 48h | >500 | 1.95 | >500 | >500 | >250 | >500 | >500 | >500 | >500 | 5.44 | |
| CK | 24h | >500 | 1.95 | 500 | >500 | >250 | 500 | 500 | >500 | >500 | 87.07 |
| 48h | >500 | 1.95 | >500 | >500 | >250 | >500 | >500 | >500 | >500 | 174.14 | |
| CG | 24h | >500 | 0.98 | 250 | >500 | >250 | 250 | 250 | >500 | >500 | 21.77 |
| 48h | >500 | 1.95 | 500 | >500 | >250 | 500 | 500 | >500 | >500 | 69.65 | |
| TA | 24h | >500 | 1.95 | 500 | >500 | >250 | 500 | 500 | >500 | >500 | 4.35 |
| 48h | >500 | 1.95 | >500 | >500 | >250 | >500 | >500 | >500 | >500 | 8.71 | |
| AF | 24h | >500 | >500 | >500 | >500 | >250 | >500 | >500 | >500 | >500 | >419 |
| 48h | >500 | >500 | >500 | >500 | >250 | >500 | >500 | >500 | >500 | >419 | |
| AC | 24h | >500 | >500 | >500 | >500 | >250 | >500 | >500 | >500 | >500 | >128 |
| 48h | >500 | >500 | >500 | >500 | >250 | >500 | >500 | >500 | >500 | >128 | |
| TM | 72h | >500 | >500 | >500 | >500 | >250 | >500 | >500 | >500 | >500 | 5.04 |
| 120h | >500 | >500 | >500 | >500 | >250 | >500 | >500 | >500 | >500 | 8.00 | |
3. Conclusions
4. Experimental
4.1. General
4.2. Synthesis
4.3. Lipophilicity HPLC determination (capacity factor k/calculated log k)
4.4. In vitro evaluation of antifungal activity
Acknowledgements
- Samples Availability: Samples of the compounds are available from the authors.
References
- Boyd, D.B. On the rhodanines and their presence in biologically active ligands. J. Mol. Struct. Theochem. 1997, 401, 227–234. [Google Scholar]
- Körner, H. Derivatives of dithiocarbaminoacetic acid. Ber. Dtsch. Chem. Ges. 1908, 41, 1901–1905, [Chem. Abstr. 1908, 2, 12300]. [Google Scholar]
- Andreasch, R. Substituted rhodaninic acids and their aldehyde condensation products. VII. Monatsh. Chem. 1908, 29, 399–419, [Chem. Abstr. 1908, 2, 14948]. [Google Scholar]
- Taniyama, H.; Yasui, B.; Takehara, N.; Uchida, H. Chemotherapeutics for Mycobacterium tuberculosis. XIX. Synthesis and antibacterial activity of some 3-substituted rhodanines. Yakugaku Zasshi 1959, 79, 1465–1468, [Chem. Abstr. 1960, 54, 34220]. [Google Scholar]
- Singh, J.; Nathan, C.F.; Bryk, R.; Samy, R.; Pupek, K.; Gurney, M. Cyclic carboxylic acid rhodanine derivatives for the treatment and prevention of tuberculosis. WO Pat. 200800 5651, 2008. [Google Scholar]
- Allan, F.J.; Allan, G.G.; Crank, G.; Jack, J. The condensation of rhodanine and derivatives with benzaldehyde sulphonic acids. Recl. Trav. Chim. Pays Bas 1960, 79, 247–254. [Google Scholar]
- Allan, F.J.; Allan, G.G. The condensation of rhodanine and derivatives with 4-antipyrinaldehyde. Can. J. Chem. 1961, 39, 1397–1399. [Google Scholar] [CrossRef]
- Allan, F.J.; Allan, G.G.; Thompson, J.B. The condensation of rhodanine and derivatives with pyridine and quinoline aldehydes. Recl. Trav. Chim. Pays Bas 1961, 80, 403–408. [Google Scholar]
- Allan, F.J.; Allan, G.G. The condensation of rhodanine and derivatives with some indole aldehydes. Monatsh. Chem. 1963, 94, 1434–4475, [Chem. Abstr. 1963, 59, 48312]. [Google Scholar]
- Allan, F.J.; Allan, G.G. The condensation of rhodanine and derivatives with aromatic aldehydes containing iodine. Recl. Trav. Chim. Pays Bas 1962, 82, 177–181. [Google Scholar]
- Allan, F.J.; Allan, G.G. Izoxazolylmethylenerhodanines. Recl. Trav. Chim. Pays Bas 1964, 83, 1299–1300. [Google Scholar]
- Orchard, M.G.; Neuss, J.C.; Galley, C.M.S. Benzylidene thiazolidinediones and their use as antimycotic agents. WO Pat. 0201 7915, 2002. [Google Scholar]
- Orchard, M.G.; Neuss, J.D. Preparation of thiazolidines as antifungal agents. WO Pat. 022 2612, 2002. [Google Scholar]
- Orchard, M.G. Preparation of 5-benzylidene-4-thiazolidinone-2-thione antifungal agents. WO Pat. 0307 0238, 2003. [Google Scholar]
- Orchard, M.G.; Neuss, J.C.; Galley, C.M.S.; Carr, A.; Porter, D.W.; Smith, P.; Scopes, D.I.C.; Haydon, D.; Vousden, K.; Stubberfield, C.R.; Young, K.; Page, M. Rhodanine-3-acetic acid derivatives as inhibitors of fungal protein mannosyl transferase 1 (PMT 1). Bioorg. Med. Chem. Lett. 2004, 14, 3975–3978. [Google Scholar]
- Dovlatyan, V.V.; Avetisyan, F.V. 2-Thioxo-4-imino(oxo)-1,3-thiazolidin-3-ylacetic acid and its alkyl esters. Armyanskii Khim. Zh. 1973, 26, 494–498, [Chem. Abstr. 1973, 79, 115482]. [Google Scholar]
- Inamori, Y.; Muro, C.; Tanaka, R.; Adachi, A.; Miyamoto, K.; Tsujibo, H. Phytogrowth-inhibitory activity of sulfur-containing compounds. I. Inhibitory activities of thiazolidine derivatives on plant growth. Chem. Pharm. Bull. 1992, 40, 2854–2856. [Google Scholar]
- Muro, C.; Yasuda, M.; Sakagami, Y.; Yamada, T.; Tsujibo, H.; Numata, A.; Inamori, Y. Inhibitory activities of rhodanine derivatives on plant growth. Biocsi. Biotechnol. Biochem. 1996, 60, 1368–1371. [Google Scholar]
- Frankov, I.A.; Kirillov, M.V.; Sokolova, T.N.; Skupskaya, R.; Kharitonovich, A.N.; Chizhevskaya, I.I. Synthesis and pharmacological properties of alkyl derivatives of 3-carboxyalkylrhodanine. Khim.-Farm. Zh. 1985, 19, 943–946, [Chem. Abstr. 1986, 104, 129831]. [Google Scholar]
- Friebe, W.G.; Krell, H.W.; Woelle, S.; Wolff, H.P. Thiazolidine carboxylic acid derivatives and their use in the treatment of cancer. WO Pat. 0157006, 2001. [Google Scholar]
- Singh, R.; Ramesh, U.V.; Goff, D.; Laidig, G.; Issakani, S.D.; Huang, J.; Payan, D.G.; Clough, J. Rhodanine derivatives and pharmaceutical compositions containing them. WO Pat. 200404 3955, 2004. [Google Scholar]
- Esswein, A.; Schaefer, W.; Tsaklakidis, C.; Honold, K.; Kaluza, K. Rhodanine carboxylic acid derivatives for the treatment and prevention of metabolic bone disorders. US Pat. App. 200303 2813, 2003. [Google Scholar]
- Esswein, A.; Schaefer, W.; Tsaklakidis, C.; Honold, K.; Kaluza, K. Rhodanine carboxylic acid derivatives for the treatment and prevention of metabolic bone disorders. US Pat. App. 667 3816, 2004. [Google Scholar]
- Smith, T.J.; Young, B.L.; Denton, H.; Hughes, D.L.; Wagner, G.W. First small molecular inhibitors of T. brucei dolicholphospate mannose synthase (DPMS), a validated target in African sleeping sickness. Bioorg. Med. Chem. Lett. 2009, 19, 1749–1752. [Google Scholar]
- Ziegler, D. Treatment of diabetic neuropathy and neuropathic pain: how far have we come? Diabetes Care 2008, 31 (Suppl. 2), S255–S261. [Google Scholar]
- Ramirez, M.A.; Borja, N.L. Epalrestat: An aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy 2008, 28, 646–655. [Google Scholar] [CrossRef]
- Tanaouchi, T.; Kawamura, M.; Ajima, A.; Mohri, T.; Hayashi, M.; Terashima, H.; Hirata, F.; Morimura, T. Rhodanine derivatives, process for their preparation, and aldose reductase inhibitor containing the rhodanine derivatives as active ingredient. EP Pat. 0047109, 1982. [Google Scholar]
- Tanaouchi, T.; Kawamura, M.; Ajima, A.; Mohri, T.; Hayashi, M.; Terashima, H.; Hirata, F.; Morimura, T. Rhodanine derivatives, process for their preparation, and aldose reductase inhibitor containing the rhodanine derivatives as active ingredient. US Pat. 4464382, 1984. [Google Scholar]
- Tomasic, T.; Masic, L.P. Rhodanine as a privileged scaffold in drug discovery. Curr. Med. Chem. 2009, 16, 1596–1629. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Avdeef, A. Physicochemical profiling (permeability, solubility, charge state). Curr. Topics Med. Chem. 2001, 1, 277–351. [Google Scholar] [CrossRef]
- Pliska, V. Lipophilicity in drug action and toxicology. In Methods and Principles in Medicinal Chemistry, 1st; Pliska, V., Testa, B., van der Waterbeemd, H., Eds.; Wiley-VCH: Weinheim, Germany, 1996; Volume 4, pp. 1–6. [Google Scholar]
- Gocan, S.; Cimpan, G.; Comer, J. Lipophilicity measurements by liquid chromatography. Adv. Chromatogr. 2006, 44, 79–176. [Google Scholar]
- Opletalova, V.; Dolezal, M.; Hartl, J. Oxidation of hydroxymethylpyrazine using sonication. In Book of Abstracts of the 23rd Conference Drug Synthesis and Analysis, the 23rd Conference Drug Synthesis and Analysis, Bratislava, Slovakia, October 4–10; 1994; p. 25. [Google Scholar]
- Opletalova, V.; Patel, A.; Boulton, M.; Dundrova, A.; Lacinova, E.; Prevorova, M.; Appeltauerova, M.; Coufalova, M. 5-Alkyl-2-pyrazinecarboxamides, 5-alkyl-2-pyrazinecarbonitriles and 5-alkyl-2-acetylpyrazines as synthetic intermediates for antiinflammatory agents. Collect. Czech Chem. Commun. 1996, 61, 1093–1101. [Google Scholar] [CrossRef]
- Opletalova, V.; Jampilek, J.; Chlupacova, M.; Dolezel, J.; Dohnal, J. Chromatographic and computational study of hydrophobic properties of ring substituted pyrazinecarbonitriles and acetylpyrazines. In Proceedings of the 9th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-9), Proceedings of the 9th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-9), November 1–30, 2005 [CD-ROM ed.]; Seijas, J.A., Tato M.P., V., Eds.; MDPI: Basel, Switzerland, 2005; p. c004. [Google Scholar]
- Jampilek, J.; Opletalova, V.; Grafnetterova, T.; Dohnal, J. Thiosemicarbazones of acetylpyrazines: preparation and their hydrophobic properties. Proceedings of the 9th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-9), November 1–30, 2005 [CD-ROM ed.]; Seijas, J.A., Tato, M.P.V., Eds.; MDPI: Basel, Switzerland, 2005; p. a001. [Google Scholar]
- Jampilek, J.; Opletalova, V.; Dohnal, J. N,N-Dimethylthiosemicarbazones of acetylpyrazines: Preparation and their hydrophobic properties. Proceedings of the 10th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-10), November 1–30, 2006 [CD-ROM ed.]; Seijas J., A., Tato M. P., V., Eds.; MDPI: Basel, Switzerland, 2006; p. a004. [Google Scholar]
- Jampilek, J.; Dolezal, M.; Osicka, Z.; Kunes, J.; Kralova, K. Substituted pyrazine-2-carboxamides: preparation and their photosynthesis-inhibiting activity. Proceedings of the 7th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-7), November 1–30, 2003 [CD-ROM ed.]; Seijas, J.A., Tato, M.P.V., Eds.; MDPI: Basel, Switzerland, 2003; p. c001. [Google Scholar]
- Chlupacova, M.; Opletalova, V.; Kunes, J.; Silva, L.; Buchta, V.; Duskova, L.; Kralova, K. Synthesis and biological evaluation of some ring-substituted (E)-3-aryl-1-pyrazin-2-ylprop-2-en-1-ones. Folia Pharm. Univ. Carol. 2005, 33, 31–43. [Google Scholar]
- Opletalova, V.; Pour, M.; Kunes, J.; Buchta, V.; Silva, L.; Kralova, K.; Chlupacova, M.; Meltrova, D.; Peterka, M.; Poslednikova, M. Synthesis and biological evaluation of (E)-3-(nitrophenyl)-1-(pyrazin-2-yl)prop-2-en-1-ones. Collect. Czech Chem. Commun. 2006, 71, 44–58. [Google Scholar] [CrossRef]
- Dolezal, M.; Palek, L.; Vinsova, J.; Buchta, V.; Jampilek, J.; Kralova, K. Substituted pyrazinecarboxamides. Synthesis and biological evaluation. Molecules 2006, 11, 242–256. [Google Scholar] [CrossRef]
- Jampilek, J.; Opletalova, V.; Dolezel, J.; Dohnal, J. Preparation and hydrophobic properties of 5-arylalkylidenerhodanines. Proceedings of the 11th International Electronic Conference on Synthetic Organic Chemistry (ECSOC-11), November 1–30, 2007 [CD-ROM ed.]; Seijas, J.A., Tato, M.P.V., Eds.; MDPI: Basel, Switzerland, 2003; p. a012. [Google Scholar]
- Kucerova-Chlupacova, M.; Opletalova, V.; Jampilek, J.; Dolezel, J.; Dohnal, J.; Kunes, J.; Pour, M.; Kunes, J.; Vorisek, V. New hydrophobicity constants of substituents in pyrazine rings derived from RP-HPLC Study. Collect. Czech Chem. Comm. 2008, 73, 1–18. [Google Scholar] [CrossRef]
- Dolezel, J.; Hirsova, P.; Opletalova, V.; Vejsova, M.; Jampilek, J. Synthesis and biological evaluation of rhodanine and N-substituted rhodanine derivatives.Book of Abstracts of the 37th Conference Drug Synthesis and Analysis. In Chem. Listy, Brno, Czech Republic, September 8–10, 2008; 2008; 102, p. S215. [Google Scholar]
- Opletalova, V.; Kalinowski, D.; Vejsova, M.; Kunes, J.; Pour, M.; Jampilek, J.; Buchta, V.; Richardson, D.R. Identification and characterization of thiosemicarbazones with anti-fungal and anti-tumor effects: Cellular iron-chelation mediating cytotoxic activity. Chem. Res. Toxicol. 2008, 21, 1878–1889. [Google Scholar]
- Zhou, J.F.; Song, Y.Z.; Zhu, F.X.; Zhu, Y.L. Facile synthesis of 5-benzylidene rhodanine derivatives under microwave irradiation. Synthetic Commun. 2006, 36, 3297–3303. [Google Scholar] [CrossRef]
- Sing, W.T.; Lee, C.L.; Yeo, S.L.; Lim, S.P.; Sim, M.M. Arylalkylidene rhodanine with bulky and hydrophobic functional group as selective HCV NS3 protease inhibitor. Bioorg. Med. Chem. Lett. 2001, 11, 91–94. [Google Scholar]
- Khodair, A.I. A convenient synthesis of 2-arylidene-5H-thiazolo[2,3-b]quinazoline-3,5[2H]-diones and their benzoquinazoline derivatives. J. Heterocycl. Chem. 2002, 39, 1153–1160. [Google Scholar] [CrossRef]
- Ohishi, Y.; Mukai, T.; Nagahara, M.; Yajima, M.; Kajikawa, N.; Miahara, K.; Takano, T. Preparations of 5-alkylmethylidene-3-carboxymethylrhodanine derivatives and their aldose reductase inhibitory activity. Chem. Pharm. Bull. 1990, 38, 1911–1919. [Google Scholar] [CrossRef]
- Whitessit, C.A.; Simon, R.L.; Reel, J.K.; Sigmund, S.K.; Phillips, M.L.; Shadle, J.K.; Heintz, L.W.; Koppel, G.A.; Hunden, D.C.; Lifer, S.L.; Berry, D.; Ray, J.; Little, S.P.; Liu, X.; Marshall, W.S.; Panetta, J.A. Synthesis and structure-activity relationships of benzophenones as inhibitors of cathepsin D. Bioorg. Med. Chem. Lett. 1996, 6, 2157–2162. [Google Scholar] [CrossRef]
- Kerns, E.H.; Li, D. Drug-Like Properties: Concept, Structure Design and Methods; Elsevier: San Diego, CA, USA, 2008. [Google Scholar]
- Valko, K. Application of high-performance liquid chromatography based measurements of lipophilicity to model biological distribution. J. Chromatogr. A 1037, 299–310. [Google Scholar]
- Valko, K.; Du, C.M.; Bevan, C.; Reynolds, D.P.; Abraham, M.H. Rapid method for the estimation of octanol/water partition coefficient (log Poct) from gradient RP-HPLC retention and a hydrogen bond acidity term (Sa2H). Curr. Med. Chem. 2001, 8, 1137–1146. [Google Scholar] [CrossRef]
- Cimpan, G.; Irimie, F.; Gocan, S.; Claessens, H.A. Role of stationary phase and eluent composition on the determination of log P values of N-hydroxyethylamide of aryloxyalkylen and pyridine carboxylic acids by reversed-phase high-performance liquid chromatography. J. Chromatogr. B 1998, 714, 247–261. [Google Scholar] [CrossRef]
- Hartmann, T.; Schmitt, J. Lipophilicity-beyond octanol/water: A short comparison of modern technologies. Drug Discov. Today Technol. 2004, 1, 431–439. [Google Scholar] [CrossRef]
- Nasal, A.; Siluk, D.; Kaliszan, R. Chromatographic retention parameters in medicinal chemistry and molecular pharmacology. Curr. Med. Chem. 2003, 10, 381–426. [Google Scholar] [CrossRef]
- Piraprez, G.; Herent, M.F.; Collin, S. Determination of the lipophilicity of aroma compounds by RP-HPLC. Flavour Fragr. J. 1998, 13, 400–408. [Google Scholar] [CrossRef]
- Yamagami, C.; Iwasaki, K.; Ishikawa, A. Hydrophobicity parameters determined by reversed-phase liquid chromatography. XII. Comparison of capacity factors and octane/methanol-water partition coefficients for monosubstituted pyrazines, and effect of octanol added to both partitioning systems. Chem. Pharm. Bull. 1997, 45, 1653–1658. [Google Scholar]
- Yamagami, C.; Araki, K.; Ohnishi, K.; Hanasato, K.; Inaba, H.; Aono, M.; Ohta, A. Measurement and prediction of hydrophobicity parameters for highly lipophilic compounds: Application of the HPLC column-switching technique to measurement of log P of diarylpyrazines. J. Pharm. Sci. 1999, 88, 1299–1304. [Google Scholar] [CrossRef]
- Yamagami, C.; Kawase, K.; Iwaki, K. Hydrophobicity parameters determined by reversed-phase liquid chromatography. XV: Optimal conditions for prediction of log Poct by using RP-HPLC procedures. Chem. Pharm. Bull. 2002, 50, 1578–1583. [Google Scholar]
- Sortino, M.; Delgado, P.; Juarez, S.; Quiroga, J.; Abonía, R.; Insuasty, B.; Nogueras, M.; Roredo, L.; Garibotto, F. M.; Enriz, R.D.; Zacchino, S.A. Synthesis and antifungal activity of (Z)-5-arylidenerhodanines. Bioorg. Med. Chem. 2007, 15, 484–494. [Google Scholar]
- Petrik, P.; Kunes, J.; Vejsova, M.; Jampilek, J.; Spaningerova, E.; Kesetovicova, D.; Vlckova, M.; Majd, M.; Kalafutova, S.; Opletalova, V. Antifungal and antimycobacterial properties of 5-arylmethylidenerhodanines and their N3-substituted analogues. Book of Abstracts of the 5th International Postgraduate Research Symposium on Pharmaceutics (IPORSIP-2007). In Acta Pharma. Sci., Istanbul, September 13-15, 2007; 2007; 49 (Suppl.), p. 77. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dolezel, J.; Hirsova, P.; Opletalova, V.; Dohnal, J.; Marcela, V.; Kunes, J.; Jampilek, J. Rhodanineacetic Acid Derivatives as Potential Drugs: Preparation, Hydrophobic Properties and Antifungal Activity of (5-Arylalkylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)acetic Acids. Molecules 2009, 14, 4197-4212. https://doi.org/10.3390/molecules14104197
Dolezel J, Hirsova P, Opletalova V, Dohnal J, Marcela V, Kunes J, Jampilek J. Rhodanineacetic Acid Derivatives as Potential Drugs: Preparation, Hydrophobic Properties and Antifungal Activity of (5-Arylalkylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)acetic Acids. Molecules. 2009; 14(10):4197-4212. https://doi.org/10.3390/molecules14104197
Chicago/Turabian StyleDolezel, Jan, Petra Hirsova, Veronika Opletalova, Jiri Dohnal, Vejsova Marcela, Jiri Kunes, and Josef Jampilek. 2009. "Rhodanineacetic Acid Derivatives as Potential Drugs: Preparation, Hydrophobic Properties and Antifungal Activity of (5-Arylalkylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)acetic Acids" Molecules 14, no. 10: 4197-4212. https://doi.org/10.3390/molecules14104197




