Bioactive Azafluorenone Alkaloids from Polyalthia debilis (Pierre) Finet & Gagnep.
Abstract
:Introduction
Results and Discussion
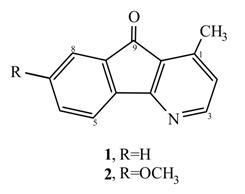
Isolation and structure elucidation

Biological activities
Antimicrobial activity
| Compound a,b,c | Microorganism | MIC e (µg/mL) |
|---|---|---|
| PC | C. diphtheriae NCTC 10356, B. subtilis ATCC 6633, | 256 |
| B. cereus, B. catarrhalis, P. shigelloides | ||
| PE | C. diphtheriae NCTC 10356, B. subtilis ATCC 6633, | 256 |
| B. cereus | ||
| C2 | B. subtilis ATCC 6633 | 256 |
| C3 | C. diphtheriae NCTC 10356, | 64 |
| B. subtilis ATCC 6633, B. catarrhalis | 128 | |
| M. lutens ATCC 10240, B. cereus, P. shigelloides, M. flavas | 256 | |
| C4 | C. diphtheriae NCTC 10356, B. subtilis ATCC 6633 | 256 |
| C5 | C. diphtheriae NCTC 10356, B. subtilis ATCC 6633 | 128 |
| B. cereus | 256 | |
| C7d | C. diphtheriae NCTC 10356, B. subtilis ATCC 6633, | 64 |
| B. cereus, B. catarrhalis, P. shigelloides | ||
| S. pyogenes | 256 | |
| C8d | C. diphtheriae NCTC 10356, B. subtilis ATCC 6633, | 64 |
| M. lutens ATCC 10240, B. cereus, B. catarrhalis, P. shigelloides, S. pyogenes | ||
| S. aureus ATCC 25923, S. epidermidis ATCC 12228, | 256 | |
| E. faecalis ATCC 29212, M. flavas, E. tarda, N. mucosa, L. monocytogenes | ||
| C9d | C. diphtheriae NCTC 10356, B. subtilis ATCC 6633, B. cereus, B. catarrhalis | 64 |
| P. shigelloides | 128 | |
| M. lutens ATCC 10240, S. epidermidis ATCC 12228, E. faecalis ATCC 29212, E. tarda | 256 | |
| E3.6 | C. diphtheriae NCTC 10356 | 256 |
| E4 | C. diphtheriae NCTC 10356, B. subtilis ATCC 6633, B. cereus, B. catarrhalis, P. shigelloides | 256 |
Antimalarial activity
| Compounda | Activity | IC50 (µg/mL) |
|---|---|---|
| PC, PE | fair | 100-1,000 |
| C2-C9, E4, E5 | fair | 100-1,000 |
| E3.6 | good | 10-100 |
Cytotoxic activity
Conclusions
| Compound | IC50 (µg/mL)a,b,c | ||
|---|---|---|---|
| HepG2 | A549 | HCC-S102 | |
| PC | 23.0 ± 1.4 | 19.0 ± 1.4 | 18.0 ± 1.4 |
| PE | >50 | >50 | >50 |
| C2 | >50 | >50 | >50 |
| C3 | 15.0 ± 4.2 | 6.0 ± 2.8 | 11.0 ± 1.4 |
| C4 | 15.0 ± 0.0 | 10.7 ± 3.3 | 14.5 ± 2.1 |
| C5 | 7.0 ± 2.5 | 14.5 ± 3.5 | 8.5 ± 0.7 |
| C6 | 15.0 ± 1.4 | 22.5 ± 0.7 | 17.5 ± 2.1 |
| C7 | 22.0 ± 7.1 | 34.5 ± 2.1 | 25.0 ± 2.8 |
| C8 | 14.5 ± 4.9 | 17.5 ± 4.9 | 17.0 ± 1.4 |
| C9 | 9.3 ± 6.6 | 6.75 ± 0.4 | 10.6 ± 1.4 |
| E3.6 | 12.5 ± 0.7 | 18.0 ± 0.0 | 16.0 ± 1.4 |
| E4 | >50 | >50 | >50 |
| E5 | 40.5 ± 7.8 | >50 | 45.0 ± 7.1 |
| Etoposide | 0.20 | 0.34 | 0.32 |
Experimental
General
Plant material
Isolation
Antimicrobial assay
Antimalarial assay
Cytotoxic assay
Acknowledgements
References
- Smitinund, T. Thai Plant Names (Botanical Names-Vernacular Names); Funny Publishing: Bangkok, 2001; p. 424. [Google Scholar]
- Kanokmedhakul, S.; Kanokmedhakul, K.; Yodbuddee, D.; Phonkerd, N. New antimalarial bis-dehydroaporphine alkaloids from Polyalthia debilis. J. Nat. Prod. 2003, 66, 616–619. [Google Scholar] [CrossRef]
- De Almeida, M.E.L.; Braz, F.R.; von Bülow, V.; Gottlieb, O.R.; Maia, J.G.S. Onychine, an alkaloid from Onychopetalum amazonicum. Phytochemistry 1976, 15, 1186–1187. [Google Scholar] [CrossRef]
- Waterman, P.G.; Muhammad, I. Sesquiterpenes and alkaloids from Cleistopholis patens. Phytochemistry 1985, 24, 523–527. [Google Scholar]
- Chakrabarty, M.; Patra, A. 6,7- Dimethoxyonychine and other alkaloids of Polyalthia longifolia. Indian J. Chem. 1990, 29B, 394–395. [Google Scholar]
- Laprévote, O.; Roblot, F.; Hocquemiller, R.; Cavé, A. Alcaloïdes des Annonacées, 87. Azafluorénones de l'Unonopsis spectabilis. J. Nat. Prod. 1988, 51, 555–561. [Google Scholar] [CrossRef]
- Goulart, M.O.F.; Santana, A.E.G.; De Oliveira, A.B.; De Oliveira, G.G.; Maia, J.G.S. Azafluorenones and azaanthraquinone from Guatteria dielsiana. Phytochemistry 1986, 25, 1691–1695. [Google Scholar]
- Chaves, M.H.; Santos, L.A.; Lago, J.H.; Roque, N.F. Alkaloids from Porcelia macrocarpa. J. Nat. Prod. 2001, 64, 240–242. [Google Scholar] [CrossRef]
- Lago, J.H.; Chaves, M.H.; Ayres, M.C.; Agripino, D.G.; Young, M.C. Evaluation of antifungal and DNA-damaging activities of alkaloids from branches of Porcelia macrocarpa. Planta Med. 2007, 73, 292–295. [Google Scholar] [CrossRef]
- Koyama, J.; Sugita, T.; Suzuta, Y. Synthesis of an alkaloid onychine and related compounds: Revised structure of onychine. Heterocycles 1979, 12, 1017–1019. [Google Scholar] [CrossRef]
- Nitta, M.; Ohnuma, M.; Iino, Y. On the reaction of N-vinyliminophosphoranes. Part 16. A new synthesis of 5H-indeno[1,2-b]pyridines and 5H-indeno[1,2-b]pyridin-5-ones. J. Chem. Soc. Perkin Trans. 1 1991, 1, 1115–1118. [Google Scholar] [CrossRef]
- Tadic, D.; Cassels, B.K.; Cavé, A. Spectral properties of ring-C-oxygenated 4-azafluorenes and 4-azafluorenones: The structures of natural onychine derivatives. Heterocycles 1988, 27, 407–421. [Google Scholar] [CrossRef]
- Cassels, B.K.; Tadic, D.; Laprévote, O.; Cavé, A. 13C-NMR Spectra of Azafluorenones. J. Nat. Prod. 1985, 52, 420–422. [Google Scholar]
- Prachayasittikul, S.; Worachartcheewan, A.; Lawang, R.; Ruchirawat, S.; Prachayasittikul, V. Activities of thiotetrahydropyridines as antioxidant and antimicrobial agents. EXCLI J. 2009, 8, 107–114. [Google Scholar]
- Hufford, C.D.; Liu, S.; Clark, A.M.; Oguntimein, B.O. Anticandidal activity of eupolauridine and onychine alkaloids from Cleistopholis patens. J. Nat. Prod. 1987, 50, 961–964. [Google Scholar] [CrossRef]
- Koyama, J.; Morita, I.; Kobayashi, N.; Osakai, T.; Usuki, Y.; Taniguchi, M. Structure-activity relations of azafluorenone and azaanthraquinone as antimicrobial compounds. Bioorg. Med. Chem. Lett. 2005, 15, 1079–1082. [Google Scholar] [CrossRef]
- Satayavivad, J.; Watcharasit, P.; Khamkong, P.; Tuntawiroon, J.; Pavaro, C.; Ruchirawat, S. The pharmacodynamic study of a potent new antimalarial (MC1). Acta Trop. 2004, 89, 343–349. [Google Scholar] [CrossRef]
- Tengchaisri, T.; Chawengkirttikul, R.; Rachaphaew, N.; Reutrakul, V.; Sangsuwan, R.; Sirisinha, S. Antitumor activity of triptolide against cholangiocarcinoma growth in vitro and in hamsters. Cancer Lett. 1998, 133, 169–175. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prachayasittikul, S.; Manam, P.; Chinworrungsee, M.; Isarankura-Na-Ayudhya, C.; Ruchirawat, S.; Prachayasittikul, V. Bioactive Azafluorenone Alkaloids from Polyalthia debilis (Pierre) Finet & Gagnep. Molecules 2009, 14, 4414-4424. https://doi.org/10.3390/molecules14114414
Prachayasittikul S, Manam P, Chinworrungsee M, Isarankura-Na-Ayudhya C, Ruchirawat S, Prachayasittikul V. Bioactive Azafluorenone Alkaloids from Polyalthia debilis (Pierre) Finet & Gagnep. Molecules. 2009; 14(11):4414-4424. https://doi.org/10.3390/molecules14114414
Chicago/Turabian StylePrachayasittikul, Supaluk, Patumporn Manam, Maneekarn Chinworrungsee, Chartchalerm Isarankura-Na-Ayudhya, Somsak Ruchirawat, and Virapong Prachayasittikul. 2009. "Bioactive Azafluorenone Alkaloids from Polyalthia debilis (Pierre) Finet & Gagnep." Molecules 14, no. 11: 4414-4424. https://doi.org/10.3390/molecules14114414