A Facile Synthesis of 2,4-Disubstituted Thiazoles Using MnO2
Abstract
:1. Introduction
2. Results and Discussion
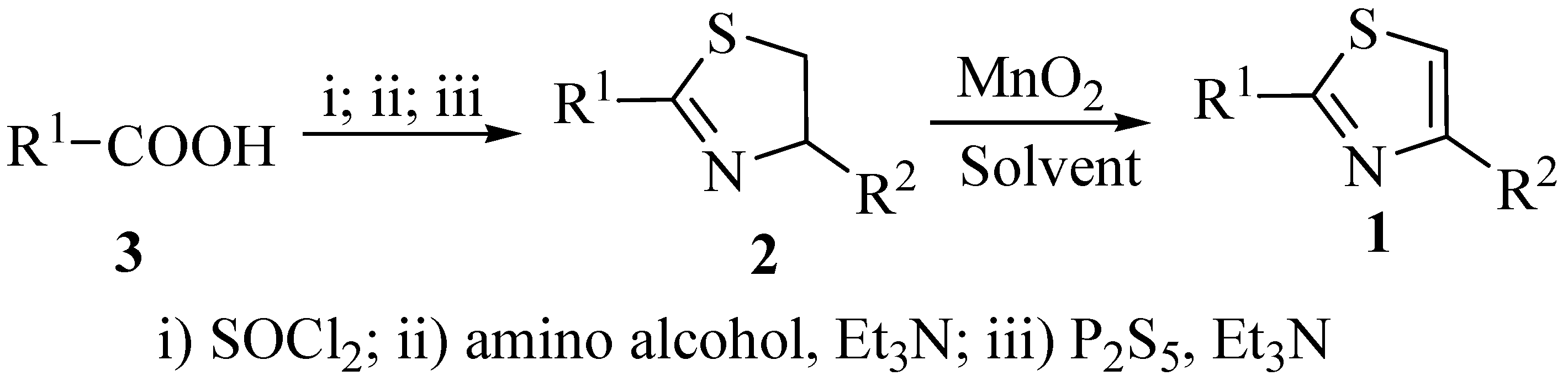
| Entry | Compd. | R1 | R2 | Solvent | Time(h) | Yield(%) |
|---|---|---|---|---|---|---|
| 1 | 1a | Ph | Me | DCM | 48 | – |
| 2 | 1a | Ph | Me | DCE | 12 | 95 |
| 3 | 1a | Ph | Me | Benzene | 12 | 90 |
| 4 | 1a | Ph | Me | CH3CN | 12 | 90 |
| 5 | 1a | Ph | Me | Toluene | 6 | 80 |
| 6 | 1b | Ph | i-Pr | DCE | 12 | 90 |
| 7 | 1c | Ph | i-Bu | DCE | 12 | 90 |
| 8 | 1d | Ph | Ph | DCE | 12 | 99 |
| 9 | 1e | 2-Py | Me | DCE | 12 | 90 |
| 10 | 1f | 2-Py | i-Pr | DCE | 12 | 77 |
| 11 | 1g | 2-Furyl | Bn | DCE | 12 | 70 |
| 12 | 1h | 2-Furyl | Ph | DCE | 12 | 95 |
| 13 | 1i | 2-thienyl | Ph | DCE | 12 | 95 |
| 14 | 1j | PhCH=CH- | i-Pr | DCE | 12 | 80 |
| 15 | 1k | PhCH=CH- | Ph | DCE | 12 | 95 |
| 16 | 1l | Me | Ph | DCE | 12 | 76 |
| 17 | 1m | Me | i-Pr | DCE | 24 | – |

| Entry | Compd. | R1 | R2 | Reaction time | Yield |
|---|---|---|---|---|---|
| 1 | 5a | 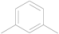 | i-Pr | 12 | 80 |
| 2 | 5b | 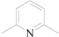 | Me | 12 | 85 |
| 3 | 5c | 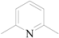 | i-Pr | 6 | 80 |
| 4 | 5d |  | Ph | 8 | 70 |
3. Conclusions
4. Experimental
4.1. Synthesis of Thiazolines
4.2. Typical Procedure for Oxidation of Thiazolines to Thiazoles
4.3. Spectral Data of Other Thiazole Compounds
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Rivkin, A.; Cho, Y.S.; Gabarda, A.E.; Yoshimura, F.; Danishefsky, S.J. Application of Ring-Closing Metathesis Reactions in the Synthesis of Epothilones. J. Nat. Prod. 2004, 67, 139–143. [Google Scholar] [CrossRef]
- Ganesh, T.; Schilling, J.K.; Palakodety, R.K.; Ravindra, R.; Shanker, N.; Bane, S.; Kingston, D.G.I. Synthesis and biological evaluation of fluorescently labeled epothilone analogs for tubulin binding studies. Tetrahedron 2003, 59, 9979–9984. [Google Scholar] [CrossRef]
- Plazzi, P.V.; Bordi, F.; Silva, C.; Morini, G.; Caretta, A.; Barocelli, E.; Vitali, T. Heteroarylaminoethyl and heteroaryithioethyl imidazoles. Synthesis and H3-receptor affinity. Eur. J. Med. Chem. 1995, 30, 881–889. [Google Scholar] [CrossRef]
- Bai, N.; Sha, Y.W.; Meng, G. Efficient and Eco-Friendly Preparation of 4-Methyl-5-formyl-thiazole. Molecules 2008, 13, 943–947. [Google Scholar] [CrossRef]
- Hu, D.J.; Liu, S.F.; Huang, T.H.; Tu, H.Y.; Zhang, A.D. Synthesis and herbicidal activities of novel 4-(4-(5-methyl-3-arylisoxazol-4-yl)thiazol-2-yl)piperidyl carboxamides and thio-carboxamides. Molecules 2009, 14, 1288–1303. [Google Scholar] [CrossRef]
- Aitken, K.M.; Aitken, R.A. Synthesis of 2,4-diacetylthiazole and 2,5-diacetylthiazole. Tetrahedron 2008, 64, 4384–4386. [Google Scholar] [CrossRef]
- Potewar, T.M.; Ingale, S.A.; Srinivasan, K.V. Efficient synthesis of 2, 4-disubstituted thiazoles using ionic liquid under ambient conditions: A practical approach towards the synthesis of Fanetizole. Tetrahedron 2007, 63, 11066–11069. [Google Scholar] [CrossRef]
- Narender, M.; Reddy, M.S.; Sridhar., R.; Nageswar, Y.V.D.; Rao, K.R. Aqueous phase synthesis of thiazoles and aminothiazoles in the presence of β-cyclodextrin. Tetrahedron Lett. 2005, 46, 5953–5955. [Google Scholar] [CrossRef]
- Hämmerle, J.; Spina, M.; Schnürch, M.; Mihovilovic, M.D.; Stanetty, P. A Comparative Study on Stille Cross-Coupling Reactions of 2-Phenylthiazoles and 2-Phenyloxazoles. Synthesis 2008, 19, 3099–3107. [Google Scholar]
- Friedrich, A.; Max, T.; Karl, G. Concomitant reaction of elementary sulfur and gaseous ammonia on ketones. XXIX. Syntheses and properties of 2-aryl-3-thiazolines and 2-arylthiazoles. Justus Liebigs Ann. Chem. 1961, 639, 133–146. [Google Scholar] [CrossRef]
- Aitken, R.A.; Armstrong, D.P.; Galt, R.H.B.; Mesher, S.T.E. Synthesis and oxidation of chiral 2-thiazolines (4,5-dihydro-1,3-thiazoles). J. Chem. Soc. Perkin Trans. 1997, 1, 935–943. [Google Scholar]
- Meyers, A.I.; Tavares, F.X. Oxidation of oxazolines and thiazolines to oxazoles and thiazoles application of the Kharasch-Sosnovsky reaction. J. Org. Chem. 1996, 61, 8207–8215. [Google Scholar] [CrossRef]
- Fernandez, X.; Duňach, E. Asymmetric synthesis of 2-alkyl-3-thiazoline carboxylates: stereochemistry of the MnO2-mediated oxidation of cis- and trans-2-alkyl-thiazolidine-(4R)-carboxylates. Tetrahedron Asymmetry 2001, 12, 1279–1286. [Google Scholar] [CrossRef]
- Fernandez, X.; Fellous, R.; Lizzani-Cuvelier, L.; Loiseau, M.; Duňach, E. Chemo- and regioselective synthesis of alkyl-3-thiazoline carboxylates. Tetrahedron Lett. 2001, 42, 1519–1521. [Google Scholar] [CrossRef]
- You, S.L.; Kelly, J.W. The total synthesis of bistratamides F–I. Tetrahedron 2005, 61, 241–249. [Google Scholar] [CrossRef]
- You, S.L.; Kelly, J.W. Highly efficient biomimetic total synthesis and structural verification of Bistratamides E and J from Lissoclinum bistratum. Chem. Eur. J. 2004, 10, 71–75. [Google Scholar] [CrossRef]
- Mislin, G.L.; Burger, A.; Abdallah, M. A. Synthesis of new thiazole analogues of pyochelin, a siderophore of Pseudomonas aeruginosa and Burkholderia cepacia. A new conversion of thiazolines into thiazoles. Tetrahedron 2004, 60, 12139–12145. [Google Scholar] [CrossRef]
- Cheng, X.M.; Zheng, Z.B.; Li, N.; Qin, Z.H.; Fu, B.; Wang, N.D. Synthesis of novel C3 symmetric tris(thiazoline) ligands and their application in the allylic oxidation reaction. Tetrahedron Asymmetry 2008, 19, 2159–2163. [Google Scholar] [CrossRef]
- Lu, X.H.; Qi, Q.Q.; Xiao, Y.M.; Li, N.; Fu, B. A convenient one-pot synthesis of arene-centered tris(thiazoline) compounds. Heterocycles 2009, 78, 1031–1039. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, Z.B.; Qin, Z.H.; Fu, B. Yuan, H.Z. Synthesis and biological activity of 2-indolyl oxazoline and thiazoline derivatives. Chin. J. Org. hem. 2008, 28, 1841–1845. [Google Scholar]
- Taku, A.; Takuya, A.; Takahide, F.; Yutaka, I.; Fumitoshi, K.; Naoto, C. Ruthenium- and Rhodium-Catalyzed Direct Carbonylation of the Ortho C-H Bond in the Benzene Ring of N-Arylpyrazoles. J. Org. Chem. 2004, 69, 4433–4440. [Google Scholar]
- Yoshihide, I.; Hideo, T. Facile preparation of thiazoles from 1H-1-(1'-alkynyl)-5-methyl-1,2,3-benziodoxathiole 3,3-dioxide with thioamides. Synlett 2008, 17, 2637–2641. [Google Scholar]
- Knott, R.F.; Breckenridge, J.G. Analogs of 2,2'-bipyridyl with isoquinoline and thiazole rings. Part I. Can. J. Chem. 1954, 32, 512–521. [Google Scholar] [CrossRef]
- Singh, S. P.; Subhash, S. Synthesis and phototoxicity of some 2-(phenyl- or 2- or 3-thienyl)-4-substituted thiazoles. Indian J. Chem. Sect. B: Org. Chem. Incl. Med. Chem. 1988, 27B, 941–943. [Google Scholar]
- Takao, S.; Hideo, N.; Yoshinori, K.; Masafumi, S.; Hiroshi, Y. Palladium-catalyzed reactions of terminal acetylenes and olefins with halo-1,3-azoles. Chem. harm. Bull. 1987, 35, 823–828. [Google Scholar] [CrossRef]
- Makoto, U.; Hideo, T. Environmentally benign preparation of heteroaromatics from ketones or alcohols, with macroporous polystyrenesulfonic acid and (diacetoxyiodo)benzene, followed by thioamide, amidine, and 2-aminopyridine. Synthesis 2004, 16, 2673–2677. [Google Scholar]
- Veli-Matti, M.; Paivi, L.; Ilkka, H.; Harri, T.; Cristina, M.; Jouko, K. Novel thiazole-containing complexing agents and luminescence of their europium(III) and terbium(III) chelates. Helv. Chim. Acta 1996, 79, 295–306. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yu, Y.-B.; Chen, H.-L.; Wang, L.-Y.; Chen, X.-Z.; Fu, B. A Facile Synthesis of 2,4-Disubstituted Thiazoles Using MnO2. Molecules 2009, 14, 4858-4865. https://doi.org/10.3390/molecules14124858
Yu Y-B, Chen H-L, Wang L-Y, Chen X-Z, Fu B. A Facile Synthesis of 2,4-Disubstituted Thiazoles Using MnO2. Molecules. 2009; 14(12):4858-4865. https://doi.org/10.3390/molecules14124858
Chicago/Turabian StyleYu, Yan-Bo, Hong-Liang Chen, Li-Yi Wang, Xin-Zheng Chen, and Bin Fu. 2009. "A Facile Synthesis of 2,4-Disubstituted Thiazoles Using MnO2" Molecules 14, no. 12: 4858-4865. https://doi.org/10.3390/molecules14124858




