Synthesis and Characterization of N-(Arylcarbamothioyl)-cyclohexanecarboxamide Derivatives: The Crystal Structure of N-(Naphthalen-1-ylcarbamothioyl)cyclohexanecarboxamide
Abstract
:Introduction
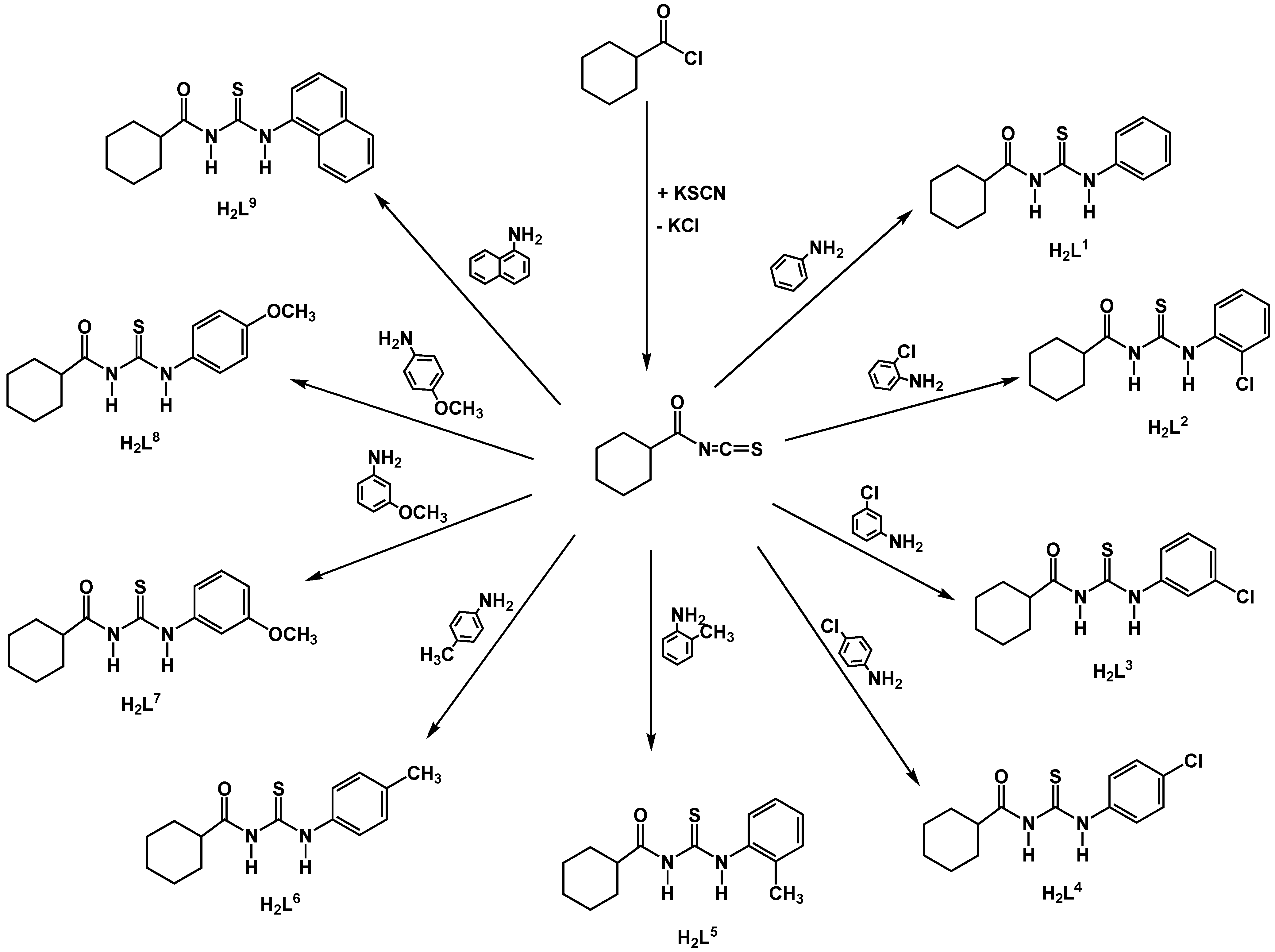
Results and Discussion
| Empirical formula | C18H20N2OS |
| Formula weight | 312.42 |
| Temperature (K) | 153(2) |
| Wavelength (Å) | 0.71073 |
| Crystal system | Triclinic |
| Space group | Pī |
| Unit cell dimensions | |
| a (Å) | 6.9921(14) |
| b (Å) | 11.002(2) |
| c (Å) | 12.381(3) |
| α (°) | 113.28(3) |
| β (°) | 99.38(3) |
| γ (°) | 101.85(3) |
| V (Å3) | 824.1(3) |
| Z | 2 |
| Dc (Mg/m3) | 1.259 |
| Absorption coefficient (mm-1) | 0.200 |
| F(000) | 332 |
| Crystal size (mm3) | 0.29 x 0.24 x 0.14 |
| θ range for data collection (°) | 3.37 to 25.05 |
| Index ranges | -8 ≤ h ≤ 8 |
| -10 ≤ k ≤ 13 | |
| -14 ≤ l ≤ 14 | |
| Reflections collected | 5439 |
| Independent reflections (Rint) | 2841 (0.0261) |
| Absorption correction | Semi-empirical from equivalents |
| Refinement method | Full-matrix least-squares on F2 |
| Data / parameters | 2841 / 199 |
| Goodness-of-fit on F2 | 1.104 |
| Final R indices [I>2σ(I)] | R1 = 0.0884, wR2 = 0.2414 |
| R indices (all data) | R1 = 0.1236, wR2 = 0.2990 |
| Largest diff. peak and hole (e.Å-3) | 1.208 and -0.578 |

| Bond lengths | ||||
| O1-C12 | 1.218(5) | N2-C11 | 1.340(6) | |
| S1-C11 | 1.670(4) | N2-C1 | 1.466(6) | |
| N1-C12 | 1.388(5) | C12-C13 | 1.504(6) | |
| N1-C11 | 1.378(5) | |||
| Bond angles | ||||
| C12-N1-C11 | 129.7(4) | N1-C11-S1 | 119.8(3) | |
| C11-N2-C1 | 122.1(4) | O1-C12-N1 | 121.7(4) | |
| N1-C11-N2 | 116.3(4) | O1-C12-C13 | 124.2(4) | |
| N2-C11-S1 | 123.8(3) | N1-C12-C13 | 114.0(3) |

Experimental
General
Synthesis of the compounds
Supplementary material
Acknowledgements
References
- Arslan, H.; Kulcu, N.; Florke, U. Synthesis and characterization of copper(II), nickel(II) and cobalt(II) complexes with novel thiourea derivatives. Trans. Metal Chem. 2003, 28, 816–819. [Google Scholar] [CrossRef]
- Binzet, G.; Arslan, H.; Florke, U.; Kulcu, N.; Duran, N. Synthesis, characterization and antimicrobial activities of transition metal complexes of N,N-dialkyl-N'-(2-chlorobenzoyl)thiourea derivatives. J. Coord. Chem. 2006, 59, 1395–1406. [Google Scholar] [CrossRef]
- Ugur, D.; Arslan, H.; Kulcu, N. Synthesis, characterization and thermal behavior of 1,1-dialkyl-3-(4-(3,3-dialkylthioureidocarbonyl)benzoyl)thiourea and its Cu(II), Ni(II), and Co(II) complexes. Russ. J. Coord. Chem. 2006, 32, 669–675. [Google Scholar] [CrossRef]
- Emen, M.F.; Arslan, H.; Kulcu, N.; Florke, U.; Duran, N. Synthesis, characterization and antimicrobial activities of some metal complexes with N '-(2-chloro-benzoyl)thiourea ligands: The crystal structure of fac-[CoL3] and cis-[PdL2]. Pol. J. Chem. 2005, 79, 1615–1626. [Google Scholar]
- Mansuroglu, D.S.; Arslan, H.; Florke, U.; Kulcu, N. Synthesis and characterization of nickel and copper complexes with 2,2-diphenyl-N-(alkyl(aryl)carbamothioyl)acetamide: The crystal structures of HL1 and cis-[Ni(L-1)(2)]. J. Coord. Chem. 2008, 61, 3134–3146. [Google Scholar]
- Habtu, M.M.; Bourne, S.A.; Koch, K.R.; Luckay, R.C. Competitive bulk liquid membrane transport and solvent extraction of some transition and post-transition metal ions using acylthiourea ligands as ionophores. New J. Chem. 2006, 30, 1155–1162. [Google Scholar] [CrossRef]
- Berhe, H.G.; Bourne, S.A.; Bredenkamp, M.W.; Esterhuysen, C.; Habtu, M.M.; Koch, K.R.; Luckay, R.C. High and selective Ag(I) bulk liquid membrane transport with N,N-diethyl-N '-camphanyl thiourea and structure of the complex. Inorg. Chem. Commun. 2006, 9, 99–102. [Google Scholar] [CrossRef]
- Henderson, W.; Nicholson, B.K.; Dinger, M.B.; Bennett, R.L. Thiourea monoanion and dianion complexes of rhodium(III) and ruthenium(II). Inorg. Chim. Acta 2002, 338, 210–218. [Google Scholar]
- Sacht, C.; Datt, M. S.; Otto, S.; Roodt, A. Synthesis, characterisation and coordination chemistry of novel chiral N,N-dialkyl-N-menthyloxycarbonylthioureas. Crystal and molecular structures of N,N-diethyl-N-(-)-(3R)-menthyloxycarbonylthiourea and cis-(S,S)-[Pt(L)Cl(DMSO)] [where HL = N-(+)-(3R)-menthyloxycarbonyl-N'-morpholinothiourea or N-benzoyl-N',N'-diethylthiourea]. J. Chem. Soc.-Dalton Trans. 2000, 24, 4579–4586. [Google Scholar] [CrossRef]
- Henderson, W.; Kemmitt, R.D.W.; Mason, S.; Moore, M.R.; Fawcett, J.; Russell, D.R. Thiadiazatrimethylenemethane and N,N',P-Triphenylphosphonothioic Diamide Complexes of Platinum(Ii). J. Chem. Soc.-Dalton Trans. 1992, 1, 59–66. [Google Scholar]
- Kemp, G.; Roodt, A.; Purcell, W.; Koch, K.R. Unprecedented N,S,O co-ordination of the doubly deprotonated anion of N-benzoyl-N '-phenylthiourea (H2L2) bridging two rhodium(I) centres: crystal structure of the acetone solvate of [(PPh3)(2)(CO)Rh(mu-L-2-kappa N ':kappa O,S)Rh(PPh3)(CO)]. J. Chem. Soc.-Dalton Trans. 1997, 23, 4481–4483. [Google Scholar]
- Koch, K.R.; Sacht, C.; Grimmbacher, T.; Bourne, S. New ligands for the platinum-group metals: Deceptively simple coordination chemistry of N-acyl-N'-alkyl- and N-acyl-N',N'-dialkyl-thioureas. S. Afr. J. Chem. 1995, 48, 71–77. [Google Scholar]
- Yuan, Y.F.; Wang, J.T.; Gimeno, M.C.; Laguna, A.; Jones, P.G. Synthesis and characterisation of copper complexes with N-ferrocenoyl-N '(alkyl)thioureas. Inorg. Chim. Acta 2001, 324, 309–317. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wei, T.B.; Xian, L.; Gao, L.M. An efficient synthesis of polymethylene-bis-aroyl thiourea derivatives under the condition of phase-transfer catalysis. Phosphorus, Sulfur Silicon Relat. Elem. 2004, 179, 2007–2013. [Google Scholar]
- Zhang, Y.M.; Wei, T.B.; Wang, X.C.; Yang, S.Y. Synthesis and biological activity of N-aroyl-N '-carboxyalkyl thiourea derivatives. Indian J. Chem., Sect. B 1998, 37, 604–606. [Google Scholar]
- Neucki, E. Zur Kenntniss des Sulfoharnstoffs. Ber. Dtsch. Chem. Ges. 1873, 6, 598–600. [Google Scholar]
- Schuster, M. The Chromatography of Metal-Chelates .16. Tlc of "N,N-Dialkyl-N'-Thiobenzoyl-Thiourea Chelates. Fresenius Z. Anal. Chem. 1986, 324, 127–129. [Google Scholar] [CrossRef]
- Konig, K.H.; Schuster, M.; Schneeweis, G.; Steinbrech, B. On the Chromatography of Metal-Chelates.14. Thin-Layer-Chromatography of N,N-Dialkyl-N'-Benzoylthiourea-Chelates. Fresenius Z. Anal. Chem. 1984, 319, 66–69. [Google Scholar] [CrossRef]
- Schuster, M.; Kugler, B.; Konig, K.H. The Chromatography of Metal-Chelates. 19. Influence of the Acyl Substituents on the Chromatographic Properties of Acylthiourea Chelates. Fresenius J. Anal. Chem. 1990, 338, 717–720. [Google Scholar] [CrossRef]
- Konig, K.H.; Schuster, M.; Steinbrech, B.; Schneeweis, G.; Schlodder, R. N,N-Dialkyl-N'-Benzoylthioureas as Reagents for Selective Extractions to Separate and Enrich Platinum-Group Metals. Fresenius Z. Anal. Chem. 1985, 321, 457–460. [Google Scholar] [CrossRef]
- Arslan, H.; Duran, N.; Sahin, N. O.; Kulcu, N. Thermal behaviour and antimicrobial activity of novel series of benzoylthiourea derivatives. Asian J. Chem. 2006, 18, 1710–1718. [Google Scholar]
- Arslan, H.; Florke, U.; Kulcu, N. Synthesis, characterization, and crystal structure of 1-(4-chloro-benzoyl)-3-naphthalen-1-yl-thiourea. J. Chem. Crystall. 2003, 33, 919–924. [Google Scholar] [CrossRef]
- Venkatachalam, T.K.; Mao, C.; Uckun, F.M. Effect of stereochemistry on the anti-HIV activity of chiral thiourea compounds. Bioorg. Med. Chem. 2004, 12, 4275–4284. [Google Scholar] [CrossRef]
- Sun, C.W.; Huang, H.; Feng, M.; Shi, X.L.; Zhang, X.D.; Zhou, P. A novel class of potent influenza virus inhibitors: Polysubstituted acylthiourea and its fused heterocycle derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 162–166. [Google Scholar] [CrossRef]
- Ozer, C.K.; Arslan, H.; Vanderveer, D.; Binzet, G. Synthesis and characterization of N-(alky(aryl)carbamothioyl)cyclohexanecarboxamide derivatives and their Ni(II) and Cu(II) complexes. J. Coord. Chem. 2009, 62, 266–276. [Google Scholar] [CrossRef]
- Mansuroglu, D.S.; Arslan, H.; VanDerveer, D.; Binzet, G. Synthesis and Characterization of N-(2,2-Diphenylacetyl) N'-Substituted Thiourea Derivatives: The Crystal Structure of N-(2,2-Diphenylacetyl)-N'-(4-chloro phenyl)-thiourea. Phosphorus, Sulfur Silicon Relat. Elem. 2009. Submitted.. [Google Scholar]
- Arslan, H.; Duran, N.; Borekci, G.; Koray Ozer, C.; Akbay, C. Antimicrobial Activity of Some Thiourea Derivatives and Their Nickel and Copper Complexes. Molecules 2009, 14, 519–527. [Google Scholar] [CrossRef]
- Binzet, G.; Emen, F.M.; Florke, U.; Yesilkaynak, T.; Kulcu, N.; Arslan, H. 4-Chloro-N-[N-(6-methyl-2-pyridyl)-carbamothioyl]benzamide. Acta Cryst. E 2009, 65, O81–O82. [Google Scholar] [CrossRef]
- Binzet, G.; Florke, U.; Kulcu, N.; Arslan, H. 3-Chloro-N-(diphenylcarbamothioyl)benzamide. Acta Cryst. E 2009, 65, O351–O352. [Google Scholar] [CrossRef]
- Binzet, G.; Florke, U.; Kulcu, N.; Arslan, H. N-(Diphenylcarbamothioyl)-3-methylbenzamide. Acta Cryst. E 2009, 65, O378–O379. [Google Scholar] [CrossRef]
- Binzet, G.; Florke, U.; Kulcu, N.; Arslan, H. 4-Bromo-N-(diethylcarbamothioyl)benzamide. Acta Cryst. E 2009, 65, O427–O428. [Google Scholar] [CrossRef]
- Binzet, G.; Florke, U.; Kulcu, N.; Arslan, H. 4-Bromo-N-(di-n-propylcarbamothioyl)benzamide. Acta Cryst. E 2009, 65, O452–O453. [Google Scholar] [CrossRef]
- Douglass, I.B.; Dains, F.B. Some Derivatives of Benzoyl and Furoyl Isothiocyanates and their Use in Synthesizing Heterocyclic Compounds. J. Am. Chem. Soc. 1934, 56, 719–721. [Google Scholar] [CrossRef]
- Su, B.Q.; Liu, G.L.; Sheng, L.; Wang, X.Q.; Xian, L. Synthesis and structure of N-ethoxycarbonyl-N '-O-methoxyphenylthiourea. Phosphorus, Sulfur Silicon Relat. Elem. 2006, 181, 745–750. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, Y.; Wang, Y.A. Synthesis and characterization of N-benzoyl-N '-carboxyalkyl substituted thiourea derivatives. Phosphorus, Sulfur Silicon Relat. Elem. 2003, 178, 293–297. [Google Scholar] [CrossRef]
- Arslan, H.; Kulcu, N.; Florke, U. Normal coordinate analysis and crystal structure of N,N-dimethyl-N '-(2-chloro-benzoyl)thiourea. Spectrochim. Acta, Part A 2006, 64, 1065–1071. [Google Scholar] [CrossRef]
- Arslan, H.; Florke, U.; Kulcu, N.; Kayhan, E. Synthesis, characterization, crystal structure and thermal behavior of N '-(4-chlorobenzoyl)-N,N-di-n-butylthiourea and its nickel complex. Turk. J. Chem. 2006, 30, 429–440. [Google Scholar]
- Yesilkaynak, T.; Florke, U.; Kulcu, N.; Arslan, H. 1-Benzoyl-3-(4-methylpyridin-2-yl)thiourea. Acta Cryst. E 2006, 62, O3934–O3935. [Google Scholar] [CrossRef]
- Arslan, H.; Florke, U.; Kulcu, N. The crystal and molecular structure of 1-(2-chloro-benzoyl)-3-p-tolyl-thiourea. Turk. J. Chem. 2004, 28, 673–678. [Google Scholar]
- Arslan, H.; Florke, U.; Kulcu, N. The crystal and molecular structure of 1-(biphenyl-4-carbonyl)-3-p-tolyl-thiourea. Acta Chim. Slov. 2004, 51, 787–792. [Google Scholar]
- Shen, X.; Shi, X.; Kang, B.; Tong, Y.; Liu, Y.; Gu, L.; Liu, Q.; Huang, Y. Preparation and crystal structure of a new Cu (II) complex derived from the desulfurization of N-(p-nitrophenyl)-N′-ethoxycarbonyl-thiourea. Polyhedron 1998, 18, 33–37. [Google Scholar] [CrossRef]
- Yamin, B.M.; Yusof, M.S.M. N-Benzoyl-N '-phenylthiourea. Acta Cryst. E 2003, 59, O151–O152. [Google Scholar] [CrossRef]
- Yusof, M.S.M.; Asroh, F.S.M.; Kadir, M.A.; Yamin, B.M. N-(3-Methylbutyryl)-N'-phenylthiourea. Acta Cryst. E 2007, 63, o1190–o1191. [Google Scholar]
- Rauf, M.K.; Badshah, A.; Bolte, M. 3-(3-Methoxyphenyl)-1-(2-methylbenzoyl)thiourea. Acta Cryst. E 2007, 63, O1676–O1678. [Google Scholar] [CrossRef]
- Cremer, D.; Pople, J.A. General definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 354–1358. [Google Scholar]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van De Streek, J. Mercury: visualization and analysis of crystal structures. J. Appl. Crystall. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXTL. Version 6.10.; Bruker AXS Inc.: Madison, WI, USA, 2000. [Google Scholar]
- Jacobson, R.A. REQABA: Empirical Absorption Correction Version 1.1.; Rigaku/Molecular Structure Corporation: The Woodlands, TX, USA, 1998. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Özer, C.K.; Arslan, H.; VanDerveer, D.; Külcü, N. Synthesis and Characterization of N-(Arylcarbamothioyl)-cyclohexanecarboxamide Derivatives: The Crystal Structure of N-(Naphthalen-1-ylcarbamothioyl)cyclohexanecarboxamide. Molecules 2009, 14, 655-666. https://doi.org/10.3390/molecules14020655
Özer CK, Arslan H, VanDerveer D, Külcü N. Synthesis and Characterization of N-(Arylcarbamothioyl)-cyclohexanecarboxamide Derivatives: The Crystal Structure of N-(Naphthalen-1-ylcarbamothioyl)cyclohexanecarboxamide. Molecules. 2009; 14(2):655-666. https://doi.org/10.3390/molecules14020655
Chicago/Turabian StyleÖzer, Cemal Koray, Hakan Arslan, Don VanDerveer, and Nevzat Külcü. 2009. "Synthesis and Characterization of N-(Arylcarbamothioyl)-cyclohexanecarboxamide Derivatives: The Crystal Structure of N-(Naphthalen-1-ylcarbamothioyl)cyclohexanecarboxamide" Molecules 14, no. 2: 655-666. https://doi.org/10.3390/molecules14020655




