2-(3,4-Dihydro-4-Oxothieno[2,3-d]pyrimidin-2-ylthio) Acetamides as a New Class of Falcipain-2 Inhibitors. 3. Design, Synthesis and Biological Evaluation
Abstract
:Introduction
Results and Discussion
Identification of Prototype (Hit) 1 by Virtual Screening
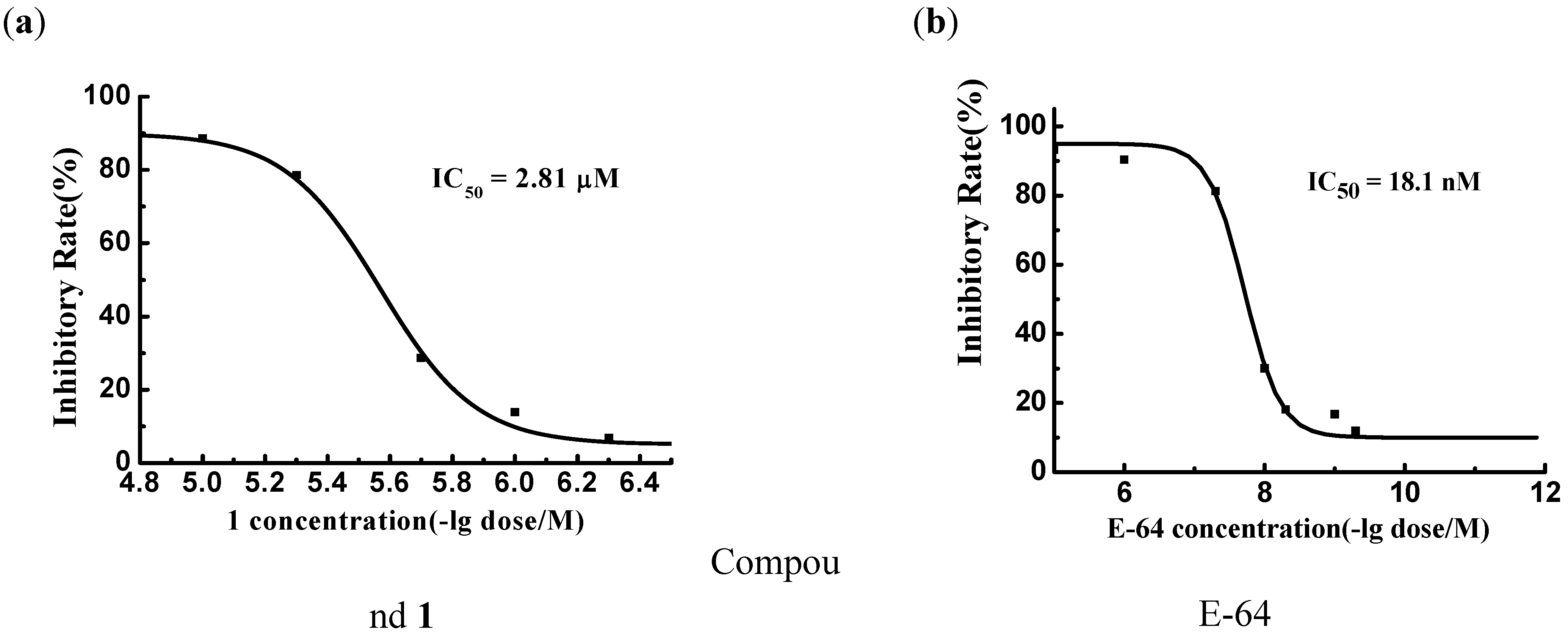
Design and Synthesis of Compounds 1-4

| Compd. | R1 | R2 | R3 | R4 | Inhibition rate at 10 μM (%) | IC50 (μM) |
| 1 | H |  |  |  | 88.7 | 2.81 |
| 2a | H |  |  |  | 92.7 | 1.46 |
| 2b | H |  |  |  | 79.0 | 2.05 |
| 2c | H |  |  |  | 85.4 | 2.77 |
| 2d | H |  |  |  | 84.7 | 4.30 |
| 2e | H |  |  |  | 90.6 | 5.74 |
| 3a | —(CH2)4— |  |  | 82.2 | 5.77 | |
| 3b | H |  |  |  | 85.7 | 2.95 |
| 3c | H |  |  |  | 53.0 | 11.8 |
| 4a | H |  |  |  | 93.3 | 6.63 |
| 4b | H |  |  |  | 94.3 | 5.70 |
| 4c | H |  |  |  | 90.3 | 3.31 |
| 4d | H |  |  |  | 93.2 | 2.49 |
| 4e | H |  |  |  | 72.0 | 5.58 |
| 4f | H |  |  |  | 92.0 | 5.43 |
Enzyme Inhibition Assay

Structure and Activity Relationships
Conclusions
Experimental
Virtual Screening by Molecular Docking
Enzyme Inhibition Assay
General procedures for the preparation of 2-(3,4-dihydro-4-oxothieno[2,3-d]pyrimidin-2-ylthio)-acetamides 1, 2a-e, 3a-c, and 4a-f, exemplified by the preparation of compound 1
Acknowledgements
References
- Vangapandu, S.; Jain, M.; Kaur, K.; Patil, P.; Patel, S.R.; Jain, R. Recent advances in antimalarial drug development. Med. Res. Rev. 2007, 27, 65–107. [Google Scholar] [CrossRef]
- Fidock, D.A.; Rosenthal, P.J.; Croft, S.L.; Brun, R.; Nwaka, S. Antimalarial drug discovery: efficacy models for compound screening. Nat. Rev. Drug Discov. 2004, 3, 509–520. [Google Scholar]
- Wellems, T.E.; Plowe, C.V. Chloroquine-resistant malaria. J. Infect. Dis. 2001, 184, 770–776. [Google Scholar]
- Trape, J.F. The public health impact of chloroquine resistance in Africa. Am. J. Trop. Med. Hyg. 2001, 64, 12–17. [Google Scholar]
- Plowe, C.V. Monitoring antimalarial drug resistance: making the most of the tools at hand. J. Exp. Biol. 2003, 206, 3745–3752. [Google Scholar] [CrossRef]
- Shenai, B.R.; Sijwali, P.S.; Singh, A.; Rosenthal, P.J. Characterization of native and recombinant falcipain-2, a principal trophozoite cysteine protease and essential hemoglobinase of Plasmodium falciparum. J. Biol. Chem. 2000, 275, 29000–29010. [Google Scholar]
- Dua, M.; Raphael, P.; Sijwali, P.S.; Rosenthal, P.J.; Hanspal, M. Recombinant falcipain-2 cleaves erythrocyte membrane ankyrin and protein 4.1. Mol. Biochem. Parasitol. 2001, 116, 95–99. [Google Scholar] [CrossRef]
- Rosenthal, P.J.; Olson, J.E.; Lee, G.K.; Palmer, J.T.; Klaus, J.L.; Rasnick, D. Antimalarial effects of vinyl sulfone cysteine proteinase inhibitors. Antimicrob. Agents Chemother. 1996, 40, 1600–1603. [Google Scholar]
- Shenai, B.R; Lee, B.J.; Alvarez-Hernandez, A.; Chong, P,Y,; Emal, C.D.; Neitz, R.J.; Roush, W.R.; Rosenthal, P J. Structure-activity relationships for inhibition of cysteine protease activity and development of Plasmodium falciparum by peptidyl vinyl sulfones. Antimicrob. Agents Chemother. 2003, 47, 154–160. [Google Scholar] [CrossRef]
- Lee, B.J.; Singh, A.; Chiang, P.; Kemp, S.J.; Goldman, E.A.; Weinhouse, M.I.; Vlasuk, G.P.; Rosenthal, P.J. Antimalarial activities of novel synthetic cysteine protease inhibitors. Antimicrob. Agents Chemother. 2003, 47, 3810–3814. [Google Scholar] [CrossRef]
- Domínguez, J.N.; López, S.; Charris, J.; Iarruso, L.; Lobo, G.; Semenov, A.; Olson, J.E.; Rosenthal, P.J. Synthesis and antimalarial effects of phenothiazine inhibitors of a Plasmodium falciparum cysteine protease. J. Med. Chem. 1997, 40, 2726–2732. [Google Scholar] [CrossRef]
- Huang, L.; Lee, A.; Ellman, J.A. Identification of potent and selective mechanism-based inhibitors of the cysteine protease cruzain using solid-phase parallel synthesis. J. Med. Chem. 2002, 45, 676–684. [Google Scholar] [CrossRef]
- Greenbaum, D.C.; Mackey, Z.; Hansell, E.; Doyle, P.; Gut, J.; Caffrey, C.R.; Lehrman, J.; Rosenthal, P.J.; McKerrow, J.H.; Chibale, K. Synthesis and structure-activity relationships of parasiticidal thiosemicarbazone cysteine protease inhibitors against Plasmodium falciparum, Trypanosoma brucei, and Trypanosoma cruzi. J. Med. Chem. 2004, 47, 3212–3219. [Google Scholar] [CrossRef]
- Desai, P.V.; Patny, A.; Sabnis, Y.; Tekwani, B.; Gut, J.; Rosenthal, P.; Srivastava, A.; Avery, M. Identification of novel parasitic cysteine protease inhibitors using virtual screening. 1. The ChemBridge database. J. Med. Chem. 2004, 47, 6609–6615. [Google Scholar] [CrossRef]
- Domínguez, J.N.; León, C.; Rodrigues, J.; Gamboa de Domínguez, N.; Gut, J.; Rosenthal, P.J. Synthesis and evaluation of new antimalarial phenylurenyl chalcone derivatives. J. Med. Chem. 2005, 48, 3654–3658. [Google Scholar] [CrossRef]
- Desai, P.V.; Patny, A.; Gut, J.; Rosenthal, P.J.; Tekwani, B.; Srivastava, A.; Avery, M. Identification of novel parasitic cysteine protease inhibitors by use of virtual screening. 2. The available chemical directory. J. Med. Chem. 2006, 49, 1576–1584. [Google Scholar] [CrossRef]
- Batra, S.; Sabnis, Y.A.; Rosenthal, P.J.; Avery, M.A. Structure-based approach to falcipain-2 inhibitors: synthesis and biological evaluation of 1,6,7-trisubstituted dihydroisoquinolines and isoquinolines. Bioorg. Med. Chem. 2003, 11, 2293–2299. [Google Scholar] [CrossRef]
- Chiyanzu, I.; Hansell, E.; Gut, J.; Rosenthal, P.J.; Mckerrow, J.H.; Chibale, K. Synthesis and evaluation of isatins and thiosemicarbazone derivatives against cruzain, falcipain-2 and rhodesain. Bioorg. Med. Chem. Lett. 2003, 13, 3527–3530. [Google Scholar] [CrossRef]
- Chipeleme, A.; Gut, J.; Rosenthal, P.J.; Chibale, K. Synthesis and biological evaluation of phenolic Mannich bases of benzaldehyde and (thio)semicarbazone derivatives against the cysteine protease falcipain-2 and a chloroquine resistant strain of Plasmodium falciparum. Bioorg. Med. Chem. 2007, 15, 273–282. [Google Scholar] [CrossRef]
- Rosenthal, P.J.; Wollish, W.S.; Palmer, J.T.; Rasnick, D. Antimalarial effects of peptide inhibitors of a Plasmodium falciparum cysteine proteinase. J. Clin. Invest. 1991, 88, 1467–1472. [Google Scholar] [CrossRef]
- Rosenthal, P.J.; Lee, G.K.; Smith, R.E. Inhibition of a Plasmodium vinckei cysteine proteinase cures murine malaria. J. Clin. Invest. 1993, 91, 1052–1056. [Google Scholar] [CrossRef]
- Sajid, M.; McKerrow, J. Cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 2002, 120, 1–21. [Google Scholar] [CrossRef]
- Hogg, T.; Nagarajan, K.; Herzberg, S.; Chen, L.; Shen, X.; Jiang, H.; Wecke, M.; Blohmke, C.; Hilgenfeld, R.; Schmidt, C.L. Structural and functional characterization of Falcipain-2, a hemoglobinase from the malarial parasite Plasmodium falciparum. J. Biol. Chem. 2006, 281, 25425–25437. [Google Scholar] [CrossRef]
- Wang, S.X.; Pandey, K.C.; Somoza, J.R.; Sijwali, P.S.; Kortemme, T.; Brinen, L.S.; Fletterick, R.J.; Rosenthal, P.J.; Mckerrow, J.H. Structural basis for unique mechanisms of folding and hemoglobin binding by a malarial protease. Proc. Natl. Acad. Sci. USA 2006, 103, 11503–11508. [Google Scholar] [CrossRef]
- Li, H.; Chen, Li.; Huang, J.; Zhu, J.; Shen, X.; Li, J.; Hilgenfeld, R.; Jiang, H. Identification of Novel Falcipain-2 Inhibitors as Potential Antimalarial Agents through Structure-Based Virtual Screening. Submitted to J. Med. Chem.
- Huang, W.; Li, J.; Tang, J.; Liu, H.; Shen, J.; Jiang, H. Microwave-assisted Synthesis of 2-Amino-thiophene-3-carboxylic Derivatives under Solvent-Free Condition. Syn. Comm. 2005, 35, 1351–1357. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Rosenthal, P.J.; McKerrow, J.H.; Rasnick, D.; Leech, J.H. Plasmodium falciparum: inhibitors of lysosomal cysteine proteinases inhibit a trophozoite proteinase and block parasite development. Mol. Biochem. Parasitol. 1989, 35, 177–183. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds reported in this paper are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhu, J.; Chen, T.; Liu, J.; Ma, R.; Lu, W.; Huang, J.; Li, H.; Li, J.; Jiang, H. 2-(3,4-Dihydro-4-Oxothieno[2,3-d]pyrimidin-2-ylthio) Acetamides as a New Class of Falcipain-2 Inhibitors. 3. Design, Synthesis and Biological Evaluation. Molecules 2009, 14, 785-797. https://doi.org/10.3390/molecules14020785
Zhu J, Chen T, Liu J, Ma R, Lu W, Huang J, Li H, Li J, Jiang H. 2-(3,4-Dihydro-4-Oxothieno[2,3-d]pyrimidin-2-ylthio) Acetamides as a New Class of Falcipain-2 Inhibitors. 3. Design, Synthesis and Biological Evaluation. Molecules. 2009; 14(2):785-797. https://doi.org/10.3390/molecules14020785
Chicago/Turabian StyleZhu, Jin, Tong Chen, Jie Liu, Ruoqun Ma, Weiqiang Lu, Jin Huang, Honglin Li, Jian Li, and Hualiang Jiang. 2009. "2-(3,4-Dihydro-4-Oxothieno[2,3-d]pyrimidin-2-ylthio) Acetamides as a New Class of Falcipain-2 Inhibitors. 3. Design, Synthesis and Biological Evaluation" Molecules 14, no. 2: 785-797. https://doi.org/10.3390/molecules14020785




