Laser Photolysis and Thermolysis of Organic Selenides and Tellurides for Chemical Gas-phase Deposition of Nanostructured Materials
Abstract
:1. Introduction
2. The nature of laser-induced processes
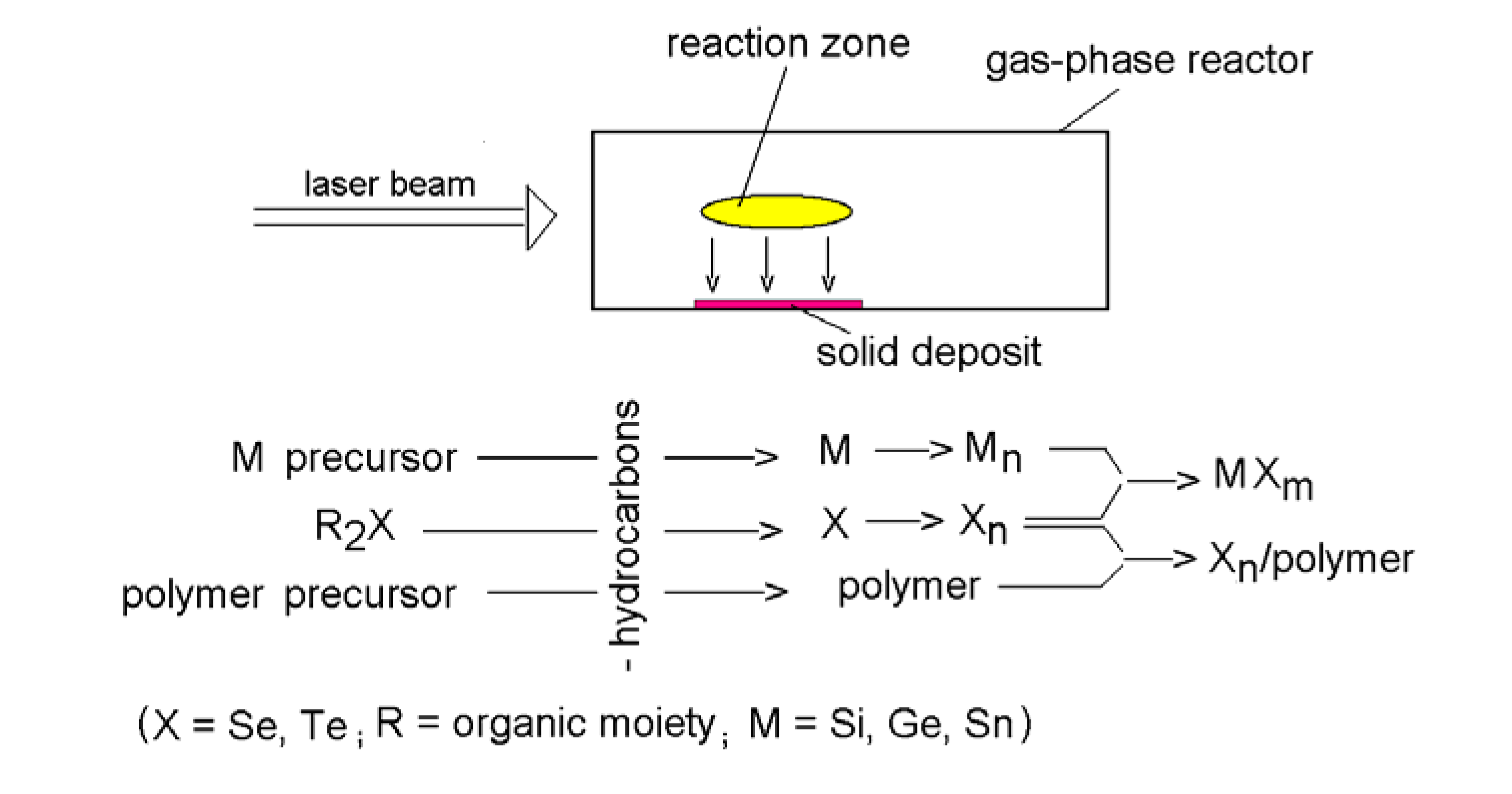
3. Deposition of chalcogen
3.1. UV laser photolysis
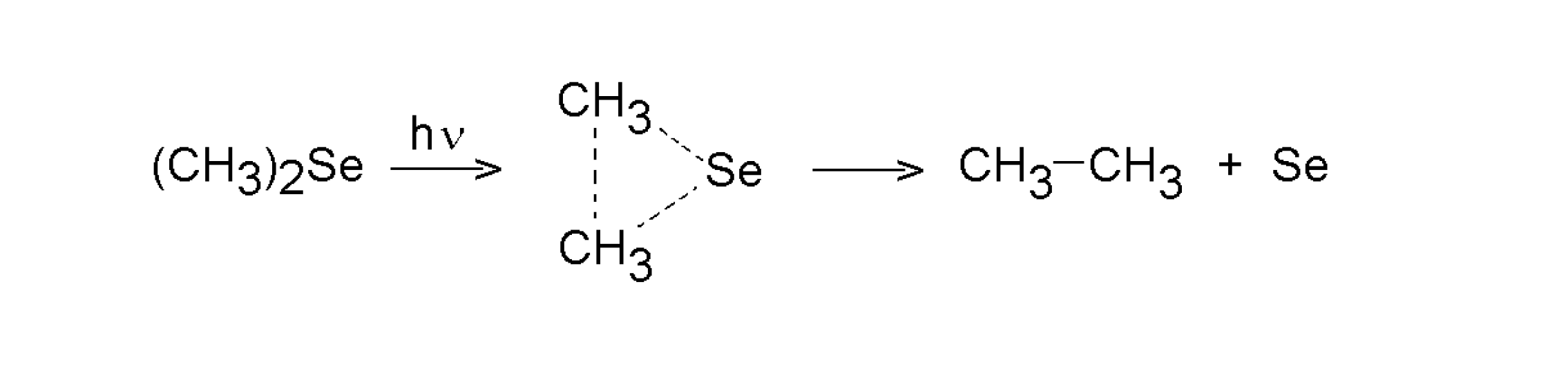
3.1.1. Dimethyl selenide and dimethyl telluride
3.1.2. Diethyl selenide and diethyl telluride


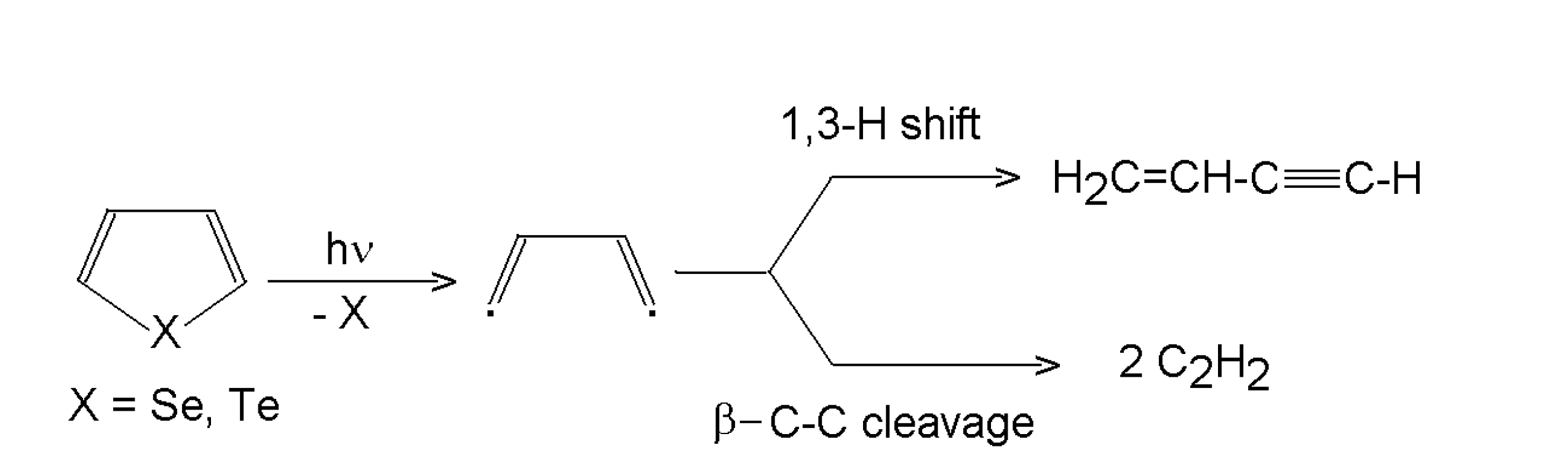
3.1.3. Selenophene and tellurophene
3.2. IR laser thermolysis
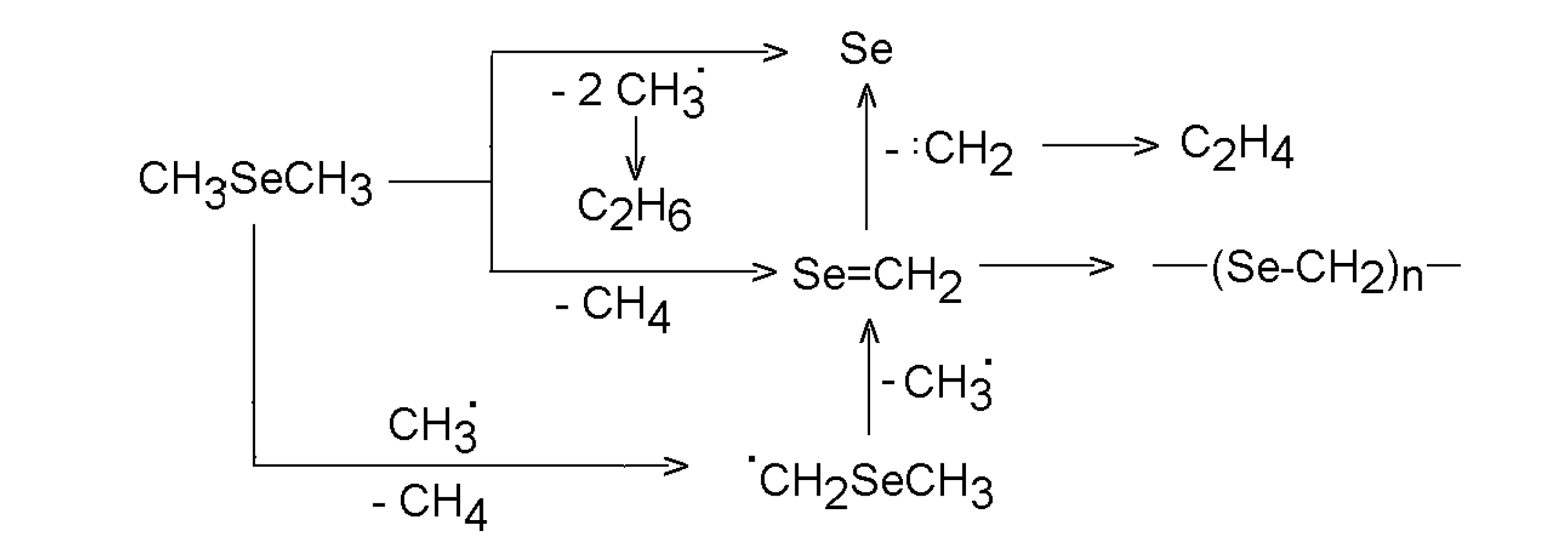
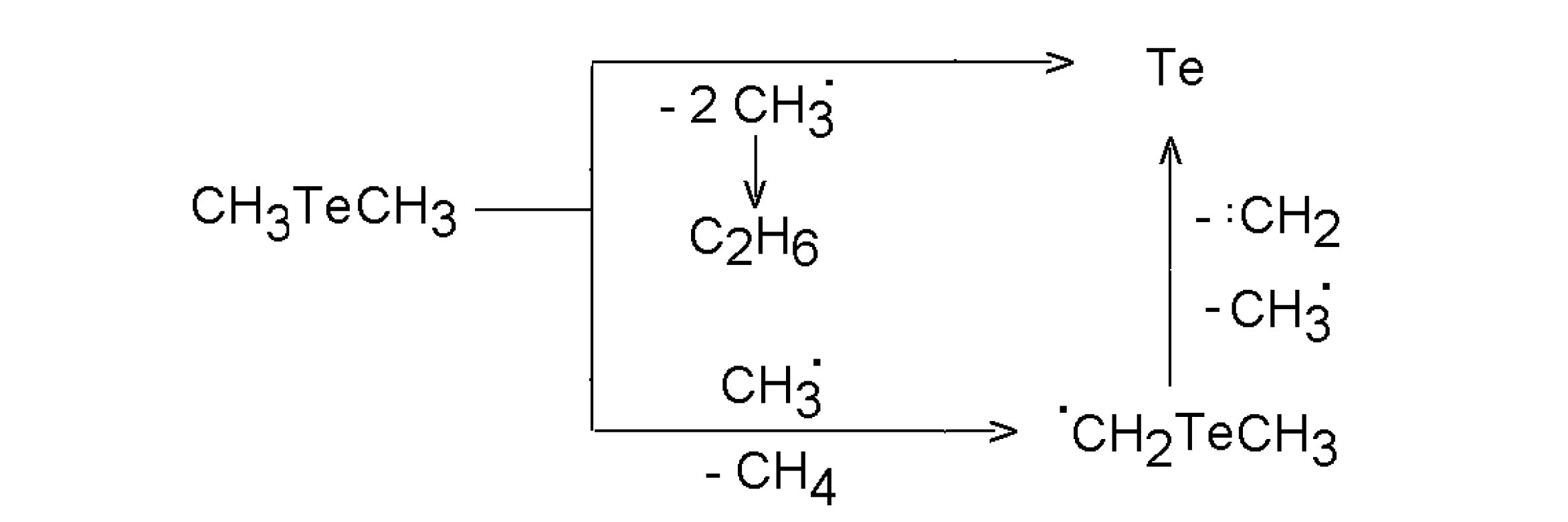
3.2.1. Dimethyl selenide and dimethyl telluride
3.2.2. Selenophene and tellurophene

4. Deposition of chalcogen/polymer composite

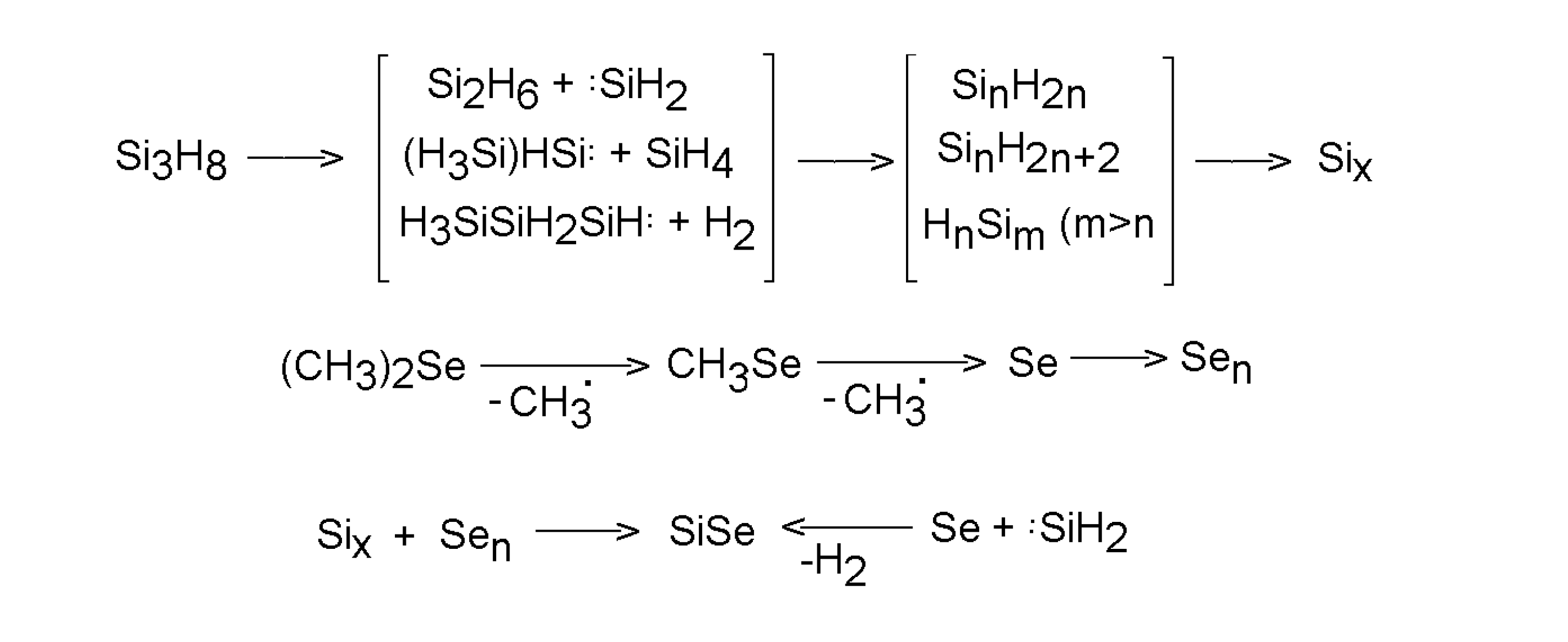
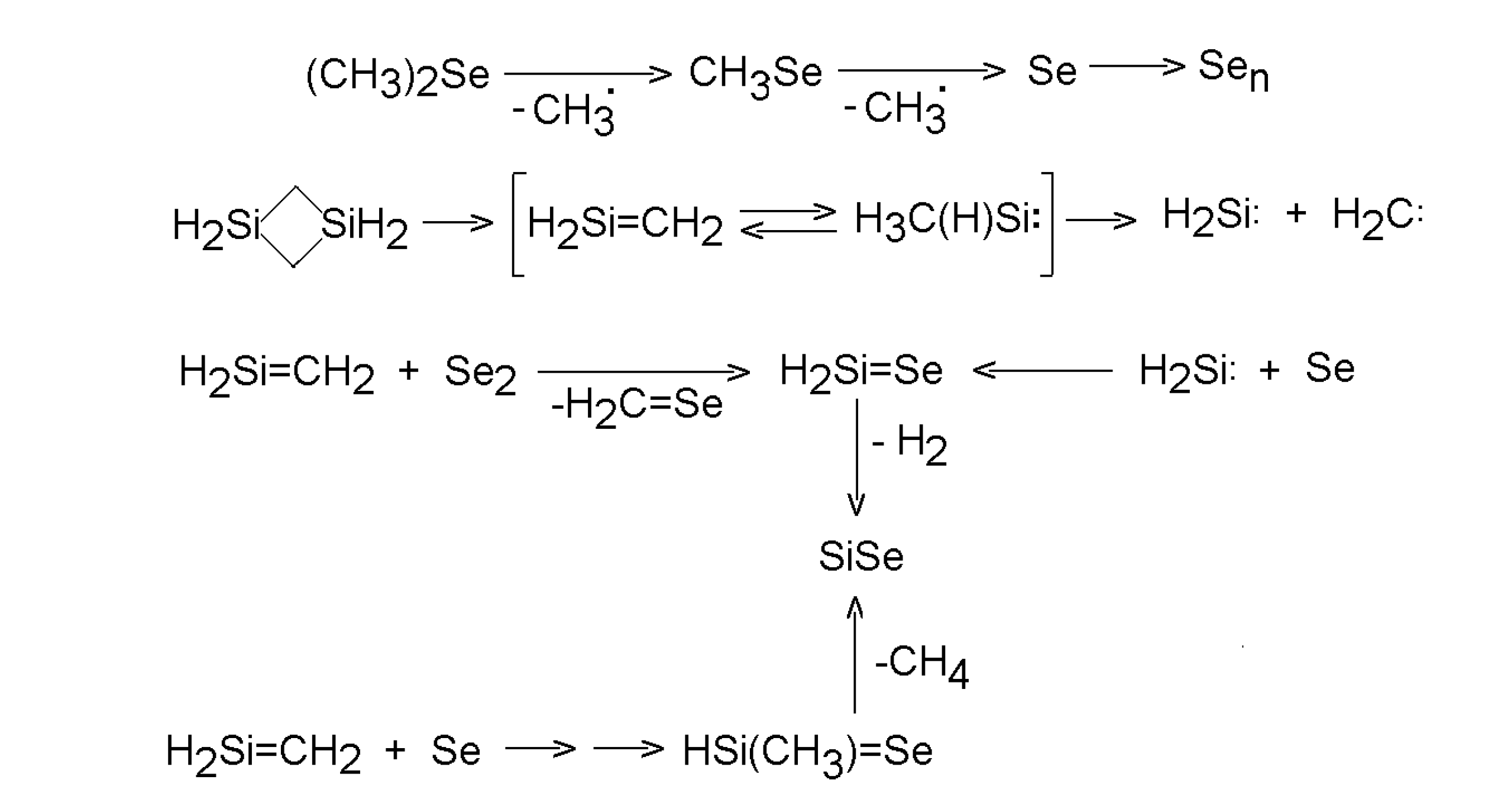
5. Deposition of metal chalcogenides
6. Summary
Acknowledgements
References and Notes
- Krief, A. Selenium. In Comprehensive Organometallic Chemistry II; Pergamon: Oxford, UK, 1995; Ch. 13. [Google Scholar]
- Petragnani, N. Tellurium. In Comprehensive Organometallic Chemistry II; Pergamon: Oxford UK, 1995; Ch. 14. [Google Scholar]
- Guziec, F.S.; Sanfilippo, L.J. Synthetically Useful Extrusion Reactions of Organic Sulfur, Selenium and Tellurium Compounds. Tetrahedron 1988, 6241–6285. [Google Scholar] [CrossRef]
- Back, T.G. Radical Reactions of Selenium Compounds. In Organoselenium Chemistry; Liotta, D.C., Ed.; Wiley-Interscience: New York, USA, 1987; Ch. 7. [Google Scholar]
- Martens, J.; Praefcke, K. Photochemistry of Organic Selenium and Tellurium Compounds. J. Organometal. Chem. 1980, 198, 321–351. [Google Scholar] [CrossRef]
- Deryagina, E.N.; Voronkov, M.G.; Korchevin, N.A. Selenium and Tellurium-Centered Radicals. Russ. Chem. Rev. 1993, 62, 1107–1117. [Google Scholar]
- Byers, J.H.; Lane, G.C. Radical Addition Reactions of 2-(Phenylseleno)Propanedioates to Alkenes and Alkynes. J. Org. Chem 1993, 58, 3355–3360. [Google Scholar] [CrossRef] and refs. therein.
- Kobayashi, K.; Shinhara, S.; Moriyama, M.; Fujii, T.; Horn, E.; Yabe, A.; Furukawa, N. Generation of Ketenes by Photolysis of Naphtha[1,8-de]-1,3-Dichalcogeninylidene 1-Oxides. Tetrahedron Lett. 1999, 40, 5211–5214. [Google Scholar]
- Batt, L. The Chemistry of Organic Selenium and Tellurium Compounds; Patai, S., Rappoport, Z., Eds.; Wiley: Chichester, U.K, 1986; Vol. 1, Ch. 4. [Google Scholar]
- Mullin, J.B.; Irwine, S.J.C. Ulraviolet Assisted Growth of II-VI Compounds. J. Vac. Sci. Technol. A 1986, 4, 700–705. [Google Scholar] [CrossRef]
- McAllister, T.J. Chemical Kinetics of Telluride Pyrolysis. Cryst. Growth 1989, 96, 552–560. [Google Scholar] [CrossRef]
- Higushi, H.; Otsubo, T.; Ogura, F.; Yamaguchi, H.; Sakata, Y.; Misumi, S. Flash Pyrolysis of Selenides. Synthesis of Bibenzyls, Olefins and Related Compounds. Bull. Chem. Soc. Jpn. 1982, 55, 182–187. [Google Scholar]
- Higuchi, H.; Tani, K.; Otsubo, T.; Sakata Y.; Misumi, S. New Synthetic Method of [2,2]Cyclophanes via Diselena[3,3]Cyclophanes. Bull. Chem. Soc. Jpn. 1987, 60, 4027–4036. [Google Scholar] [CrossRef]
- Ahlgren, G.; Åkermark, B.; Nils, M. Bialkyls from Dialkylmetals. I. Photochemistry and Mass Spectra of some (2-cyanoethyl)- and [2(methoxycarbonyl)ethyl]Metals. J. Organometal Chem. 1971, 30, 303–313. [Google Scholar] [CrossRef]
- Bergman, J. Deselenation and the Mass Spectra of 3,3’-di-indolyl Selenides. Acta Chim. Scand. 1968, 22, 1882–1887. [Google Scholar]
- Magnus, P.D. Organic Selenium and Tellurium Compounds. In Comprehensive Organic Chemistry; Jones, D. N., Ed.; Pergamon Press: Oxford, UK, 1979; Volume. 3, pp. 529–530. [Google Scholar]
- Brown, D.H.; Cross, R.J.; Millington, D. Photolysis of Diorganoditellurides in the Presence of Tertiary Phosphines. J. Organometal. Chem. 1977, 125, 219–223. [Google Scholar] [CrossRef]
- Spencer, H.K.; Cava, M.P. Organotellurium Chemistry. 2. Dibenzyl Ditelluride: some Transformations Involving Loss of Tellurium. J. Org. Chem. 1977, 42, 2937–2939. [Google Scholar] [CrossRef]
- Cuthbertson, E.; MacNicol, D.D. Tellurium Extrusion: a Novel Method for Carbon–Carbon Bond Formation. J. Chem. Soc., Chem. Commun. 1974, 498–499. [Google Scholar] [CrossRef]
- Cuthbertson, E.; MacNicol, D.D. Tellurium Extrusion: Synthesis of Benzocyclobutene and Naphto[b]Cyclobutene. Tetrahedron Lett. 1975, 16, 1893–1894. [Google Scholar] [CrossRef]
- Clive, D.L.J.; Anderson, P.C.; Moss, N.; Singh, A. New Method for Coupling Allylic Halides: Use of Telluride(2-) ion Species. J. Org. Chem. 1982, 47, 1641–1647. [Google Scholar] [CrossRef]
- Connor, J.; Greig, G.; Strausz, O.P. The Reactions of Tellurium Atoms. J. Am. Chem. Soc. 1969, 91, 5695–5696. [Google Scholar] [CrossRef]
- Strausz, O.P.; Connor, J.; Van Roodselaar, A.; Fair, R.W. Addition of Group Via atoms to Tetramethylene. Addition Reaction with a Negative Activation Energy. J. Am. Chem. Soc. 1971, 93, 560–562. [Google Scholar] [CrossRef]
- Grunwald, E.; Dever, D.F.; Keehn, P.M. Megawatt Infrared Laser Chemistry; Wiley: New York, USA, 1978. [Google Scholar]
- Kleinermanns, K.; Wolfrum, J. Laser Chemistry – What Is Its Current Status? Angew. Chem. Int. Ed. Engl. 1987, 26, 38–58. [Google Scholar] [CrossRef]
- Proceedings on Laser Induced Chemistry, Bechyně, Czechoslovakia, 1989. Spectrochim. Acta 1990, 46A(No. 4).
- Shaub, W.M.; Bauer, S.H. Laser-Powered Homogeneous Pyrolysis. Int. J. Chem. Kinet. 1975, 7, 509–529. [Google Scholar] [CrossRef]
- Gritsenko, K.P. Metal-Polymer Optical Storage Media Produced by PECVD. Thin Solid Films 1993, 227, 1–2. [Google Scholar] [CrossRef]
- Kolobov, A.V.; Tominaga, J. Chalcogenide Glasses in Optical Recording: Recent Progress. J. Optoelectron. Adv. Mater. 2002, 4, 679–686. [Google Scholar]
- Yannopoulos, S.N.; Andrikopoulos, K.S. Raman Scattering Study on Structural and Dynamical Features of Noncrystalline Selenium. J. Chem. Phys. 2004, 121, 4747–4758. [Google Scholar] [CrossRef]
- Martin, M. Passive colling; Cook, J., Ed.; MIT Press: Cambridge, MA, USA, 1989. [Google Scholar]
- Tsiulyanu, D.; Marian, S.; Miron, V.; Liess, H.D. High Sensitive Tellurium Based NO2 Gas Sensor. Sens. Actuators B 2001, 73, 35–39. [Google Scholar] [CrossRef]
- Lou, D.Y.; Blom, G.M.; Kenney, G.C. Bit Oriented Optical Storage with Thin Tellurium Films. J. Vac. Sci. Technol. 1981, 18, 78–86. [Google Scholar] [CrossRef]
- Judge, R.H.; Moule, D.C. Detection of a3A2(n,.pi.*) Selenoformaldehyde by Flash Pyrolysis. J. Am. Chem. Soc. 1984, 106, 5406–5407. [Google Scholar] [CrossRef]
- Didenkulova, I.I.; Dyagileva, L.M.; Tsyganova, E.I.; Alexandrov, Yu.A. Kinetics of Gas-Phase Pyrolysis of Dimethyl Selenide. Zh. Obshch. Khim. 1984, 54, 2288–2290, [Chem. Abstr. 102:45357m]. [Google Scholar]
- Yablokov, V.A.; Dozorov, A.V.; Zorin, A.D.; Feshchenko, I.A.; Ronina, O.V.; Karataev, E.N. Kinetics of Gas-Phase Pyrolysis of Dimethyl Telluride and Dimethyl Selenide. Zh. Obshch. Khim. 1986, 56, 1751–1754, [Chem. Abstr. 1987, 106, 213284q]. [Google Scholar]
- McAllister, T. Chemical Kinetics of Telluride Pyrolysis. J. Cryst. Growth 1989, 96, 552–560. [Google Scholar] [CrossRef]
- Jackson, D.A. The Onset of Pyrolysis for a Group of Organometallics. J. Cryst. Growth 1988, 87, 205–212. [Google Scholar] [CrossRef]
- McQueen, A.E.D.; Culshaw, P.N.; Walton, J.C.; Shenai-Khatkhate, D.V.; Mullin, J.B. Synthesis and Decomposition Studies of Dialkyltellurides of Type RTeR’. J. Cryst. Growth 1991, 107, 325–330. [Google Scholar] [CrossRef]
- BelBruno, J.L.; Spacek, J.; Christophy, E. Multiphoton Dissociation Dynamics of Dimethyl Selenide. J. Phys. Chem. 1991, 95, 6928–6932. [Google Scholar]
- Pola, J.; Bastl, Z.; Šubrt, J.; Ouchi, A. Atmospheric Pressure Chemical Vapor Deposition of Selenium Films by KrF Laser Photolysis of Dimethyl Selenium. Appl. Surf. Sci. 2001, 172, 220–224. [Google Scholar] [CrossRef]
- Pola, J.; Urbanová, M.; Volnina, E.A.; Bakardjieva, S.; Šubrt, J.; Bastl, Z. Polymer-Stabilized Nano-Sized Tellurium Films by Laser-Induced Chemical Vapour Co-Deposition Process. J. Mater. Chem. 2003, 13, 394–398. [Google Scholar] [CrossRef]
- Brewer, P.D.; Jensen, J.E.; Plson, G.L.; Tutt, L.W.; Zinck, J.J. Photodissociation Dynamics of Alkyltellurides. Mat. Res. Symp. Proc. (Materials Research Society) 1988, 101, 327–330. [Google Scholar]
- Brewer, P.D. The Production of Te Atoms by the Multiphoton Dissociation of Diethyl Telluride. Chem. Phys. Lett. 1987, 141, 301–305. [Google Scholar] [CrossRef]
- Fantoni, R.; Stuke, M. Laser Multiphoton Mass Spectroscopy of Organometallic Compounds: State Selective and Mass Resolved Detection of Neutral Fragment Tellurium Atoms from C2H5TeC2H5. Appl. Phys. B 1985, 38, 209–218. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujii, S.; Iuchi, T. Ultraviolet spectra of II-VI Organometallic Compounds and their Application to in situ Measurements of the Photolysis in a Organometallic Vapor Deposition Reactor. J. Vac. Sci. Technol. A 1989, 7, 276–280. [Google Scholar] [CrossRef]
- Jensen, J.E.; Brewer, P.D.; Olson, G.L.; Tutt, L.W.; Zinck, J.J. Excimer Laser-Assisted Metalorganic Vapor Phase Epitaxy of CdTe and HgTe on (100) GaAs. J. Vac. Sci. Technol. A 1988, 6, 2808–2812. [Google Scholar] [CrossRef]
- Pola, J.; Ouchi, A. UV Laser-Induced Photolysis of Diethyl Selenium and Diethyl Tellurium: Extrusion of Selenium and Tellurium via Molecular Elimination of Ethene. J. Organometal. Chem. 2001, 629, 93–96. [Google Scholar] [CrossRef]
- Pola, J.; Bastl, Z.; Šubrt, J.; Ouchi, A. Atmospheric Pressure Chemical Vapor Deposition od Selenium and Tellurium Films by UV Laser Photolysis of Diethyl Selenium and Diethyl Tellurium. Appl. Organometal. Chem. 2001, 15, 924–930. [Google Scholar]
- Gibian, M.J.; Corley, R.C. Organic Radical-Radical Reactions. Disproportionation vs. Combination. Chem. Rev. 1973, 73, 441–464. [Google Scholar] [CrossRef]
- Pola, J.; Ouchi, A. ArF and KrF Laser-Induced Gas-Phase Photolysis of Selenophene and Tellurophene: Extrusion of Te and Se and Intramoleculr 1,3-H Shift Competing with b-C-C Bond Cleavage in C4H4 residue. J. Org. Chem. 2000, 65, 2759–2762. [Google Scholar] [CrossRef]
- Pola, J.; Bastl, Z.; Šubrt, J.; Ouchi, A. Chemical Vapor Deposition of Selenium and Tellurium Films by UV Laser Photolysis of Selenophene and Tellurophene. Appl. Organometal. Chem. 2000, 14, 715–720. [Google Scholar] [CrossRef]
- Pokorná, D.; Urbanová, M.; Bastl, Z.; Šubrt, J.; Pola, J. Laser-Induced Gas-Phase Pyrolysis of Dimethyl Selenium: Chemical Deposition of Selenium and poly(Selenoformaldehyde). J. Anal. Appl. Pyrol. 2004, 71, 635–644. [Google Scholar]
- Pola, J.; Pokorná, D.; Boháček, J.; Bastl, Z.; Ouchi, A. Nano-Structured Crystalline Te Films by Laser Gas-Phase Pyrolysis of Dimethyl Tellurium. J. Anal. Appl. Pyrol. 2004, 71, 739–746. [Google Scholar]
- Urbanová, M.; Pokorná, D.; Ouchi, A.; Pola, J. Laser Powered Homogeneous Decomposition of Selenophene And Tellurophene. J. Anal. Appl. Pyrol. 2005, 73, 101–106. [Google Scholar]
- Pola, J. IR and UV laser-Induced Decomposition of Organosilanes for CVD of Si/C/H Phases. Res. Chem. Intermed. 1999, 25, 351–366. [Google Scholar] [CrossRef]
- Santos, M.; Diaz, L.; Pola, J. Transient Detection in Infrared Multiphoton Decomposition of (Chloromethyl)Silane and 1,3-Disilacyclobutane: Evidence for Cleavage of SiCH4 Intermediates. J. Photochem. Photobiol. A 2002, 152, 17–24. [Google Scholar] [CrossRef]
- Gritsenko, K.P. Polymeric Metal-Filled Films for Photothermal Optical Recording. Proc. of SPIE 1998, 3347, 165–173. [Google Scholar]
- Herman, I.P. Laser-assisted deposition of thin films from gas-phase and surface-adsorbed molecules. Chem. Rev. 1989, 89, 1323–1357. [Google Scholar] [CrossRef]
- Pokorná, D.; Boháček, J.; Vorlíček, V.; Šubrt, J.; Bastl, Z.; Volnina, E.A.; Pola, J. IR laser-co-pyrolysis of (CH3)2Te and (CH3)4Sn: Gas-Phase Formation and Deposition of Nanostructured SnTe. J. Anal. Appl. Pyrol. 2006, 75, 65–68. [Google Scholar]
- Pola, J.; Pokorná, D.; Diánez, M.J.; Sayagués, M.J.; Bastl, Z.; Vorlíček, V. IR Laser-Induced Synthesis of Nanostructured Germanium Telluride in the Gas Phase. Appl. Organometal. Chem. 2005, 19, 854–858. [Google Scholar] [CrossRef]
- Santos, M.; Díaz, L.; Urbanová, M.; Bastl, Z.; Šubrt, J.; Pola, J. IR Laser-Induced Co-Decomposition of Dimethyl Selenide and Trisilane: Gas-Phase Formation of SiSe and Chemical Vapor Deposition of Nanostructured H/Si/Se/C Polymers. J. Photochem. Photobiol. A 2007, 188, 399–408. [Google Scholar] [CrossRef]
- Díaz, L.; Santos, M.; Pola, J. Gas-phase formation of SiSe in IR Laser-Co-Decomposition of Dimethyl Selenide and 1,3-Disilacyclobutane. J. Organometal. Chem. 2007, 692, 3841–3845. [Google Scholar] [CrossRef]
- Santos, M.; Díaz, L.; Urbanová, M.; Pokorná, D.; Bastl, Z.; Šubrt, J.; Pola, J. IR Laser-Induced Process for Chemical Vapor Deposition of Polyselenocarbosilane Films. J. Anal. Appl. Pyrol. 2006, 76, 178–185. [Google Scholar]
- Ouchi, A.; Bastl, Z.; Boháček, J.; Orita, H.; Miyazaki, K.; Miyashita, S.; Bezdička, P.; Pola, J. Room –Temperature Reaction Between Laser Chemical Vapor Deposited Selenium and some Metals. Chem. Mater. 2004, 16, 3439–3445. [Google Scholar] [CrossRef]
- Sample availability: Not available.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pola, J.; Ouchi, A. Laser Photolysis and Thermolysis of Organic Selenides and Tellurides for Chemical Gas-phase Deposition of Nanostructured Materials. Molecules 2009, 14, 1111-1125. https://doi.org/10.3390/molecules14031111
Pola J, Ouchi A. Laser Photolysis and Thermolysis of Organic Selenides and Tellurides for Chemical Gas-phase Deposition of Nanostructured Materials. Molecules. 2009; 14(3):1111-1125. https://doi.org/10.3390/molecules14031111
Chicago/Turabian StylePola, Josef, and Akihiko Ouchi. 2009. "Laser Photolysis and Thermolysis of Organic Selenides and Tellurides for Chemical Gas-phase Deposition of Nanostructured Materials" Molecules 14, no. 3: 1111-1125. https://doi.org/10.3390/molecules14031111



