Association Mechanism of S-Dinitrophenyl Glutathione with Two Glutathione Peroxidase Mimics: 2, 2¢-Ditelluro- and 2, 2¢-Diseleno-bridged b-cyclodextrins
Abstract
:Introduction
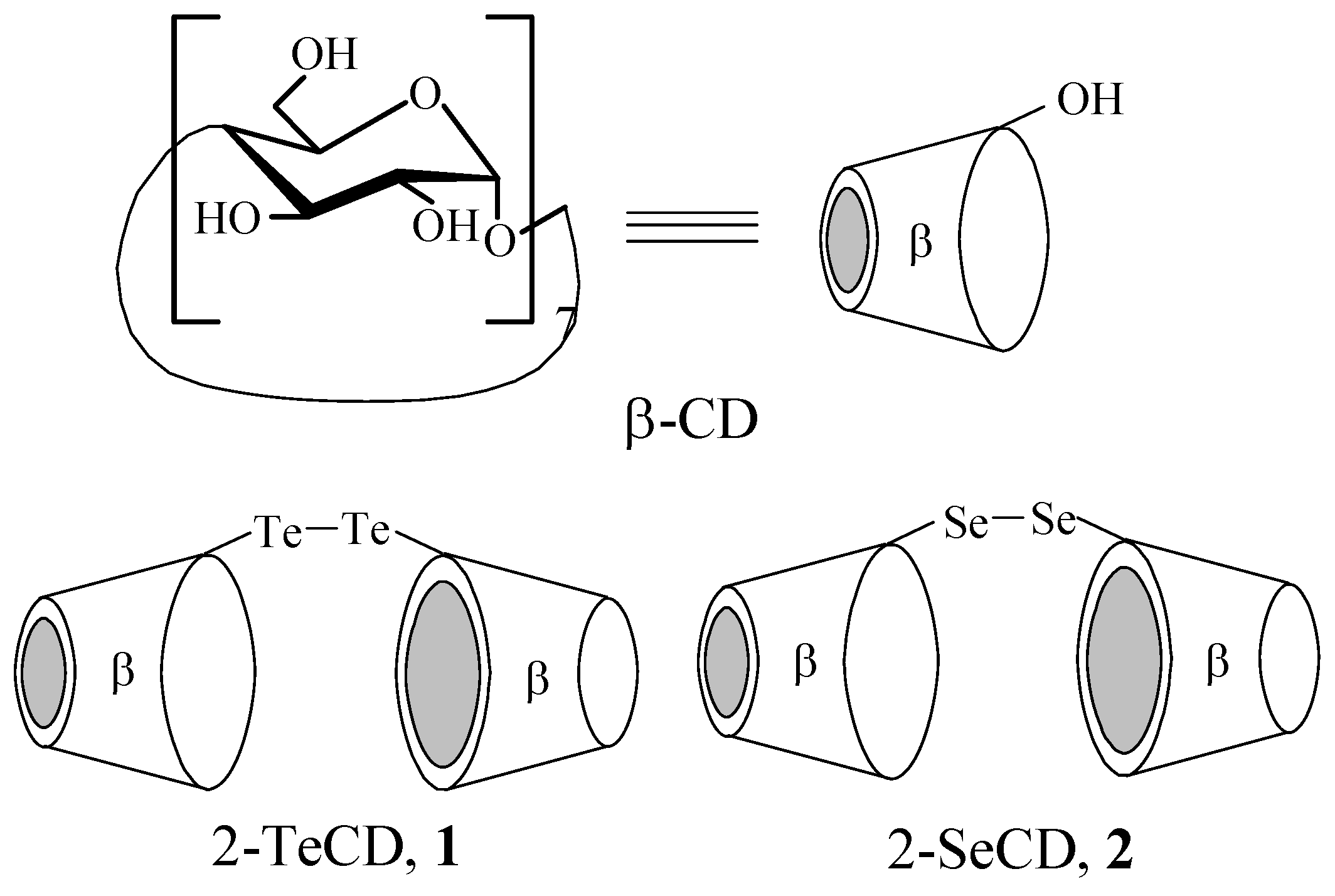
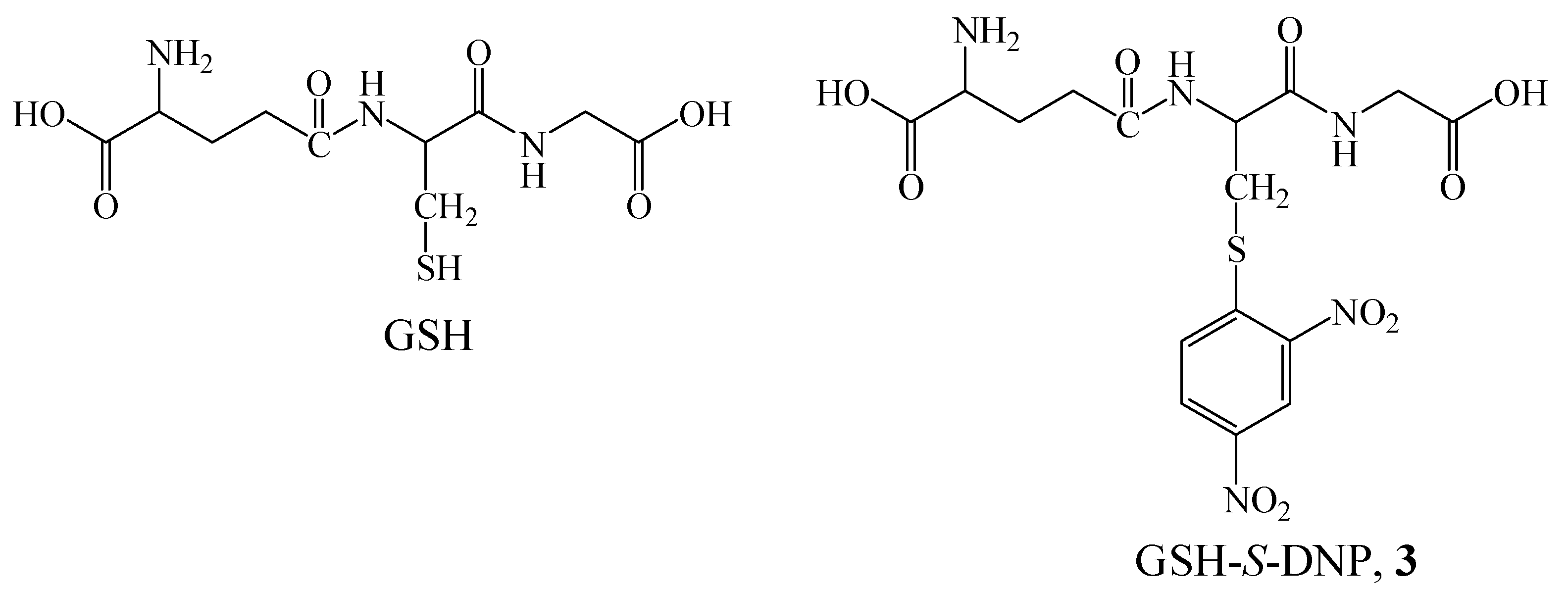
Results and Discussion
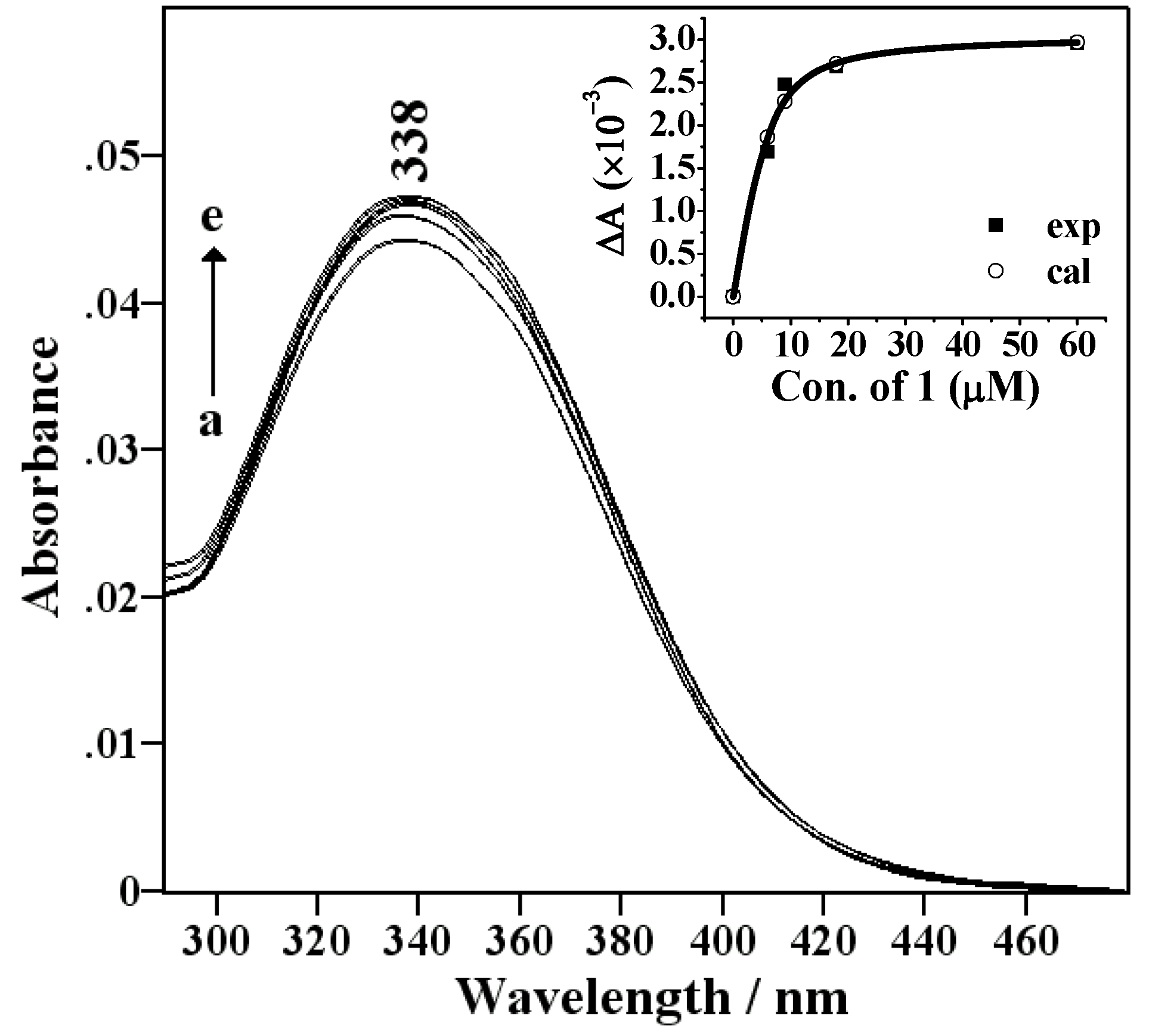
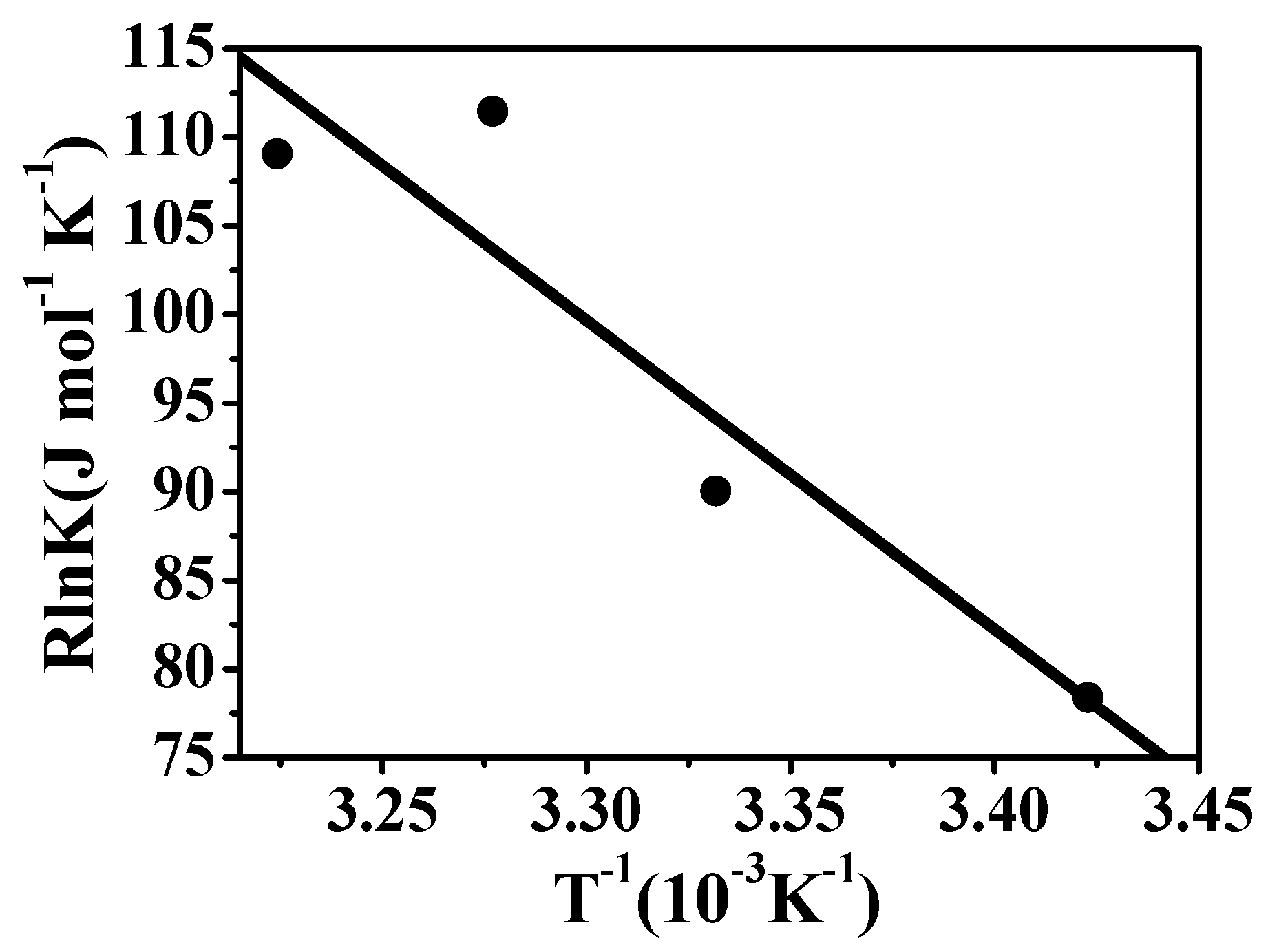
Spectral titration and thermodynamic measurements


| Hosts | Ks [M-1] | Ks(X)/Ks(β-CD)* | log Ks | Δε |
|---|---|---|---|---|
| β-CD | 1.49×104 | ≡1 | 4.17 | 1050 |
| 1 | 6.64×104 | 4.46 | 4.82 | 510 |
| 2 | 2.21×104 | 1.48 | 4.34 | 960 |

| Hosts | -ΔG° [kJ mol-1] | ΔH° [kJ mol-1] | TΔS° [kJ mol-1] |
|---|---|---|---|
| β-CD | 22.095 | 81.649 | 103.744 |
| 1 | 26.908 | 174.497 | 201.404 |
| 2 | 22.402 | 261.049 | 283.451 |
1H-NMR spectroscopy

Molecular mechanics (MM2) modeling calculations


Conclusions
Experimental
General
UV-Vis spectra
MM2 calculations
Acknowledgements
References
- Mugesh, G.; Singh, H.B. Synthetic organoselenium compounds as antioxidants: glutathione peroxidese activity. Chem. Soc. Rev. 2000, 29, 347–357. [Google Scholar] [CrossRef]
- Mugesh, G.; du Mont, W.-W. Structure-Activity Correlation between Natural Glutathione Peroxidase (GPx) and Mimics: A Biomimetic Concept for the Design and Synthesis of More Efficient GPx Mimics. Chem. Eur. J. 2001, 7, 1365–1370. [Google Scholar] [CrossRef]
- Wilson, S.R.; Zucker, P.A.; Huang, R.R.C.; Spector, A. Development of synthetic compounds with glutathione peroxidase activity. J. Am. Chem. Soc. 1989, 111, 5936–5939. [Google Scholar] [CrossRef]
- McNaughton, M.; Engman, L.; Birmingham, A.; Powis, G.; Cotgreave, I.A. Cyclodextrin-derived diorganyl tellurides as glutathione peroxidase mimics and inhibitors of thioredoxin reductase and cancer cell growth. J. Med. Chem. 2004, 47, 233–239. [Google Scholar] [CrossRef]
- Andersson, C.-M.; Hallberg, A.; Brattsand, R.; Cotgreave, I.A.; Engman, L.; Persson, J. Glutathione peroxidase-like activity of diaryl tellurides. Bioorg. Med. Chem. Lett. 1993, 3, 2553–2558. [Google Scholar] [CrossRef]
- Engman, L.; Stern, D.; Pelcman, M.; Andersson, C. M. Thiol Peroxidase Activity of Diorganyl Tellurides. J. Org. Chem. 1994, 59, 1973–1979. [Google Scholar] [CrossRef]
- Vessman, K.; Ekstrom, M.; Berglund, M.; Andersson, C.M.; Engman, L. Catalytic Antioxidant Activity of Diaryl Tellurides in a Two-Phase Lipid Peroxidation Model. J. Org. Chem. 1995, 60, 4461–4467. [Google Scholar] [CrossRef]
- Malmström, J.; Jonsson, M.; Cotgreave, I.A.; Hammarström, L.; Sjödin, M.; Engman, L. The antioxidant profile of 2,3-dihydrobenzo[b]furan-5-ol and its 1-thio, 1-seleno, and 1-telluro analogues. J. Am. Chem. Soc. 2001, 123, 3434–3440. [Google Scholar]
- Kanda, T.; Engman, L.; Cotgreave, I.A.; Powis, G. Novel Water-Soluble Diorganyl Tellurides with Thiol Peroxidase and Antioxidant Activity. J. Org. Chem. 1999, 64, 8161–8169. [Google Scholar] [CrossRef]
- Dsouza, V.P.; Bender, M.L. Miniature organic models of enzymes. Acc. Chem. Res. 1987, 20, 146–152. [Google Scholar] [CrossRef]
- Breslow, R.; Dong, S.D. Biomimetic Reactions Catalyzed by Cyclodextrins and Their Derivatives. Chem. Rev. 1998, 98, 1997–2012. [Google Scholar] [CrossRef]
- Zhang, B.; Breslow, R. Enthalpic domination of the chelate effect in cyclodextrin dimmers. J. Am. Chem. Soc. 1993, 115, 9353–9354. [Google Scholar] [CrossRef]
- Venema, F.; Rowan, A.E.; Nolte, R.J.M. Binding of Porphyrins in Cyclodextrin Dimers. J. Am. Chem. Soc. 1996, 118, 257–258. [Google Scholar] [CrossRef]
- French, R.R.; Wirz, J.; Woggon, W.-D. A Synthetic Receptor for β,β-Carotene: Towards an enzyme mimic for central cleavage. Helv. Chim. Acta 1998, 81, 1521–1527. [Google Scholar] [CrossRef]
- Breslow, R.; Greenspoon, N.; Guo, T.; Zarzycki, R. Very strong binding of appropriate substrates by cyclodextrin dimmers. J. Am. Chem. Soc. 1989, 111, 8296–8297. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; You, C.C.; Wada, T.; Inoue, Y. Molecular recognition studies on supramolecular systems. 32. Molecular recognition of dyes by organoselenium-bridged bis(beta-cyclodextrin)s. J. Org. Chem. 2001, 66, 225–232. [Google Scholar] [CrossRef]
- Breslow, R.; Zhang, B. Very fast ester hydrolysis by a cyclodextrin dimer with a catalytic linking group. J. Am. Chem. Soc. 1992, 114, 5882–5883. [Google Scholar] [CrossRef]
- Zhang, B.; Breslow, R. Ester Hydrolysis by a Catalytic Cyclodextrin Dimer Enzyme Mimic with a Metallobipyridyl Linking Group. J. Am. Chem. Soc. 1997, 119, 1676–1681. [Google Scholar] [CrossRef]
- Ren, X.; Xue, Y.; Liu, J.; Zhang, K.; Zheng, J.; Luo, G.; Guo, C.; Mu, Y.; Shen, J. A novel cyclodextrin-derived tellurium compound with glutathione peroxidase activity. ChemBioChem. 2002, 3, 356–363. [Google Scholar] [CrossRef]
- Ren, X.; Xue, Y.; Zhang, K.; Liu, J.; Luo, G.; Zheng, J.; Mu, Y.; Shen, J. A novel dicyclodextrinyl ditelluride compound with antioxidant activity. FEBS Lett. 2001, 507, 377–380. [Google Scholar] [CrossRef]
- Liu, J. Q.; Gao, S.J.; Luo, G.M.; Yan, G.L.; Shen, J.C. Artificial imitation of glutathione peroxidase with 6-selenium-bridged beta-cyclodextrin. Biochem. Biophys. Res. Commun. 1998, 247, 397–400. [Google Scholar] [CrossRef]
- Becker, H.-C.; Norden, B. DNA Binding Properties of 2, 7-Diazapyrene and Its N-Methylated Cations Studied by Linear and Circular Dichroism Spectroscopy and Calorimetry. J. Am. Chem. Soc. 1997, 119, 5798–5803. [Google Scholar] [CrossRef]
- Inoue, Y.; Yamamoto, K.; Wada, T.; Everitt, S.; Gao, X.-M.; Hou, Z.-J.; Tong, L.-H.; Jiang, S. -K.; Wu, H. -M. Inclusion complexation of (cyclo)alkanes and (cyclo)alkanols with 6-O-modified cyclodextrins. J. Chem. Soc., Perkin Trans. 2 1998, 1807–1816. [Google Scholar]
- Liu, Y.; Li, L.; Li, X.-Y.; Zhang, H.-Y.; Wada, T.; Inoue, Y. Synthesis of phosphoryl-tethered beta-cyclodextrins and their molecular and chiral recognition thermodynamics. J. Org. Chem. 2003, 68, 3646–3657. [Google Scholar] [CrossRef]
- Merino, C.; Junquera, E.; Jiménez-Barbero, J.; Aicart, E. Effect of the Presence of β-Cyclodextrin on the Solution Behavior of Procaine Hydrochloride. Spectroscopic and Thermodynamic Studies. Langmuir 2000, 16, 1557–1565. [Google Scholar]
- Rekharsky, M.V.; Inoue, Y. Complexation and chiral recognition thermodynamics of 6-amino-6-deoxy-beta-cyclodextrin with anionic, cationic, and neutral chiral guests: counterbalance between van der Waals and coulombic interactions. J. Am. Chem. Soc. 2002, 124, 813–826. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Chiral Recognition Thermodynamics of β-Cyclodextrin: The Thermodynamic Origin of Enantioselectivity and the Enthalpy-Entropy Compensation Effect. J. Am. Chem. Soc. 2000, 122, 4418–4435. [Google Scholar] [CrossRef]
- Hauser, S.L.; Johanson, E.W.; Green, H.P.; Smith, P.J. Aryl phosphate complexation by cationic cyclodextrins. An enthalpic advantage for guanidinium over ammonium and unusual enthalpy-entropy compensation. Org. Lett. 2000, 2, 3575–3578. [Google Scholar] [CrossRef]
- Piel, G.; Dive, G.; Evrard, B. Molecular modeling study of beta- and gamma-cyclodextrin complexes with miconazole. Eur. J. Pharm. Sci. 2001, 13, 271–279. [Google Scholar] [CrossRef]
- Taraszewska, J.; Migut, K.; Kozblio, M. Complexation of flutamide by native and modifiedcyclodextrins. J. Phys. Org. Chem. 2003, 16, 121–126. [Google Scholar] [CrossRef]
- Breslow, R.; Halfon, S.; Zhang, B. Molecular recognition by cyclodextrin dimers. Tetrahedron 1995, 51, 377–388. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, J.; Mao, S.; Huang, X.; Yang, B.; Ren, X.; Luo, G.; Shen, J. Aryl Thiol Substrate 3-Carboxy-4-Nitrobenzenethiol Strongly Stimulating Thiol Peroxidase Activity of Glutathione Peroxidase Mimic 2, 2'-Ditellurobis(2-Deoxy-β-Cyclodextrin). J. Am. Chem. Soc. 2004, 126, 16395–16404. [Google Scholar]
- Luo, G.M.; Zhu, Z.Q.; Ding, L.; Gao, G.; Sun, Q.A.; Liu, Z.; Yang, T.S.; Shen, J.C. Generation of selenium-containing abzyme by using chemical mutation. Biochem. Biophys. Res. Commun. 1994, 198, 1240–1247. [Google Scholar] [CrossRef]
- Estrada, E.; Perdomo-López, I.; Torres-Labandeira, J.J. Molecular modeling (MM2 and PM3) andexperimental (NMR and thermal analysis) studies on the inclusion complex of salbutamol and beta-cyclodextrin. J. Org. Chem. 2000, 65, 8510–8517. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hao, Y.-Q.; Liu, X.-C.; Liu, J.-Q.; Wu, Y.-Q. Association Mechanism of S-Dinitrophenyl Glutathione with Two Glutathione Peroxidase Mimics: 2, 2¢-Ditelluro- and 2, 2¢-Diseleno-bridged b-cyclodextrins. Molecules 2009, 14, 904-916. https://doi.org/10.3390/molecules14030904
Hao Y-Q, Liu X-C, Liu J-Q, Wu Y-Q. Association Mechanism of S-Dinitrophenyl Glutathione with Two Glutathione Peroxidase Mimics: 2, 2¢-Ditelluro- and 2, 2¢-Diseleno-bridged b-cyclodextrins. Molecules. 2009; 14(3):904-916. https://doi.org/10.3390/molecules14030904
Chicago/Turabian StyleHao, Ya-Qiong, Xing-Chen Liu, Jun-Qiu Liu, and Yu-Qing Wu. 2009. "Association Mechanism of S-Dinitrophenyl Glutathione with Two Glutathione Peroxidase Mimics: 2, 2¢-Ditelluro- and 2, 2¢-Diseleno-bridged b-cyclodextrins" Molecules 14, no. 3: 904-916. https://doi.org/10.3390/molecules14030904




