Flavonoids with Gastroprotective Activity
Abstract
:Introduction
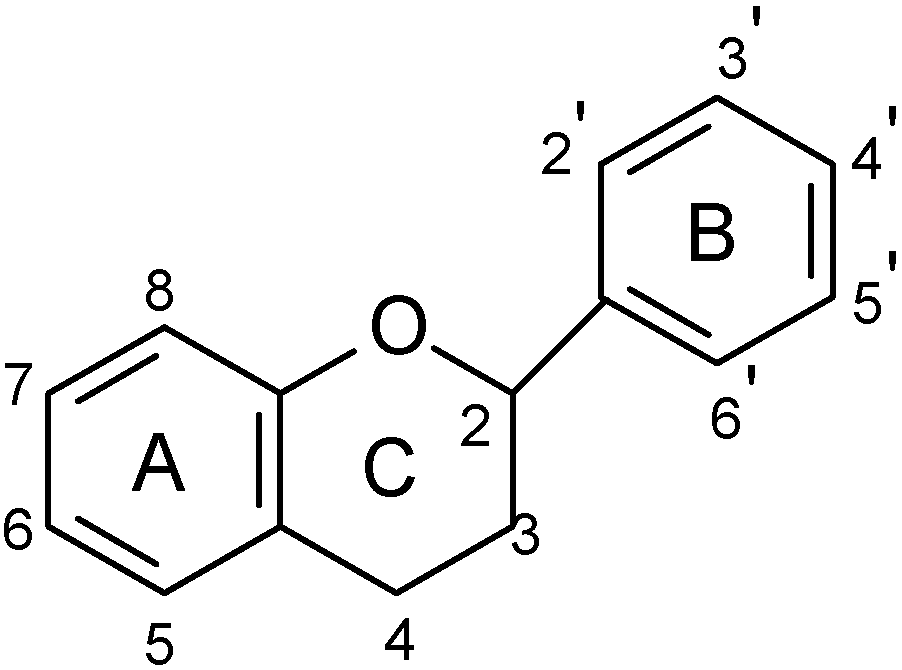
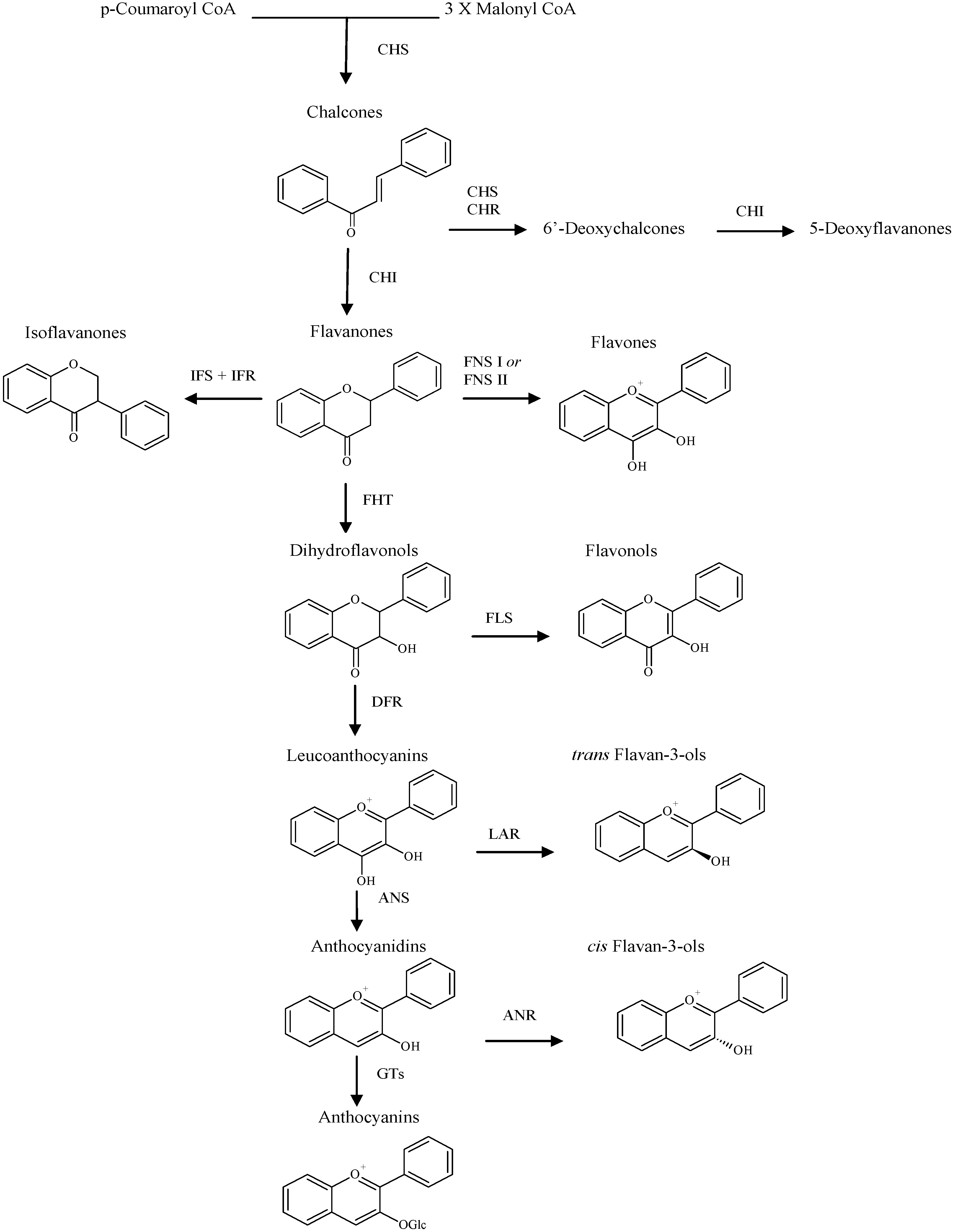
Flavonoids studied in models that investigate anti-ulcer activity
| Substance | Experimental assay/Administration route | Animal tested | Dose | Activity |
|---|---|---|---|---|
| Chalcones | ||||
Butein
 | HCl/ethanol-induced ulcers/intragastric | Rat | 10 mg/kg | Active [65] |
| NaOH-induced ulcers/intragastric | Rat | 50.0 mg/kg | Inactive [65] | |
2',3,4,4',6'-pentahydroxychalcone
 | HCl/ethanol-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] |
| NaOH-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] | |
2',3,4-trihydroxychalcone
 | HCl/ethanol-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] |
| NaOH-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] | |
2',4',6'-trihydroxychalcone
 | HCl/ethanol-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] |
| NaOH-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] | |
2',4'-dihydroxy-3',5'-diprenyl-4-O-prenyl- chalcone
 | Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Active [42] |
2',4'-dihydroxy-3'-methoxychalcone
 | Ethanol-induced ulcers/intragastric | Mouse | * | Weak Active [66] |
| Ethanol-induced ulcers/intragastric | Rat | 100.0 mg/kg | Active [67] | |
| Ethanol-induced ulcers/intragastric | Rat | * | Active [66] | |
2',4'-dihydroxy-5'-prenyl-4-O-prenyl- chalcone
 | Pylorus ligation-induced ulcers/i.p. | Rat | 100.0 mg/kg | Active [42] |
| Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Active [42] | |
2',4'-dihydroxychalcone
 | Stress-induced ulcers (water-immersion)/intragastric | Rat | 10.0 mg/kg | Active [65] |
| Acetic acid-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] | |
| HCl/ethanol-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] | |
| NaOH-induced ulcers/intragastric | ||||
| Ethanol-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] | |
| Ethanol-induced ulcers/intragastric | Mouse | * | Active [66] | |
| Ethanol-induced ulcers/intragastric | Rat | 100 mg/kg | Active [67] | |
| Rat | * | Active [66] | ||
2',4,4',6'-tetrahydroxychalcone
 | HCl/ethanol-induced ulcers/intragastric | Rat | 10.0 mg/kg | Inactive [65] |
| NaOH-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] | |
2',4,4'-trihydroxy-3,3',5'-tris-(3-methyl-but-2-enyl) chalcone
 | */* | Rat | * | Active [68] |
2',4,4'-trihydroxy-3,3',5,5'-tetrakis-(3-methyl-but-2-enyl)-4,4'-bis-(O-3-methyl-but-2-enyl) chalcone
 | */* | Rat | * | Active [69] |
2',4,4'-trihydroxy-3,3',5,5'-tetrakis-3-methyl-but-2-enyl) chalcone
 | */* | Rat | * | Active [68] |
2',4,4'-trihydroxy-3,3',5-tris-(3-methyl-but-2-enyl)-4-4'-di-O-allyl chalcone
 | */* | Rat | * | Active [69] |
2',4,4'-trihydroxy-3,3'-bis-(3-methylbut-2-enyl) chalcone
 | */* | Rat | * | Active [68] |
2',4,4'-trihydroxy-3,3'-diprenylchalcone
 | Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Active [42] |
2',4,4'-trihydroxy-3,5,5'-tris-(3-methyl-but-2-enyl)-4'-O-(3-methylbut-2-enyl) chalcone
 | */* | Rat | * | Active [69] |
2',4,4'-trihydroxychalcone
 | HCl/ethanol-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] |
| NaOH-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] | |
2',4-dihydroxy-3-prenyl-4'-O-prenyl- chalcone
 | Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Active [42] |
2',4-dihydroxy-4'-methoxy-3-5-bis-(3-methyl-but-2-enyl) chalcone
 | */* | Rat | * | Active [42] |
2'-carbomethoxy-4,4'-bis-(3-methyl-2-butenyl-oxy) chalcone (sofalcone)
 | Histamine-induced ulcers/i.p. | Rat | 100.0 mg/kg | Active [45] |
| Acetic acid-induced ulcers/gastric intubation | Rat | 20-50 mg/kg | Active [45] | |
| Histamine-induced ulcers/gastric intubation | Guinea pig | 100.0 mg/kg | Active [45] | |
| Pylorus ligation-induced ulcers/i.p. | Rat | 50.0 mg/kg | Active [45] | |
| Stress-induced ulcers (water-immersion)/i.p. | Rat | 50.0 mg/kg | Active [45] | |
| Phenylbutazone-induced ulcers/gastric ntubation | Rat | 300.0 mg/kg | Active [45] | |
| Acetic acid-induced ulcers/gastric intubation | Rat | 50.0 mg/kg | Active [44] | |
| HCl induced gastric lesions/i.p. | Rat | 100.0 mg/kg | Active [46] | |
| HCl induced gastric lesions/gastric intubation | Rat | 100.0 mg/kg | Active [46] | |
| Pretreatment with indomethacin vs HCl induced gastric lesions/gastric intubation | Rat | 300.0 mg/kg | Active [46] | |
| Pretreatment with indomethacin vs HCl induced gastric lesions/i.p. | Rat | 100.0 mg/kg | Active [46] | |
| H. pylori induced ulcer/p.o. | Human adult | 100.0 mg/kg | Active [49] | |
2'-hydroxy-4,4'-di-O-prenylchalcone
 | Pylorus ligation-induced ulcers/i.p. | Rat | 100.0 mg/kg | Strong activity [42] |
| Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Strong activity [42] | |
2,4'-di-O-prenylchalcone
 | Pylorus ligation-induced ulcers/i.p. | Rat | 100.0 mg/kg | Active [42] |
| Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Weak activity [42] | |
2,4,4'-trihydroxy-3,3',5'-tris-(3-methyl-but-2-enyl)-4-O-allyl-4-O-propargyl-chalcone
 | */* | Rat | * | Active [69] |
3',5'-dihydroxy-4'-prenyl-5-O-prenyl- chalcone
 | Pylorus ligation-induced ulcers/i.p. | Rat | 100.0 mg/kg | Strong activity [42] |
| Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Strong activity [42] | |
3,3',4-trihydroxychalcone
 | HCl/ethanol-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] |
| NaOH-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] | |
3,4,4'-trihydroxychalcone
 | HCl/ethanol-induced ulcers/intragastric | Rat | 10.0 mg/kg | Inactive [65] |
| NaOH-induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [65] | |
4'-hydroxy-3'-prenyl-4-O-prenylchalcone
 | Pylorus ligation-induced ulcers/i.p. | Rat | 100.0 mg/kg | Active [42] |
| Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Strong activity [42] | |
4,4'-di-O-geranyl chalcone
 | Pylorus ligation-induced ulcers/i.p. | Rat | 100.0 mg/kg | Weak activity [42] |
| Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Weak activity [42] | |
4,4'-di-O-prenylchalcone
 | Pylorus ligation-induced ulcers/i.p. | Rat | 100.0 mg/kg | Active [42] |
| Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Strong activity [42] | |
4,4'-dihydroxy-3,3'-diprenylchalcone
 | Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Active [42] |
4,4'-dimethoxy-3,3'-diprenylchalcone
 | Pylorus ligation-induced ulcers/i.p. | Rat | 100.0 mg/kg | Weak activity [42] |
| Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Active [42] | |
4-hydroxy-3-prenyl-4'-O-prenylchalcone
 | Pylorus ligation-induced ulcers/i.p. | Rat | 100.0 mg/kg | Active [42] |
| Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Weak activity [42] | |
2',4-bis-(carbomethoxy)-4'-(3-carboxy-2-butenyl-oxy) dihydrochalcone
 | Pylorus ligation-induced ulcers/i.p. | Rat | 100.0 mg/kg | Weak activity [70] |
| Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Weak activity [70] | |
| Histamine-induced ulcers/i.p. | Rat | 100.0 mg/kg | Weak activity [70] | |
2',4-bis-(carboxymethoxy)-4'-(3-methyl-2-butenyl-oxy) dihydrochalcone
 | Pylorus ligation-induced ulcers/i.p. | Rat | 100.0 mg/kg | Active [70] |
| Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Weak activity [70] | |
| Histamine-induced ulcers/i.p. | Rat | 100.0 mg/kg | Active [70] | |
2'-carboxymethoxy-4-4'-bis-(3-methyl-2-butenyl-oxy) dihydro-chalcone
 | Pylorus ligation-induced ulcers/i.p. | Rat | 100.0 mg/kg | Active [70] |
| Stress-induced ulcers (water-immersion)/i.p. | Rat | 100.0 mg/kg | Active [70] | |
| Histamine-induced ulcers/i.p. | Rat | 100.0 mg/kg | Active [70] | |
Garcinol
 | Stress-induced (restraint) ulcers/intragastric | Rat | 200.0 mg/kg | Active [52] |
| Indomethacin-induced ulcers/intragastric | Rat | 200.0 mg/kg | Active [52] | |
Sophoradin
 | Pylorus ligation-induced ulcers/p.o. | Rat | * | Active [43] |
| Stress-induced ulcers/p.o. | Rat | * | Active [43] | |
| Pylorus ligation-induced ulcers/p.o. | Rat | 100.0 mg/kg | Strong activity [42] | |
| Stress-induced ulcers/p.o. | Rat | 100.0 mg/kg | Strong activity [42] | |
Xanthoangelol E
 | Stress-induced (restraint) ulcers/intragastric | Rat | 100.0 mg/kg | Active [71] |
| Flavanones | ||||
3',4',5,7-tetrahydroxy-3-methoxy- flavanone
 | stress-induced (restraint) ulcers/* | Rat | * | Active [72] |
2',4',7-trihydroxy-5-methoxy-8-(5-hydroxy-5-methyl-2-iso-propenyl-hexyl) flavanone
 | */p.o. | Human adult | * | Active [73] |
Hesperidin
 | Cold stress-induced ulcers/intragastric | Rat | 100.0 mg/kg | Active [74] |
| Ethanol-induced ulcers/intragastric | Rat | 100.0 mg/kg | Inactive [74] | |
Naringenin
 | Acetic acid-induced ulcers/intragastric | Rat | 100.0 mg/kg | Active [62] |
| Stress-induced ulcers (water-immersion)/intragastric | Rat | 100.0 mg/kg | Weak active [56] | |
| Pylorus ligation-induced ulcers/intragastric | Rat | 100.0 mg/kg | Active [56] | |
| Pylorus ligation-induced ulcers /intragastric | Rat | 100.0 mg/kg | Active [56] | |
| Pylorus ligation-induced ulcers/gastric intubation | Rat | ED50 132 mg/kg | Active [75] | |
| Stress-induced (restraint) ulcers/gastric intubation | Rat | ED50 42.0 mg/kg | Active [75] | |
| Aspirin-induced ulcers/gastric intubation | Rat | * | Active [75] | |
| Phenylbutazone-induced ulcers/gastric intubation | Rat | * | Active [75] | |
| Reserpine-induced ulcers/gastric intubation | Rat | * | Active [75] | |
Naringin
 | Aspirin-induced ulcers/intragastric | Rat | 200.0 mg/kg | Active [76] |
| Acid-ethanol-induced ulcers/i.p. | Rat | 100.0 mg/kg | Inactive [37] | |
| Acid-ethanol-induced ulcers/i.p. | Rat | 200.0 mg/kg | Active [37] | |
| Acid-ethanol-induced ulcers/i.p. | Rat | 400.0 mg/kg | Active [37] | |
| Ethanol-induced gastric injury/intragastric | Rat | 400.0 mg/kg | Active [37] | |
Sigmoidin A
 | Stress-induced ulcers (water-immersion)/gastric intubation | Rat | 50.0 mg/kg | Active [78] |
| Stress-induced (restraint) ulcers/gastric intubation | Rat | 50.0 mg/kg | Active [78] | |
Sigmoidin B
 | Stress-induced ulcers (water-immersion)/gastric intubation | Rat | 50.0 mg/kg | Active [78] |
| Stress-induced (restraint) ulcers/gastric intubation | Rat | 50.0 mg/kg | Active [78] | |
Sophoranone
 | Pylorus ligation-induced ulcers/p.o. | Rat | * | Active [43] |
| Stress-induced ulcers/p.o. | Rat | * | Active [43] | |
| Flavane and Flavanols | ||||
(+) catechin
 | HCl/ethanol-induced stomach ulcers/intragastric | Rat | * | Inactive [79] |
| */pathway oral (p.o.) | Rat | 100.0 mg/kg | Active [80] | |
| Reserpine-induced ulcers/gastric intubation | Mouse | 49.7 mg/kg | Equivocal [57] | |
| Reserpine-induced ulcers/gastric intubation | Mouse | 72.5 mg/kg | Inactive [57] | |
| Stress-induced ulcers (water-immersion)/gastric intubation | Mouse | 500.0 mg/kg | Weak Active [81] | |
(dl) catechin
 | Stress-induced (restraint) ulcers/subcutaneous (s.c.) | Mouse | 300.0 mg/kg | Active [82] |
| Stress-induced (restraint) ulcers/intragastric | Mouse | 300.0 mg/kg | Active [82] | |
| Stress-induced ulcers (water-immersion)/s.c. | Mouse | 300.0 μmol/kg | Active [82] | |
| Stress-induced ulcers (water-immersion)/intragastric | Mouse | 300.0 mg/kg | Active [82] | |
3-O-methyl: (+) catechin
 | Pylorus ligation-induced ulcers/s.c. | Rat | ED50 60.0 mg/kg | Active [83] |
| Stress-induced (restraint) ulcers/s.c. | Rat | ED50 13.2 mg/kg | Active [83] | |
| */s.c. | Rat | * | Active [83] | |
| Phenylbutazone-induced ulcers/s.c. | Rat | * | Active [83] | |
| Reserpine-induced ulcers/s.c. | Rat | * | Active [83] | |
| */* | * | * | Active [84] | |
(-) Epicatechin
 | Stress-induced ulcers (water-immersion)/gastric intubation | Mouse | 500.0 mg/kg | Weak Active [81] |
(+) Cyanidan-3-beta- ol
 | Pylorus ligation-induced ulcers/s.c. | Rat | ED50 62 mg/kg | Active [85] |
| Restraint-induced ulcers/s.c. | Rat | ED50 18 mg/kg | Active [85] | |
| Aspirin-induced ulcers/gastric intubation | Rat | 100.0 mg/kg | Active [85] | |
| Phenylbutazone-induced ulcers/gastric intubation | Rat | 100.0 mg/kg | Active [85] | |
| Ibuprofen-induced ulcers/gastric intubation | Rat | 100.0 mg/kg | Active [85] | |
| Reserpine-induced ulcers/gastric intubation | Rat | 100.0 mg/kg | Active [85] | |
Leucocyanidin
 | Aspirin-induced ulcers/* | Rat | 5.0 mg/day | Active [50] |
| */intragastric | Rat | * | Active [51] | |
| Flavanolols | ||||
Taxifolin
 | Ethanol induced gastric ulcers/intragastric | Rat | 50.0 mg/kg | Active [86] |
Taxifolin,(dl)
 | HCl /ethanol-induced stomach ulcers/intragastric | Rat | * mg/kg | Inactive [79] |
| Anthocyanidines | ||||
Benzopyrylium chloride,1: 3,5,7- trihydroxy-2-(3-4-dihydroxyphenyl)
 | Pylorus ligation-induced ulcers/intragastric | Rat | 12.5 mg/kg | Active [87] |
| Stress-induced (restraint) ulcers/intragastric | Rat | 100.0 mg/kg | Active [87] | |
| Phenylbutazone-induced ulcers/intragastric | Rat | 22.0 mg/kg | Active [87] | |
| Indomethacin-induced ulcers/intragastric | Rat | 100.0 mg/kg | Active [87] | |
| Reserpine-induced ulcers/intragastric | Rat | 100.0 mg/kg | Active [87] | |
| Ethanol induced lesion/intragastric | Rat | 200.0 mg/kg | Active [87] | |
| Histamine-induced ulcers/intragastric | Rat | 24.0 mg/kg | Active [87] | |
| Cysteamine-induced ulcers/intragastric | Rat | 200.0 mg/kg | Active [87] | |
| Cysteamine-induced ulcers/intraperitoneal (i.p.) | Rat | 50.0 mg/kg | Active [87] | |
| Acetic acid-induced ulcers/intragastric | Rat | 50.0 mg/kg | Active [87] | |
| Flavones | ||||
Acacetin
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Inactive [36] |
Apigenin
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Inactive [36] |
Cynaroside
 | */intragastric | Rat | * | * [88] |
Dactylin
 | Reserpine-induced ulcers/gastric intubation | Mouse | * | Inactive [35] |
| Stress-induced (restraint) ulcers/gastric intubation | Mouse | * | Inactive [35] | |
Eupatilin
 | */ Intragastric | Rat | * | Active [89] |
Gnaphaloside A
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Active [36] |
Gossypin
 | */oral | Rat | 100.0 mg/kg | Active [80] |
Hyperoside
 | Reserpine-induced ulcers/gastric intubation | Mouse | * | Weak activity [35] |
| Stress-induced (restraint) ulcers/gastric intubation | Mouse | * | Weak activity [35] | |
Hypolaetin-8-O-beta-d-glucoside
 | Cold stress-induced ulcers/i.p. | Rat | ED50 573mg/kg | Active [90] |
| Cold stress-induced ulcers/* | * | ED50 57.3mg/kg | Active [91] | |
| Ethanol-induced gastric lesions/s.c. | Rat | ED50 68.0mg/kg | Active [92] | |
Kaempferol rhamnoside
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Active [36] |
Linarin
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Inactive [36] |
Luteolin
 | */intragastric | Rat | * | Active [88] |
| Reserpine-induced ulcers/gastric intubation | Mouse | 47.4 mg/kg | Active [57] | |
| Reserpine-induced ulcers/gastric intubation | Mouse | 474 mg/kg | Active [57] | |
Myricetin rhamnoside
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Active [36] |
Nobiletin
 | Ethanol-induced gastric ulcer/intragastric | Rat | ED50 8.0 mg/kg | Active [38] |
| Ethanol-induced ulcers/intragastric | Rat | ED50 8.0 mg/kg | Active [93] | |
| Aspirin-induced ulcers/intragastric | Rat | 50.0 mg/kg | Weak active [93] | |
| HCl/ethanol-induced gastric ulcers/intragastric | Rat | 25.0 mg/kg | Active [97] | |
Pectolinarigenin
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Inactive [36] |
Pectolinarin
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Inactive [36] |
Acetyl pectolinarin
 | Reserpine-iduced ulcers/gastric itubation | Mouse | 0.05 mL/g | Inactive [36] |
Quercetin rhamnoside
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Active [36] |
Quercitrin
 | Reserpine-induced ulcers/gastric intubation | Mouse | 50.0 mg/g | Active [57] |
Robinin
 | Reserpine-induced ulcers/gastric intubation | Mouse | * | Inactive [36] |
| Stress-induced (restraint) ulcers/gastric intubation | Mouse | * | Inactive [36] | |
Rutin
 | Acid-ethanol-induced ulcers/i.p. | Mouse | 12.5 mg/kg | Inactive [37] |
| Acid-ethanol-induced ulcers/i.p. | Rat | 25.0 mg/kg | Active [37] | |
| Acid-ethanol-induced ulcers/i.p. | Rat | 50.0 mg/kg | Active [37] | |
| Pretreatment with indomethalin vs ethanol induced-ulcers/intragastric | Rat | 25.0 mg/kg | Weak activity [53] | |
| Ethanol-induced ulcers/intragastric | Rat | 50.0 mg/kg | Active [53] | |
| Ethanol-induced ulcers/ intragastric | Rat | 200.0 mg/kg | Active [34] | |
| */intragastric | Mouse | 7.0 mg/kg | Active [95] | |
| */intragastric | Mouse | * | Active [96] | |
| Reserpine-induced ulcers/gastric intubation | Mouse | * | Weak activity [35] | |
| Stress-induced (restraint) ulcers/gastric intubation | Mouse | * | Weak activity [35] | |
Salvigenin
 | Pylorus ligation-induced ulcers/i.p. | Rat | 100.0 mg/kg | Inactive [97] |
Scoparin
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Inactive [36] |
Ternatin
 | Cold stress-induced ulcers/i.p. | Rat | 25.0 mg/kg | Inactive [98] |
| Ethanol-induced ulcers/i.p. | Rat | 25.0 mg/kg | Inactive [98] | |
| Indomethacin-induced ulcers/i.p. | Rat | 25.0 mg/kg | Inactive [98] | |
Vexibinol
 | HCl-ethanol induced ulcers/intragastric | Rat | 10.0 mg/kg | Active [99] |
| Stress-induced ulcers (water-immersion)/intragastric | Rat | 10.0 mg/kg | Active [99] | |
| Pylorus ligation-induced ulcers/intragastric | Rat | 100.0 mg/kg | Active [99] | |
| Indomethacin-induced ulcers/intragastric | Rat | 100.0 mg/kg | Active [99] | |
| Histamine-induced ulcers/intragastric | Rat | 100.0 mg/kg | Inactive [99] | |
| 5-Ht-induced ulcers/intragastric | Rat | 300.0 mg/kg | Inactive [99] | |
| Phenylbutazone induced ulcers/intragastric | Rat | 300.0 mg/kg | Active [99] | |
| Isoflavones | ||||
Genistin
 | */intragastric | Rat | * | Active [88] |
| Flavonols | ||||
Kaempferol
 | Acid-ethanol-induced ulcers/i.p. | Rat | 250.0 mg/kg | Inactive [37] |
| Acid-ethanol-induced ulcers/i.p. | Rat | 50.0 mg/kg | Active [37] | |
| Acid-ethanol-induced ulcers/i.p. | Rat | 100.0 mg/kg | Active [37] | |
| Ethanol-induced ulcers/i.p. | Rat | 100.0 mg/kg | Active [100] | |
| Cold stress-induced ulcers/i.p. | Rat | 200.0 mg/kg | Active [100] | |
| Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Inactive [36] | |
| Pylorus ligation-induced ulcers/i.p. | Rat | 200.0 mg/kg | Active [101] | |
| Stress-induced (restraint) ulcers/i.p. | Rat | 200.0 mg/kg | Active [101] | |
| Reserpine-induced ulcers/gastric intubation | Mouse | * | Inactive [35] | |
| Stress-induced (restraint) ulcers/gastric | Mouse | * | Inactive [35] | |
Myricetin
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Inactive [36] |
| Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Active [36] | |
| Reserpine-induced ulcers/gastric intubation | Mouse | * | Active [55] | |
| Stress-induced (restraint) ulcers/gastric intubation | Mouse | * | Active [55] | |
| Reserpine-induced ulcers/gastric intubation | Mouse | * | Active [35] | |
| Stress-induced (restraint) ulcers/gastric intubation | Mouse | * | Active [35] | |
Patuletin
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Inactive [36] |
Patulitrin
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Weak active [36] |
Phellavin
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Inactive [36] |
Quercetin
 | Ethanol-induced gastric lesions/intragastric | Rat | 200.0 mg/kg | Active [59] |
| Acetic acid-induced ulcers/intragastric | Rat | 100.0 mg/kg | Active [62] | |
| Stress-induced ulcers (water-immersion)/intragastric | Rat | 100.0 mg/kg | Active [56] | |
| Pylorus ligation-induced ulcers/intragastric | Rat | 100.0 mg/kg | Active [56] | |
| Pylorus ligation-induced ulcers/intragastric | Rat | 100.0 mg/kg | Active [56] | |
| */intragastric | Rat | 200.0 mg/kg | Active [58] | |
| Acid-ethanol-induced ulcers/i.p. | Rat | 12.5 mg/kg | Inactive [37] | |
| Acid-ethanol-induced ulcers/i.p. | Rat | 25.0 mg/kg | Active [37] | |
| Acid-ethanol-induced ulcers/i.p. | Rat | 50.0 mg/kg | Active [37] | |
| Ethanol-induced gastric ulcers/i.p. | Rat | 12.5 mg/kg | Active [104] | |
| */ intragastric | Mouse | * | Active [96] | |
| Ethanol-induced ulcers/intragastric | Rat | 100.0 mg/kg | Active [54] | |
| Stress-induced (restraint) ulcers/intragastric | Rat | 100.0 mg/kg | Active [54] | |
| Ethanol-induced ulcers/intragastric | Rat | 200.0 mg/kg | Active [60] | |
| Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/gm | Inactive [36] | |
| Reserpine-induced ulcers/gastric intubation | Mouse | 50.0 mg/kg | * [57] | |
| Reserpine-induced ulcers/gastric intubation | Mouse | * | Active [55] | |
| Stress-induced (restraint) ulcers/gastric | Mouse | * | Active [55] | |
| Reserpine-induced ulcers/gastric intubation | Mouse | * | Active [35] | |
| Stress-induced (restraint) ulcers/gastric intubation | Mouse | * | Active [35] | |
Quercetin-3'-o-beta-d-glucoside
 | Reserpine-induced ulcers/gastric intubation | Mouse | 0.05 mL/g | Inactive [36] |
| Biflavonoids | ||||
Cinnamtannin B-1
 | Stress-induced ulcers (water-immersion)/gastric intubation | Mouse | 500.0 mg/kg | Inactive [81] |
Cinnamtannin D-1
 | Stress-induced ulcers (water-immersion)/gastric intubation | Mouse | 500.0 mg/kg | Inactive [81] |
Procyanidin B-1
 | Stress-induced ulcers (water-immersion)/gastric intubation | Mouse | 500.0 mg/kg | Weak activity [81] |
Procyanidin B-2
 | Stress-induced ulcers (water-immersion)/gastric intubation | Mouse | 200.0 mg/kg | Active [81] |
Procyanidin B-3
 | HCl/ethanol-induced stomach ulcers/intragastric | Rat | 200.0 mg/kg | Inactive [79] |
Procyanidin B-4
 | Stress-induced ulcers (water-immersion)/gastric intubation | Mouse | 500.0 mg/kg | Active [81] |
Conclusions
Acknowledgements
References and Notes
- Mayty, P.; Biswas, K.; Roy, S.; Barnergee, R. K.; Bandyopadhyay, U. Smoking and pathogenesis of gastroduodenal ulcer-recent mechanistic update. Mol. Cell. Biochem. 2003, 253, 329–338. [Google Scholar]
- Prados, C. M.A.; Miquel, D.B. Úlcera péptica. Rev. Esp. Enferm. Dig. 2004, 96, 81–82. [Google Scholar]
- Bandyopadhyay, D.; Biswas, K.; Bhattacharyya, M.; Reiter, R.J.; Banerjee, R.K. Gastric toxicity and mucosal ulceration induced by oxygen-derived reactive species, protection by melatonin. Curr. Mol. Med. 2001, 1, 501–513. [Google Scholar]
- Bhattacharjee, M.; Bhattacharjee, S.; Gupta, A.; Banerjee, R.K. Critical role of an endogenous gastric peroxidase in controlling oxidative damage in H. pylori-mediated and non-mediated gastric ulcer. Free Radical Biol. Med. 2002, 32, 731–743. [Google Scholar]
- Konturek, P.C.; Konturek, S. Role of Helicobacter pylori infection in gastro-duodenal secretion and in pathogenesis of peptic ulcer and gastritis. J. Physiol. Pharmacol. 1994, 45, 333–350. [Google Scholar]
- Wallace, J.L.; Granger, D.N. The cellular and molecular basis of gastric mucosal defense. FASEB J. 1996, 10, 731–740. [Google Scholar]
- Calam, J.; Baron, J. H. Pathophysiology of duodenal and gastric ulcer and gastric cancer. Brit Med J. 2001, 323, 980–983. [Google Scholar]
- Liu, C.; Crawford, J. M. Patologia–Bases patológicas das doenças. In Robbins & Cotran.; Kumar, V., Abbas, A.K., Fausto, N., Eds.; Elsevier: Rio de Janeiro, Brazil, 2005; Volume 7, pp. 851–857. [Google Scholar]
- Rao, C.H.V.; Ojha, S.K.; Radhakrishnan, K.; Govindarajan, R.; Rastogi, S.; Mehrotra, S.; Pushpangadan, P. Antiulcer activity of Utleria salicifolia rhizome extract. J. Ethnopharmacol. 2004, 91, 243–249. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Biswas, K.; Bhattacharyya, M.; Reiter, R.J.; Banerjee, R.K. Involvement of reactive oxygen species in gastric ulceration, protection by melatonin. Indian J. Exp. Biol. 2002, 40, 693–705. [Google Scholar]
- Lehne, R.A. Pharmacology for Nursing Care; W.B. Saunders: Philadelphia, USA, 1998; pp. 781–783. [Google Scholar]
- Cechinel-Filho, V.; Yunes, R.A. Breve análise histórica da Química de Plantas Medicinais: Sua importância na atual concepção de fármaco segundo os paradigmas Ocidental e Oriental. In Plantas medicinais sob a ótica da química medicinal moderna; Yunes, R.A., Calixto, J.B., Eds.; Argos Editora Universitária: Chapecó, Brazil, 2001. [Google Scholar]
- Kanner, J.; Lapidot, T. The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plantderived antioxidants. Free Radical Biol. Med. 2001, 31, 1388–1395. [Google Scholar]
- Gurbuz, I.; Akyuz, C.; Yesilada, E.; Sener, B. Anti-ulcerogenic effect of Momordica charantia L. fruits on various ulcer models in rats. J. Ethnopharmacol. 2000, 71, 77–82. [Google Scholar]
- Cechinel Filho, V.; Yunes, R.A. Estratégias para a obtenção de compostos farmacologicamente ativos a partir de plantas medicinais. Conceitos sobre modificação estrutural para otimização da atividade. Quim. Nova 1998, 21, 1. [Google Scholar]
- Czinner, E.; Hagymasi, K.; Blazovics, A.; Kery, A.; Szoke, E.; Lemberkovics, E. The in vitro effect of Helichysi flos on microsomal lipid peroxidation. J. Ethnopharmacol. 2001, 77, 31–35. [Google Scholar] [CrossRef]
- Harborne, J.B.; Baxter, H. Handbook of Natural Flavonoids; Wiley: Chichester, UK, 1999; p. 2. [Google Scholar]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Hassig, A.; Liang, W.X.; Schwabl, H.; Stampfli, K. Flavonoids and tannins plant-based antioxidants with vitamin character. Med. Hypotheses 1999, 52, 479–481. [Google Scholar] [CrossRef]
- Martens, S.; Mithofer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar]
- Dewick, P. M. Medicinal Natural Products: a biosynthetic approach; John Wiley: Chichester, UK, 2002; p. 150. [Google Scholar]
- Buchanan, B.; Gruissem, W.; Jones, R. Biochemistry & Molecular Biology of Plants; American Society of Plant: Physiologists, USA, 2000; p. 1304. [Google Scholar]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Beecher, G.R. Overview of dietary flavonoids: nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar]
- Ross, J.A.; Kasum. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar]
- Manach, C.; Morand, C.; Demigne, C.; Texier, O.; Regerat, F.; Rémésy, C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997, 409, 12–16. [Google Scholar] [CrossRef]
- Critchfield, J.W.; Butera, S.T.; Folks, T.M. Inhibition of HIV activation in latently infected cells by flavonoid compounds. AIDS Res. Hum. Retrovir. 1996, 12, 39–46. [Google Scholar] [CrossRef]
- Cheong, H.; Ryu, S.Y.; Oak, M.H.; Cheon, S.H.; Yoo, G.S.; Kim, K.M. Studies of structure activity relationship for the anti-allergic actions. Arch. Pharmacal Res. 1998, 21, 478–480. [Google Scholar] [CrossRef]
- Carotenuto, A.; Fattorusso, E.; Lanzotti, V.; Magno, S.; De Feo, V.; Cicala, C. The flavonoids of Allium neapolitanum. Phytochemistry 1997, 44, 949–957. [Google Scholar] [CrossRef]
- Lima, J.T.; Almeida, J.R.G.S.; Barbosa-Filho, J.M.; Assis, T.S.; Silva, M.S.; Dacunha, E.V.L.; Braz-Filho, R.; Silva, B.A. Spasmolytic action of diplotropin, a furanoflavan from Diplotropis ferruginea Benth., involves calcium blockade in ginea-pig ileum. Z. Naturforsch. B 2005, 60, 1–8. [Google Scholar]
- Di Carlo, G.; Autore, G.; Izzo, A.A.; Maiolino, P.; Mascolo, N.; Viola, P.; Diurno, M.V.; Capasso, F. Inhibition of intestinal motility and secretion by flavonoids in mice and rats: structure-activity relationships. J. Pharm. Pharmacol. 1993, 45, 1054–1059. [Google Scholar] [CrossRef]
- La Casa, C.; Villegas, I.; Alarcon De La Lastra, C.; Motilva, V.; Martin Calero, M.J. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric Lesions. J. Ethnopharmacol. 2000, 71, 45–53. [Google Scholar] [CrossRef]
- Barnaulov, O.D.; Manicheva, O.A.; Komissarenko, N.F. Comparative evaluation of the effect of some flavonoids on changes in the gastric wall of reserpine-treated or immobilized mice. Pharm Chem. 1983, 17, 946–951. [Google Scholar]
- Barnaulov, O.D.; Manicheva, O.A.; Shelyuto, V.L.; Konopleva, M.M.; Glyzin, V.I. Effect of flavonoids on development of experimental gastric dystrophies in mice. Khim Farm Zh. 1985, 18, 935–941. [Google Scholar]
- Izzo, A.A.; Di Carlo, G.; Mascolo, N.; Capasso, F. Antiulcer effect of flavonoids: role of endogenous PAF. Phytother Res. 1994, 8, 179–181. [Google Scholar]
- Hirano, H.; Takase, H.; Yamamoto, K.; Abe, K.; Saito, J. Nobiletin as ulcer inhibitor. Patent-Japan Kokai Tokkyo Koho-06 72 1994. 870, 4. [Google Scholar]
- Larson, R.A. The antioxidants of higher plants. Phytochemistry 1998, 27, 969–978. [Google Scholar]
- Sahu, S. Pro-oxidant activity of flavonoids: effects on glutathione and glutathione S-transferase in isolated rat liver nuclei. Cat. Inist. 1996, 104, 193–196. [Google Scholar]
- Yoshino, K.; Suzuki, M.; Sasaki, K.; Miyase, T.; Sano, M. Formation of antioxidants from (-)-epigallocatechin gallate in mild alkaline fluids, such as authentic intestinal juice and mouse plasma. J. Nutr. Biochem. 1999, 10, 223–229. [Google Scholar] [CrossRef]
- Kyogoku, K.; Hatayama, K.; Yokomori, S.; Saziki, R.; Nakane, S.; Sasajima, M.; Sawada, J.; Ohzeki, M.; Tanaka, I. Anti-ulcer effect of isoprenyl flavonoids. II. Synthesis and anti-ulcer activity of new chalcones related to sophoradin. Chem. Pharm. Bull. 1979, 27, 2943–2953. [Google Scholar] [CrossRef]
- Sasajima, M.; Nakane, S.; Saziki, R.; Saotome, H.; Hatayama, K.; Kyogoku, K.; Tanaka, I. Studies on the anti-ulcer effects of isoprenyl flavonoids. I. The anti-ulcer effects of isoprenyl chalcone extracted from Sophora subprostrata. Nippon Yakurigaku Zasshi 1978, 74, 897–905. [Google Scholar]
- Kimura, M.; Saziki, R.; Arai, I.; Tarumoto, Y.; Nakane, S. Effect of z-carboxymethoxy-4,4'-bis (3-methyl-2-butenyloxy) chalcone (sofalcone) on chronic gastric ulcers in rats. JPN. J. Pharmacol. 1984, 35, 389–396. [Google Scholar] [CrossRef]
- Saziki, R.; Kyogoku, K.; Hatayama, K.; Suwa, T.; Sawada, J. Anti-ulcer activities of su-88, a compound related to sophoradin isolated from guang-dou-gen. J. Pharmacobio-Dynam. 1983, 6, S-59. [Google Scholar]
- Saziki, R.; Arai, I.; Isobe, Y.; Hirose, H.; Aihara, H. Effects of sofalcone on necrotizing agents-induced gastric lesions and on endogenous prostaglandins in rat stomachs. J. Pharmacobio-Dynam. 1984, 7, 791–797. [Google Scholar]
- Sunairi, M.; Watanabe, K.; Suzuki, T.; Tanaka, N.; Kuwayama, H.; Nakajima, M. Effects of anti-ulcer agents on antibiotic activity against Helicobacter pylori. Eur J Gastroenterol Hepatol. 1994, 6 (Suppl 1), S121–S124. [Google Scholar]
- Nagate, T.; Numata, K.; Hanada, K.; Kondo, I. The susceptibility of Campylobacter pylori to agents and antibiotics. J Clin Gastroenterol. 1990, 12 (Suppl 1), S135–S138. [Google Scholar] [CrossRef]
- Isomoto, H.; Furusu, H.; Ohnita, K.; Wen, Chun-Yang.; Inoue, K.; Kohno, S. Sofalcone, a mucoprotective agent, increases the cure rate of Helicobacter pylori infection when combined with rabeprazole, amoxicillin and clarithromycin. World J. Gastroenterol. 2005, 11, 1629–1633. [Google Scholar]
- Lewis, D.A.; Fields, W.N.; Shaw, G.P. A natural flavonoid present in unripe plantain banana pulp (Musa sapientum L. var. paradisiaca) protects the gastric mucosa from aspirin-induced erosions. J. Ethnopharmacol. 1999, 65, 283–288. [Google Scholar] [CrossRef]
- Lewis, D.A.; Shaw, G.P. A natural flavonoid and synthetic analogues protect the gastric mucosa from aspirin-induced erosions. J. Nutr. Biochem. 2001, 12, 95–100. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Saito, M.; Ariga, T.; Yoshimura, Y.; Nakazawa, H. Free radical scavenging activity and antiulcer activity of garcinol from Garcinia indica Fruit Rind. J. Agric. Food Chem. 2000, 48, 2320–2325. [Google Scholar]
- Guerrero, C.P.; Martin, M.J.; Marhuenda, E. Prevention by rutin of gastric lesions induced by ethanol in rats, role of endogenous prostaglandins. Gen. Pharmacol. 1994, 25, 575–580. [Google Scholar]
- Rao, C.V.; Govindarajan, S.K.O.R.; Rawat, A.K.S.; Mehrotra, S.; Pushpangadan, P. Quercetin, a bioflavonoid, protects against oxidative stress-related gastric mucosal damage in rats. Nat. Prod. Sci. 2003, 9, 68–72. [Google Scholar]
- Barnaulov, O.D.; Manicheva, O.A.; Yasinov, R.K.; Yakovlev, G.P. Evaluation of the effect of flavonoids from the aerial parts of Astragalus quisqualis bunge and A. floccosifolius sumn on the development of experimental lesions in the mouse stomach. Rast. Resur. 21, 85–90.
- Martin, M.J.; Motilva, V.; Alarcon De La Lastra, C. Quercetin and naringenin, effects on ulcer formation and gastric secretion in rats. Phytother. Res. 1993, 7, 150–153. [Google Scholar] [CrossRef]
- Barnaulov, O.D.; Manicheva, O.A.; Zapesochnaya, G.G.; Shelyuto, V.L.; Glyzin, V.I. Effects of certain flavonoids on the ulcerogenic action of reserpine in mice. Khim. Farm. Zh. 1982, 16, 300–303. [Google Scholar]
- Alarcon De La Lastra, C.; Martin, M.J.; Motilva, V. Antiulcer and gastroprotective effects of quercetin., a gross and histologic study. Pharmacology 1994, 48, 56–62. [Google Scholar]
- Martin, M.J.; La-Casa, C.; Alarcon De La Lastra, C.; Cabeza, J.; Villegas, I.; Motilva, V. Anti-oxidant mechanisms involved in gastroprotective effects of quercetin. Z. Naturforsch. C J. Biosci. 1998, 53, 82–88. [Google Scholar]
- Kahraman, A.; Erkasap, N.; Koken, T.; Serteser, M.; Aktepe, F.; Erkasap, S. The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicology 2003, 183, 133–142. [Google Scholar] [CrossRef]
- Beil, W.; Birkhoiz, C.; Sewing, K.F. Effects of flavonoids on parietal cell acid secretion, gastric mucosal prostaglandin production and Helicobacter pylori growth. Arzneim. Forsch. 1995, 45, 697–700. [Google Scholar]
- Motilva, V.; Alarcon De La Lastra, C.; Calero, M.J.M. Effects of naringenin and quercetin on experimental chronic gastric ulcer in rat studies on the histological findings. Phytother. Res. 1992, 6, 168–170. [Google Scholar]
- Russo, A.; Acquaviva, R.; Campisi, A.; Sorrenti, V.; Di Giacomo, C.; Virgata, G.; Barcellona, M.L.; Vanella, A. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biol. Toxicol. 2000, 16, 91–98. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidant. J. Nat. Prod. 2000, 62, 1035–1042. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kakegawa, H.; Ueda, H.; Matsumoto, H.; Sudo, T.; Miki, T.; Satoh, T. Gastric cytoprotective anti-ulcerogenic actions of hydroxychalcones in rats. Planta Med. 1992, 58, 389–393. [Google Scholar]
- Ortega, C.A.; Maria, A.O.M.; Gianello, J.C. Chemical components and biological activity of Bidens subalternans, B. aurea (Astereaceae) and Zuccagnia puntacta (Fabaceae). Molecules 2000, 5, 465–467. [Google Scholar]
- De La Rocha, N.; Maria, A.O.M.; Gianello, J.C.; Pelzer, L. Cytoprotective effects of chalcones from Zuccagnia punctata and melatonin on the gastroduodenal tract in rats. Pharmacol. Res. 2003, 48, 97–99. [Google Scholar] [CrossRef]
- Kyogoku, K.; Hatayama, K.; Yokomori, S.; Seki, T. Isoprenylchalcones. Patent-Japan Kokai-75 1975, 24, 257. [Google Scholar]
- Kyogoku, K.; Hatayama, K.; Yokomori, S.; Seki, T. Isoprenylchalcones. Patent Japan Kokai 75 1975b. [Google Scholar]
- Hatayama, K.; Yokomori, S.; Kawashima, Y.; Saziki, R.; Kyogoku, K. Anti-ulcer effect of isoprenyl flavonoids. III. Synthesis and anti-ulcer activity of metabolites of 2'-carboxymethoxy-4,4'-Bis(3-methyl-2-butenyloxy) chalcone. Chem. Pharm. Bull. 1985, 33, 1327–1333. [Google Scholar] [CrossRef]
- Murakami, S.; Kotomo, S.; Ozawa, M.; Baba, K. Novel chalcone derivative as antiulcer agent. Patent Japan Kokai Koho 04 1992. [Google Scholar]
- Parmar, S.; Hennings, G. Perfect of 3-methoxy-5,7,3'4'-tetrahydroxyflavan on the healing of restraint ulcers in albino rats. IRCS Med. Sci-Biochem. 1984, 12, 393–394. [Google Scholar]
- Kyogoku, K.; Tachi, Y.; Hatayama, K.; Ohtake, T. Novel flavanone from Sophora angustifolia. Patent Japan Kokai 74 1974. [Google Scholar]
- Suarez, J.; Herrera, M.D.; Marhuenda, E. Hesperidin and neohesperidin dihydrochalcone on different experimental models of induced gastric ulcer. Phytother. Res. 1996, 10, 616–618. [Google Scholar] [CrossRef]
- Parmar, N.S. The Gastric Anti-Ulcer Activity of Naringenin, a specific histidine decarboxylase inhibitor. Int. J. Tissue React. 1984, 5, 415–420. [Google Scholar]
- Galati, E.M.; Monforte, M.T.; D'aquino, A.; Miceli, N.; Di Mauro, D.; Sanogo, R. Effects of naringin on experimental ulcer in rats. Phytomedicine 1998, 5, 361–366. [Google Scholar]
- Martin, M.J.; Marhuenda, E.; Perez-Guerrero, C.; Franco, J.M. Antiulcer effect of naringin on gastric lesions induced by ethanol in rats. Pharmacology 1994, 49, 144–150. [Google Scholar]
- Fomum, Z.T.; Ayafor, J.F.; Mbafortj; Mbi, C.N. Erythrina studies. Part 6. Pharmacological studies on sigmoidin a, b and c, novel flavanones isolated from Erythrina sigmoidea. Rev. Sci. Technol. (Health Sci. Ser.) 1984, 1, 55–68. [Google Scholar]
- Saito, M.; Hosoyama, H.; Ariga, T.; Kataoka, S.; Yamaji, N. Antiulcer activity of grape seed extract and procyanidins. J. Agric. Food Chem. 1998, 46, 1460–1464. [Google Scholar] [CrossRef]
- Parmar, N.S.; Ghosh, M.N. The antiinflammatory and anti-gastric ulcer activities of some bioflavonoids. Bull Jawaharlal Inst. Post-Grad. Med. Educ. Res. 1976, 1, 6–11. [Google Scholar]
- Ezaki, N.; Kato, M.; Takizawa, N.; Morimoto, S.; Nonaka, G.I.; Nishioka, I. Pharmacological studies on linderae umbellatae ramus, IV. Effects of condensed tannin related compounds on peptic activity and stress-induced gastric lesions in mice. Planta Med. 1985, 51, 34–38. [Google Scholar]
- Yamazaki, M.; Okuyama, E.; Matsudo, T.; Takamaru, T.; Kaneko, T. Principles of indonesian herbal drugs having an antiulcerogenic activity. I. Isolation and identification of (+)-catechin from Artocarpus integra merr. Yakugaku Zasshi. 1987, 107, 914–916. [Google Scholar]
- Parmar, N.S.; Ghosh, M.N. Gastric antiulcer activity of methyl-3-(+)-catechin. Stud. Org. Chem. (Amsteram). 1982, 11, 513–521. [Google Scholar]
- Balint, G. A.; Gabor, M.; Nafradi, J.; Varro, V. Interaction of 3-o-mehtyl-(+)-catechin and H2-receptor blockers in piroxican- and indomethacin-induced gastric ulcer models of rat. Stud. Org. Chem. 1985, 23, 453–456. [Google Scholar]
- Parmar, N.S.; Ghosh, M.N. Gastric anti-ulcer activity of (+)-cyanidanol-3, a histidine decarboxylase inhibitor. Eur. J. Pharmacol. 1981, 69, 25–32. [Google Scholar] [CrossRef]
- Kolhir, V.K.; Bykov, V.A.; Baginskaja, A.I.; Sokolov, S.Y.; Glazova, N.G.; Leskova, T.E.; Sakovich, G.S.; Tjukavkina, N.A.; Kolesnik, Y.A.; Rulenko, I.A. Antioxidant activity of a dihydroquercetin isolated from Larix gmelinii(Rupr.) Rupr. wood. Phytother. Res. 1996, 10, 478–482. [Google Scholar]
- Magistretti, M.J.; Conti, M.; Cristoni, A. Antiulcer activity of an anthocyanidin from Vaccinium myrtillus. Arzneim. Forsch. 1988, 38, 686–690. [Google Scholar]
- Rainova, L.; Nakov, N.; Bogdanova, S.; Minkov, E.; Staneva-Stoytcheva, D. Ulceroprotective activity of the flavonoids of Genista rumelica Vel. Phytother. Res. 1988, 2, 137–139. [Google Scholar] [CrossRef]
- Lee, E.B.; Kang, S.S. Antigastritis and antiulcer agent containing eupatilin, and isolation thereof. Patent Repub. Korea 1994. [Google Scholar]
- Villar, A.; Gasco, M.A.; Alcaraz, M.J. Anti-inflammatory and anti-ulcer properties of hypolaetin-8-glucoside, a novel plant flavonoid. J. Pharm. Pharmacol. 1984, 36, 820–823. [Google Scholar] [CrossRef]
- Villar, A.; Gasco, M.A.; Alcaraz, M.J.; Manez, S.; Cortes, D. Hypolaetin-8-o-glucoside, an anti-inflammatory flavonoid from Sideritis mugronensis. Planta Med. 1985, 51, 70–71. [Google Scholar] [CrossRef]
- Alcaraz, M.J.; Tordera, M. Studies on the gastric anti-ulcer activity of hypolaetin-8-glucoside. Phytother. Res. 1988, 2, 85–88. [Google Scholar] [CrossRef]
- Takase, H.; Yamamoto, K.; Hirano, H.; Saito, Y.; Yamashita, A. Pharmacological profile of gastric mucosal protection by marmin and nobiletin from a traditional herbal medicine, Aurantii fructus immaturus. JPN J. Pharmacol. 1994, 66, 139–147. [Google Scholar]
- Hirano, H.; Takase, H.; Yamamoto, K.; Yanase, T.; Abe, K.; Saito, Y. The anti-ulcer effects of Aurantii fructus immaturus, Aurantii fructus and the principles in Aurantii fructus immaturus. Nat. Med. 1997, 51, 190–193. [Google Scholar]
- Cen, D.Y.; Chen, Z.W.; Wang, Y.L.; Wang, Q.; Li, Q.J.; Song, B.W. Protective effects of rutoside on gastric mucosa and influence on nitric oxide and prostaglandin. Chin. Pharm. Bull. 1999, 15, 360–362. [Google Scholar]
- Meng, D.S.; Wnag, S.L. Protective effects of quercetin, rutoside, and flavone against intestinal mucosal injury induced by burns in mice. Chin. Pharm. Bull. 2000, 16, 673–675. [Google Scholar]
- Okuyama, E.; Yamazaki, M.; Ishii, Y. Isolation and identification of ursolic acid-related compounds as the principles of glechoma hederacea having an antiulcerogenic activity. Shoyakugaku zasshi 1983, 37, 52–55. [Google Scholar]
- Rao, V.S.N.; Santos, F.A.; Sobriera, T.T.; Souza, M.F.; Melo, C.L.; Silveira, E.R. Investigations on the gastroprotective and antidiarrhoeal properties of ternatin, a tetramethoxyflavone from Egletes viscosa. Planta Med. 1997, 63, 146–149. [Google Scholar] [CrossRef]
- Yamahara, J.; Mochizuki, M.; Chisaka, T.; Fujimura, H.; Tamai, Y. The antiulcer action of sophora and the active constituent in sophora. II. The antiulcer action of vexibinol. Chem. Pharm. Bull. 1990, 38, 1039–1044. [Google Scholar] [CrossRef]
- Goel, R.K.; Maiti, R.N.; Tavares, I.A. Role of endogenous eicosanoids in the antiucler effect of kaempferol. Fitoterapia 1996, 67, 548–552. [Google Scholar]
- Goel, R.K.; Pandey, V.B.; Dwivedi, S.P.D.; Rao, Y.V. Antiinflammatory and antiulcer effects of kaempferol, a flavone, isolated from Rhamnus procumbens. Indian J. Exp. Bio. 26, 1988, 121–124. [Google Scholar]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Effects of quercetin on the gastrointestinal tract in rats and mice. Phytother. Res. 1994, 8, 42–45. [Google Scholar]
- Sample availability: Not available.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Lira Mota, K.S.; Dias, G.E.N.; Pinto, M.E.F.; Luiz-Ferreira, Â.; Monteiro Souza-Brito, A.R.; Hiruma-Lima, C.A.; Barbosa-Filho, J.M.; Batista, L.M. Flavonoids with Gastroprotective Activity. Molecules 2009, 14, 979-1012. https://doi.org/10.3390/molecules14030979
De Lira Mota KS, Dias GEN, Pinto MEF, Luiz-Ferreira Â, Monteiro Souza-Brito AR, Hiruma-Lima CA, Barbosa-Filho JM, Batista LM. Flavonoids with Gastroprotective Activity. Molecules. 2009; 14(3):979-1012. https://doi.org/10.3390/molecules14030979
Chicago/Turabian StyleDe Lira Mota, Kelly Samara, Guilherme Eduardo Nunes Dias, Meri Emili Ferreira Pinto, Ânderson Luiz-Ferreira, Alba Regina Monteiro Souza-Brito, Clélia Akiko Hiruma-Lima, José Maria Barbosa-Filho, and Leônia Maria Batista. 2009. "Flavonoids with Gastroprotective Activity" Molecules 14, no. 3: 979-1012. https://doi.org/10.3390/molecules14030979




