Synthesis of New Naphtho[2,3-f]quinoxaline-2,7,12(1H)-trione and Anthra-9,10-quinone Dyes from Furan-2,3-diones
Abstract
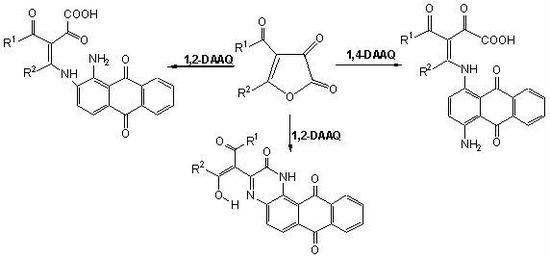
:1. Introduction
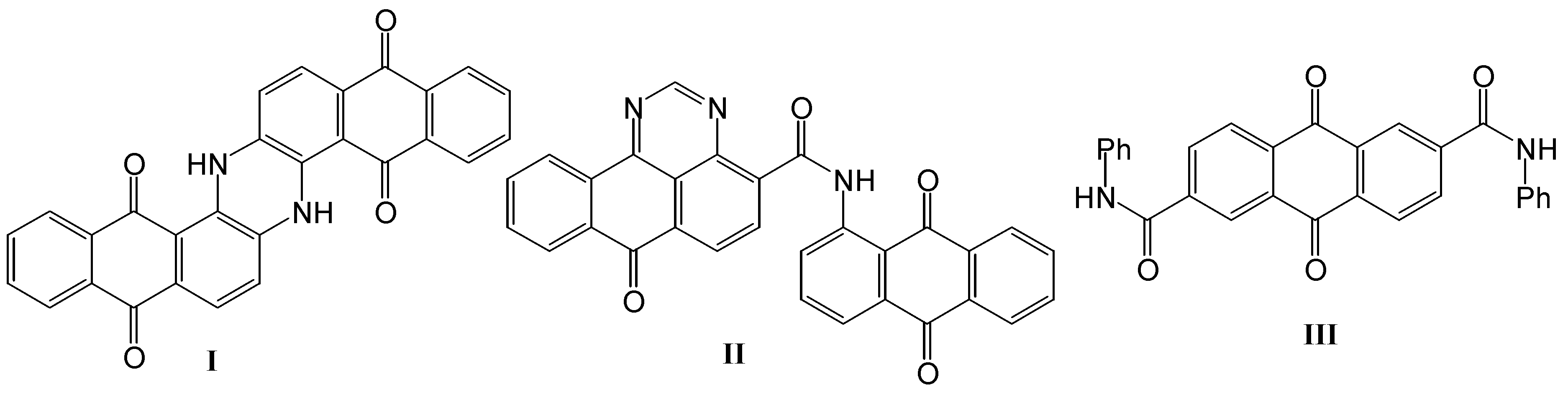
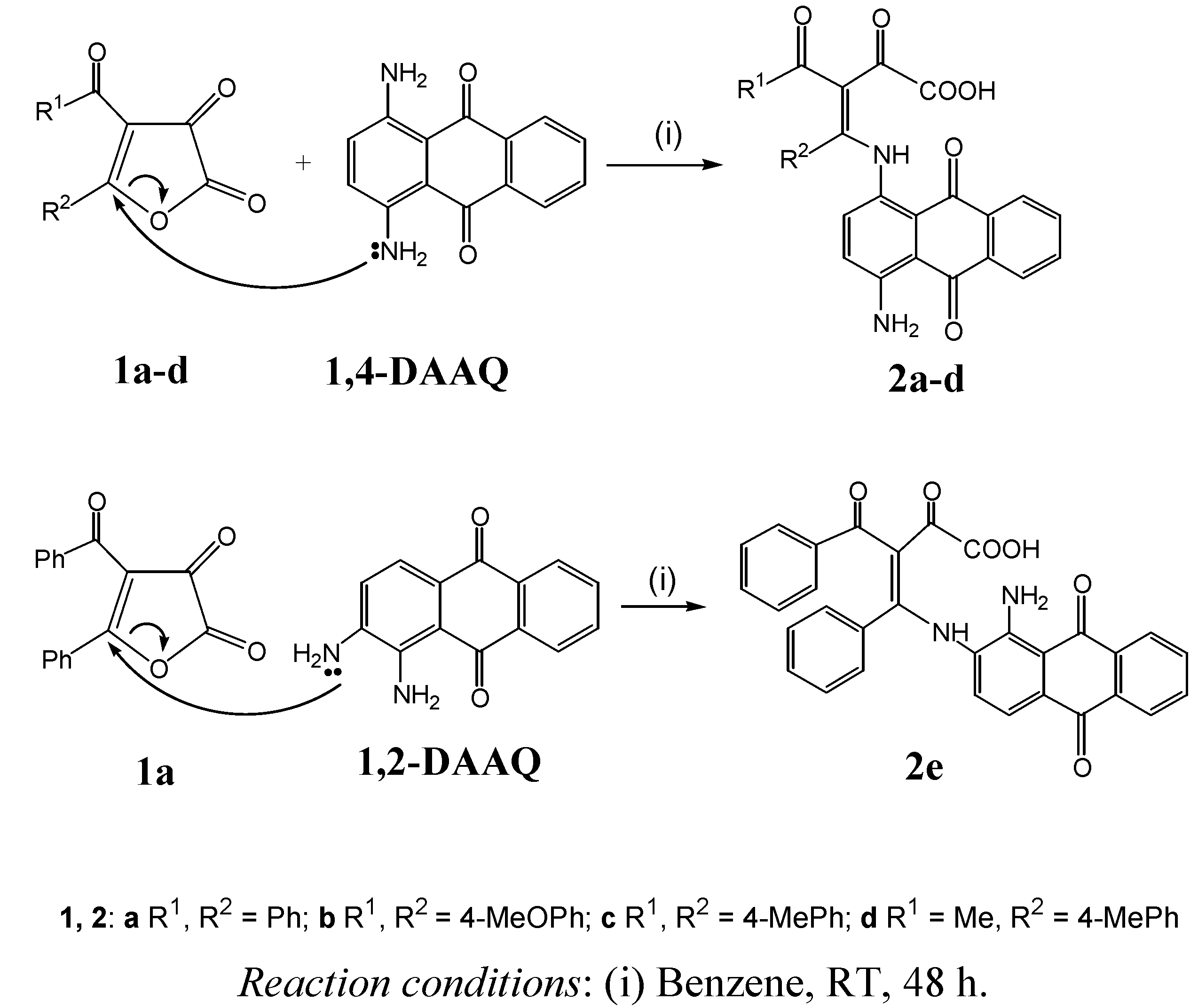
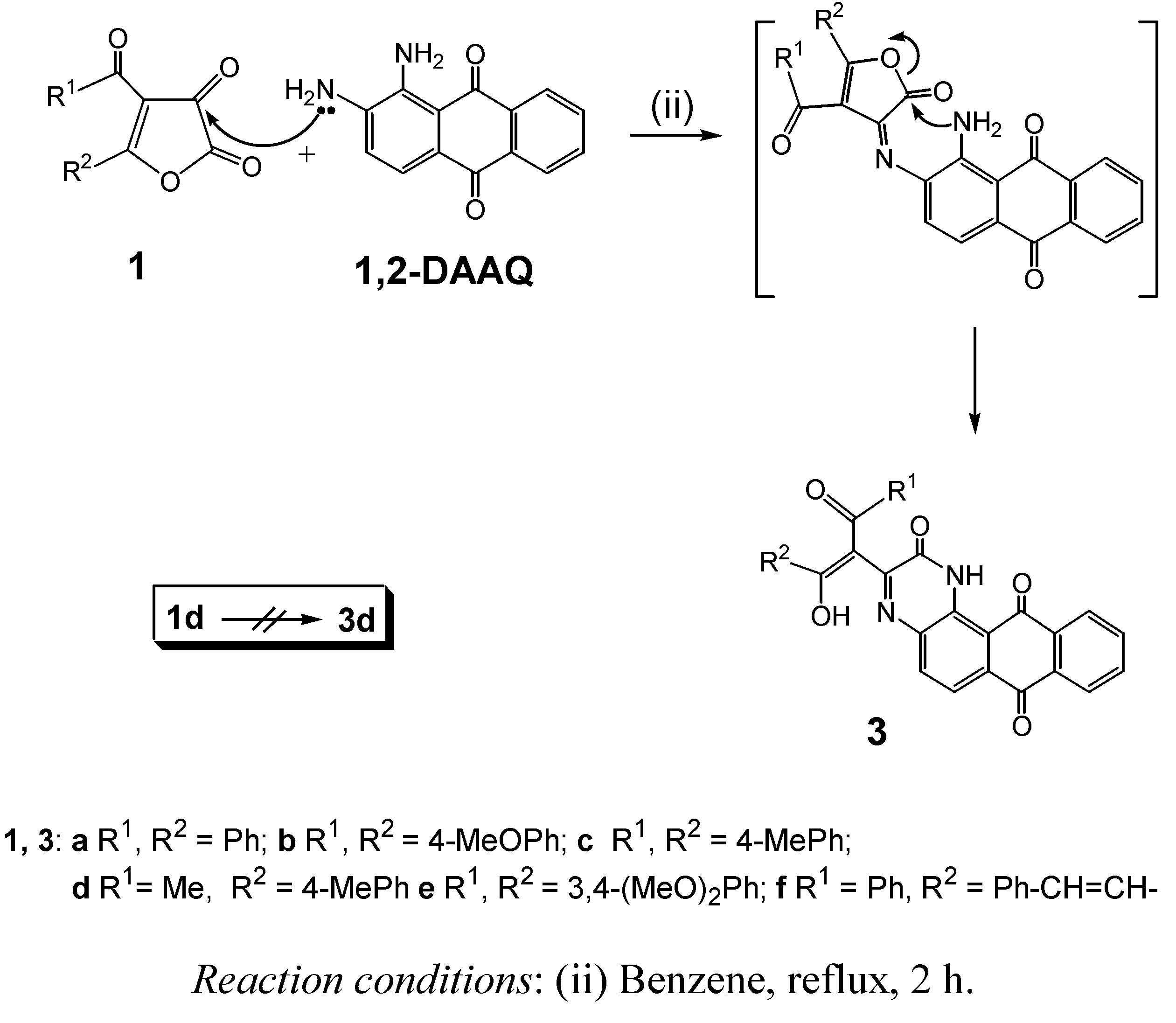
2. Results and Discussion
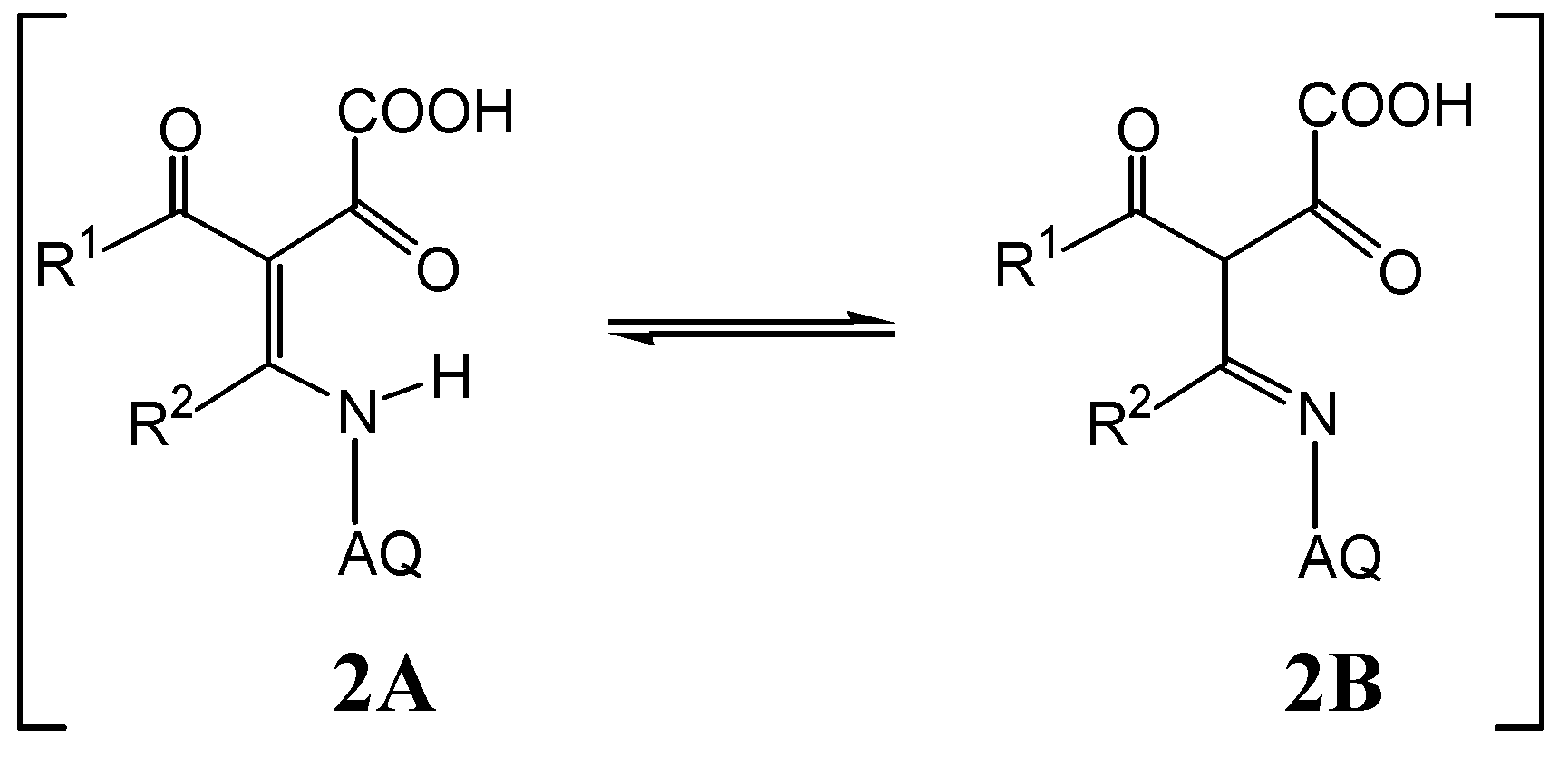
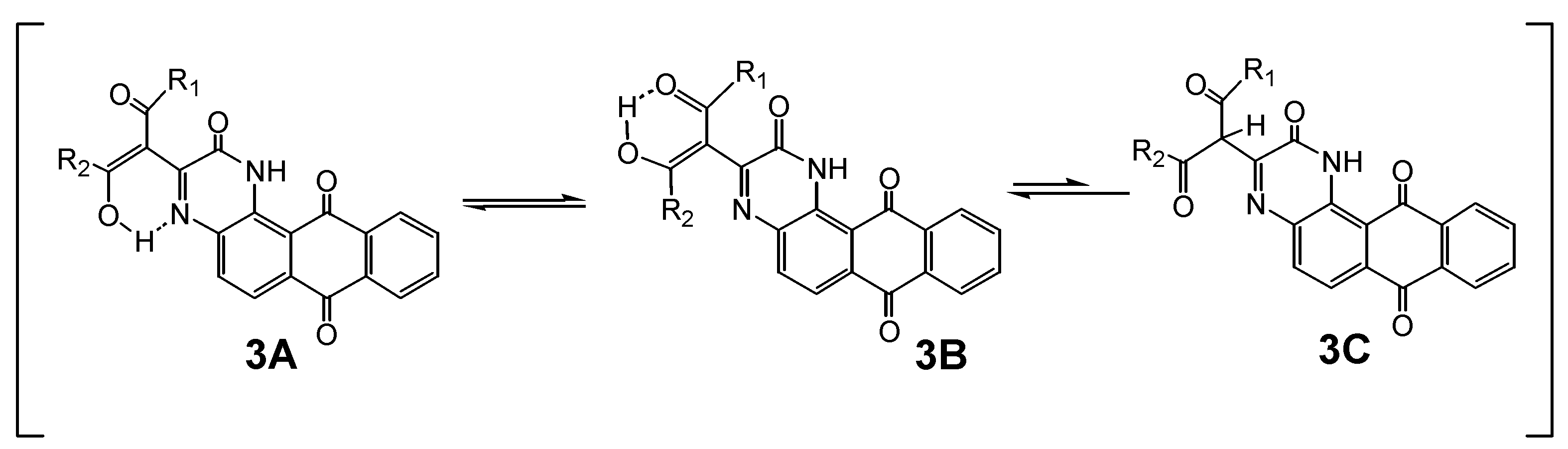
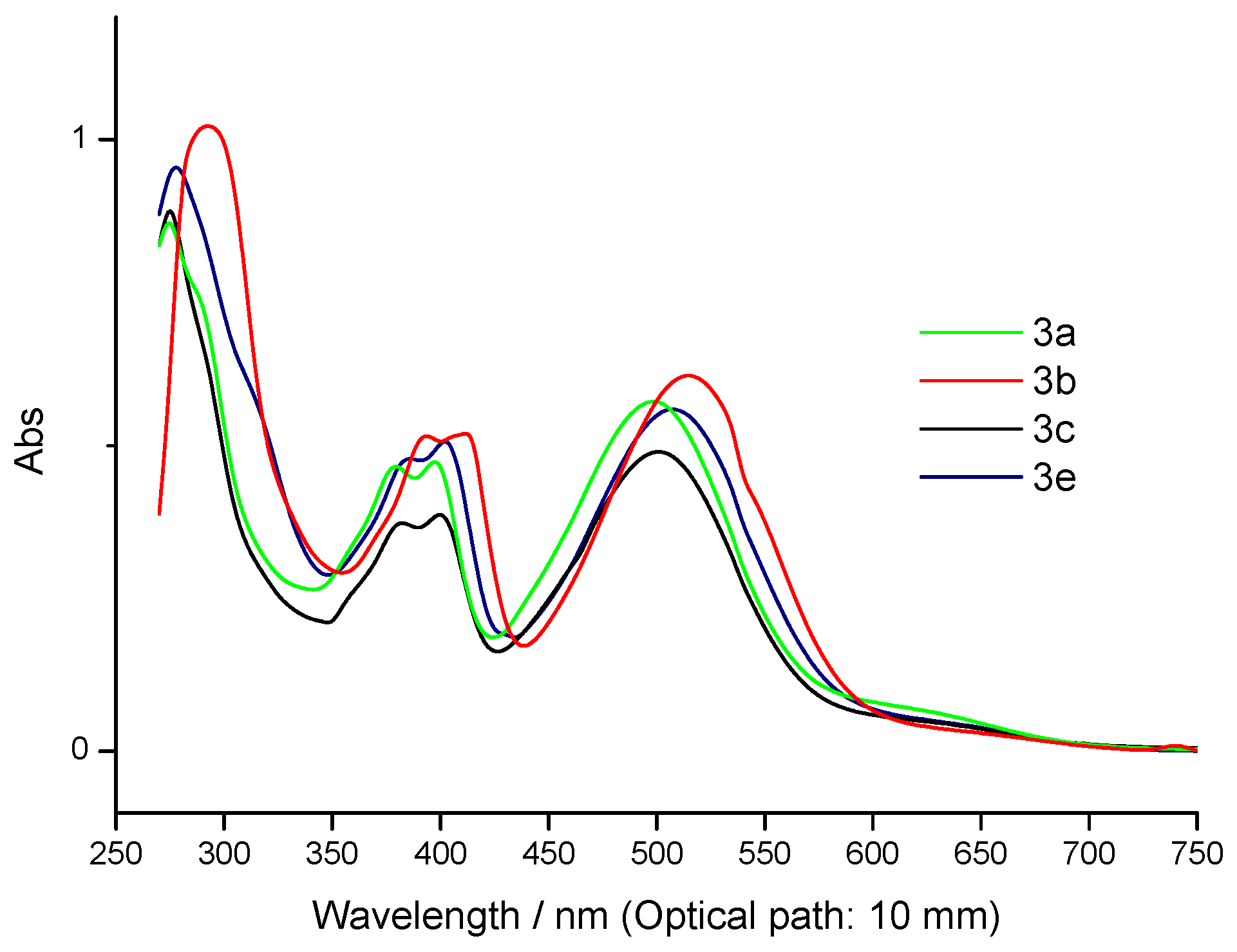
| Compounds | UV-Vis | |||
|---|---|---|---|---|
| λ1-4max(nm), | ||||
| ε1-4 (liter mol-1 cm-1) | ||||
| 3a | 274, | 380, | 397, | 498, |
| 2.592x104 | 1.398x104 | 1.416x104 | 1.716x104 | |
| 3b | 293, | 394, | 412, | 515, |
| 3.066x104 | 1.545x104 | 1.557x104 | 1.842x104 | |
| 3c | 275, | 382, | 400, | 501, |
| 2.652x104 | 1.119x104 | 1.161x104 | 1.467x104 | |
| 3e | 278, | 386, | 402, | 507, |
| 2.865x104 | 1.434x104 | 1.518x104 | 1.677x104 | |
3. Experimental Section
3.1. General
3.2. General Procedure for the Preparation of Compounds 2a-e
3.3. General Procedure for the Preparation of Compounds 3a-c,e,f
4. Conclusions
Acknowledgements
References and Notes
- Thetford, D.; Chorlton, A.P. Investigation of vat dyes as potential high performance pigments. Dyes Pigments 2004, 61, 49–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, W.; Tan, Y. Structure and dyeing properties of some anthraquinone violet acid dyes. Dyes Pigments 1996, 34, 25–35. [Google Scholar] [CrossRef]
- Orban, N.; Boldizsar, I.; Szucs, Z.; Danos, B. Influence of different elicitors on the synthesis of anthraquinone derivatives in Rubia tinctorum L. cell suspension cultures. Dyes Pigments 2007, 77, 249–257. [Google Scholar] and references therein.
- Matsui, M.; Taniguchi, S.; Suzuki, M.; Wang, M.; Funabiki, K.; Shiozaki, H. Dyes produced by the reaction of 1,2,3,4-tetrafluoro-9,10-anthraquinones with bifunctional nucleophiles. Dyes Pigments 2005, 65, 211–220. [Google Scholar] [CrossRef]
- Kollenz, G.; Heilmayer, W. Furan-2,3-diones-versatile synthons in heterocyclic chemistry. Trends Heterocycl. Chem. 1993, 3, 379–395. [Google Scholar]
- Üngören, Ş.H.; Saçmacı, M.; Akçamur, Y.; Arıcı, C.; Ülkü, D. Synthesis and characterization of new α,β-unsaturated γ-lactones: Alkyl 3-acetyl-4-hydroxy-2-methyl-5-oxo-2,5-dihydrofuran-ylcarbamates. J. Heterocycl. Chem. 2005, 42, 685–689. [Google Scholar] [CrossRef]
- Şener, A.; Akbaş, E.; Şener, M.K. Synthesis and some reactions of 4-benzoyl-5-phenyl-1-pyridin-2-yl-1H-pyrazole-3-carboxylic acid. Turk. J. Chem. 2004, 28, 271–277. [Google Scholar]
- Akbaş, E.; Aslanoğlu, F. Syntheses of some new 1H-pyrazole, pyridazin-3(2H)-one, and oxazin-4-one derivative. Heteroatom Chem. 2006, 17, 8–12. [Google Scholar] [CrossRef]
- Amer, A.; Ventura, M.; Zimmer, H. Substituted γ-lactones. XXX (1). Reactions of α-keto-β-substituted-γ-lactones with diamines. J. Heterocycl. Chem. 1983, 20, 359–364. [Google Scholar] [CrossRef]
- Ziegler, E.; Eder, M.; Belegratis, C.; Prewedourakis, E. Synthesen von heterocyclen, 103. mitt.: Über reaktionen mit oxalylchlorid. Monatsh. Chem. 1967, 98, 2249–2251. [Google Scholar]
- Hökelek, T.; Sarıpınar, E.; Yıldırım, İ.; Akkurt, M.; Akçamur, Y. 4-(4-Methoxybenzoyl)-5-(4-methoxyphenyl)-2,3-dihydro-2,3-furandione. Acta Cryst. E 2002, 58, 30–32. [Google Scholar]
- Yıldırım, İ.; Koca, İ. Synthesis, characterization and some reactions of novel 4-aroyl-5-aryl-2,3-dihydro-2,3-furandiones. Kuwait J. Sci. Eng. 2005, 32, 49–60. [Google Scholar]
- Üngören, Ş.H.; Deniz, B.; Altural, B. Synthesis of novel 2,3-dihydro-2,3-furandione and 1H-pyrazole-3-carboxylic acid derivatives. Asian J. Chem. 2004, 16, 805–810. [Google Scholar]
- Kappe, C.O.; Terpetschnig, E.; Penn, G.; Kollenz, G.; Peters, K.; Peters, E.M.; von Schnering, H.G. Reactions of cyclic oxalyl compounds .37. substituent effects on the site of nucleophilic-attack at 1H-pyrrole-2,3-diones. Liebigs Ann. 1995, 37, 537–543. [Google Scholar]
- Lopez, R.; Boys, D.; Loeb, B.; Zuloaga, F. Synthesis, crystal structure, molecular orbital calculations and electronic properties of 2,3-di(2-pyridyl)naphtho[2,3-f]quinoxaline-7,12-quinone(Aqdpp). J. Chem. Soc., Perkin Trans. 2 1998, 877–883. [Google Scholar]
- Sample Availability: Samples of the compounds 2a-e and 3a,b,c,e,f are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Üngören, Ş.H. Synthesis of New Naphtho[2,3-f]quinoxaline-2,7,12(1H)-trione and Anthra-9,10-quinone Dyes from Furan-2,3-diones. Molecules 2009, 14, 1429-1437. https://doi.org/10.3390/molecules14041429
Üngören ŞH. Synthesis of New Naphtho[2,3-f]quinoxaline-2,7,12(1H)-trione and Anthra-9,10-quinone Dyes from Furan-2,3-diones. Molecules. 2009; 14(4):1429-1437. https://doi.org/10.3390/molecules14041429
Chicago/Turabian StyleÜngören, Şevket Hakan. 2009. "Synthesis of New Naphtho[2,3-f]quinoxaline-2,7,12(1H)-trione and Anthra-9,10-quinone Dyes from Furan-2,3-diones" Molecules 14, no. 4: 1429-1437. https://doi.org/10.3390/molecules14041429