Transformations of Organic Molecules with F-TEDA-BF4 in Ionic Liquid Media
Abstract
:1. Introduction

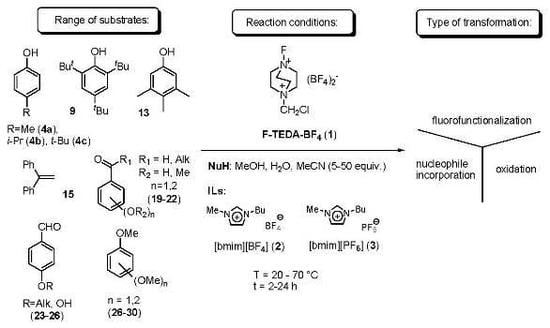
2. Results and Discussion

| Entry | R | ILa | [bmim][BF4] | [bmim][PF6] | ||
|---|---|---|---|---|---|---|
| T, t | Conv.b | Product distribution (5 : 6 : 7 : 8) | Conv.b | Product distribution (5 : 6 : 7 : 8) | ||
| 1 | Me ( 4a) | 70 °C, 4 h | complex reaction mixture | complex reaction mixture | ||
| 2 | 22 °C, 22 h | 10% | 6 : 0 : 4 : 0 | 0c | ||
| 3 | 50 °C, 22 h | 20% | 100 : 0 : 0 : 0 | |||
| 4 | i-Pr (4b) | 70 °C, 24 h | 71% | 59 : 2 : 0 : 10 | 36% | 100 : 0 : 0 : 0d |
| 5 | t-Bu (4c) | 70 °C, 4 h | 69% | 37 : 4 : 0 : 28 | 14% | 10 : 0 : 0 : 4 |
| 6 | 70 °C, 24 h | 87% | 44 : 11 : 0 : 32 | 46% | 31 : 0 : 0 : 15e | |





| Entry | ILb | NuHc | T [°C] | Product distributiond | |||||
|---|---|---|---|---|---|---|---|---|---|
| 15 | 16a | 16b | 17 | 18a | 18b | ||||
| 1 | [bmim][PF6] | / | 22 | 100% | / | / | / | / | / |
| 2 | / | 50 | 64% | / | / | 36% | / | / | |
| 3 | MeOH | 50 | 7% | / | / | 60% | 22% | 12% | |
| 4 | H2O | 50 | 13% | / | / | 78% | / | 9% | |
| 5 | MeCN | 50 | 30% | / | / | 70% | / | / | |
| 6 | [bmim][BF4] | / | 22 | 58% | / | 25% | 17% | / | / |
| 7 | / | 50 | 2% | / | 65% | 14% | / | 19% | |
| 8 | MeOH | 50 | / | 76% | 6% | 1% | 17% | / | |
| 9 | H2O | 50 | 3% | / | 76% | / | / | 21% | |

| Entry | Substrate | Ionic liquid | t (h) | Conv.b | Products | Product distributionc |
|---|---|---|---|---|---|---|
| 1 |  | [bmim][BF4] | 5 | 10% |  | 19a: 9%, 19: 90%d |
| 2 | 18 | 14% | 19a: 11%, 19b: 2%, 19: 86%d | |||
| 3 | [bmim][PF6] | 5 | 6% | 19a: 6%, 19: 94% | ||
| 4 | 18 | 10% | 19a: 9%, 19: 90%d | |||
| 5 |  | [bmim][BF4] | 20 | 28% |  | 20a: 28%, 20: 72% |
| 6 | [bmim][PF6] | 20 | 7% | 20a: 7%, 20: 93% | ||
| 7 |  | [bmim][BF4] | 5 | 29% |  | 21a: 17%, 21b: 5%, 21c: 7%, 21: 71% |
| 8 | 18 | 47% | 21a: 27%, 21b: 7%, 21c: 13%, 21: 53% | |||
| 9 | [bmim][PF6] | 5 | 18% | 21a: 12%, 21b: 3%, 21c: 3%, 21: 82% | ||
| 10 | 18 | 10% | 21a: 7%, 21b: 2%, 21c: 1%, 21: 90% | |||
| 11 |  | [bmim][BF4] | 5 | 49% |  | 22a: 29%, 22b: 6%, 22c: 11%, 22d: 3%, 22: 51% |
| 12 | 18 | 61% | 22a: 30%, 22b: 7%, 22c: 16%, 22d: 8%, 22: 39% | |||
| 13 | [bmim][PF6] | 5 | 22% | 22a: 16%, 22b: 3%, 22c: 3%, 22: 78% | ||
| 14 | 18 | 11% | 22a: 8%, 22b: 2%, 22c: 1%, 22: 89% |

3. Experimental
3.1. General
4. Conclusions
Acknowledgements
References
- Malhotra, S.V. Ionic Liquids in Organic Synthesis; ACS Symposium Series 950, ACS: Washington, DC, USA, 2007. [Google Scholar]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, Germany, 2008; volumes 1-2. [Google Scholar]
- Earle, M.J.; Seddon, K.R. Ionic Liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Davis, J.H., Jr.; Fox, P.A. From curiosities to commodities: ionic liquids begin the transition. Chem. Commun. 2003, 1209–1212. [Google Scholar] [CrossRef]
- Chiappe, C.; Pieraccini, D. Ionic liquids: Solvent properties and organic reactivity. J. Phys. Org. Chem. 2005, 18, 275–297. [Google Scholar] [CrossRef]
- Handy, S.T. Room Temperature Ionic Liquids: Different Classes and Physical Properties. Curr. Org. Chem. 2005, 9, 959–988. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef]
- Zhao, H.; Malhotra, S.V. Applications of Ionic Liquids in Organic Synthesis. Aldrichim. Acta 2002, 35, 75–83. [Google Scholar] [CrossRef]
- Pârvulescu, V.I.; Hardacre, C. Catalysis in Ionic Liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar]
- Hapiot, P.; Lagrost, C. Electrochemical Reactivity in Room-Temperature Ionic Liquids. Chem. Rev. 2008, 108, 2238–2264. [Google Scholar] [CrossRef]
- Harper, J.B.; Kobrak, M.N. Understanding Organic Processes in Ionic Liquids: Achievements So Far and Challenges Remaining. Mini-Rev. Org. Chem. 2006, 3, 253–269. [Google Scholar] [CrossRef]
- Scammells, P.J.; Scott, J.L.; Singer, R.D. Ionic Liquids: The Neglected Issues. Aust. J. Chem. 2005, 58, 155–169. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mohan, R.S.; Scott, J.L. Reactivity of ionic liquids. Tetrahedron 2007, 63, 2363–2389. [Google Scholar] [CrossRef]
- Ranke, J.; Stolte, S.; Störmann, R.; Arning, J.; Jastorff, B. Design of Sustainable Chemical Products – The Example of Ionic Liquids. Chem. Rev. 2007, 107, 2183–2206. [Google Scholar] [CrossRef]
- Smart, B.E. Fluorine substituent effects (on bioactivity). J. Fluor. Chem. 2001, 109, 3–11. [Google Scholar] [CrossRef]
- Banks, R.E.; Smart, B.E.; Tatlow, J.C. Organofluorine chemistry: Principles and Commercial Applications; Plenum Press: New York, NY, USA, 1994. [Google Scholar]
- Hiyama, T. Organofluorine Compounds: Chemistry and Applications; Springer-Verlag: Berlin, Germany, 2000. [Google Scholar]
- Singh, R.P.; Shreeve, J.M. Recent Highlights in Electrophilic Fluorination With 1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane Bis(tetrafluoroborate). Acc. Chem. Res. 2004, 37, 31–44. [Google Scholar] [CrossRef]
- Stavber, S.; Zupan, M. SelectfluorTM F-TEDA-BF4 as a Versatile Mediator or Catalyst in Organic Chemistry. Acta Chim. Slov. 2005, 52, 13–26. [Google Scholar]
- Nyffeler, P.T.; Durón, S.G.; Burkart, M.D.; Vincent, S.P.; Wong, C.H. Selectfluor: Mechanistic Insight and Applications. Angew. Chem. Int. Ed. 2005, 44, 192–212. [Google Scholar] [CrossRef]
- Stavber, S.; Zupan, M. N-Fluoro-Diazonia-Bicyclo[2.2.2]Octane Dication Salts; Efficient Fluorination Agents and Functionalization Mediators for Organic Compounds. In Advances in Organic Synthesis, Modern Organofluorine Chemistry-Synthetic Aspects; Rahman, A., Laali, K.K., Eds.; Bentham Science Publishers Ltd.: Hilversum, The Netherlands, 2006; Vol. 2, pp. 213–268. [Google Scholar]
- Laali, K.K.; Borodkin, G.I. First application of ionic liquids in electrophilic fluorination of arenes; Selectfluor™ (F-TEDA-BF4) for “green” fluorination. J. Chem. Soc., Perkin Trans. 2 2002, 953–957. [Google Scholar]
- Baudoux, J.; Salit, A.F.; Cahard, D.; Plaquevent, J.C. Ionic liquids as solvents of choice for electrophilic fluorination: fluorination of indoles by F-TEDA-BF4. Tetrahedron Lett. 2002, 43, 6573–6574. [Google Scholar] [CrossRef]
- Heravi, M.R.P. Fluorination of activated aromatics systems with Selectfluor™ F-TEDA-BF4 in ionic liquids. J. Fluor. Chem. 2008, 129, 217–221. [Google Scholar] [CrossRef]
- Baudequin, C.; Plaquevent, J.C.; Audouard, C.; Cahard, D. Enantioselective electrophilic fluorination in ionic liquids. Green Chem. 2002, 4, 584–586. [Google Scholar] [CrossRef]
- Baudequin, C.; Loubassou, J.F.; Plaquevent, J.C.; Cahard, D. Enantioselective electrophilic fluorination: a study of the fluorine-transfer from achiral N-F reagents to cinchona alkaloids. J. Fluor. Chem. 2003, 122, 189–193. [Google Scholar] [CrossRef]
- Pravst, I.; Papež Iskra, M.; Jereb, M.; Zupan, M.; Stavber, S. The role of F-N reagent and reaction conditions on fluoro functionalisation of substituted phenols. Tetrahedron 2006, 62, 4474–4481. [Google Scholar]
- Stavber, S.; Jereb, M.; Zupan, M. The effect of the reaction conditions on the course of the reactions of hindered phenols with N-F reagents. Arkivoc 2001, (v). 98–107. [Google Scholar]
- Stavber, S.; Jereb, M.; Zupan, M. Kinetic investigation of the reactions of hindered phenols with N-fluoro-1,4-diazoniabicyclo[2.2.2]octane salt analogues. J. Phys. Org. Chem. 2002, 15, 56–61. [Google Scholar] [CrossRef]
- Stavber, S.; Jereb, M.; Zupan, M. Efficient Synthesis of 4-Fluorocyclohexa-2,5-dienone Derivatives Using N-Fluoro-1,4-diazoniabicyclo[2.2.2]octane Salt Analogues. Synlett 1999, 1375–1378. [Google Scholar] [CrossRef]
- Stavber, S.; Sotler, T.; Zupan, M. A Mild, Selective Method for Preparation of Vicinal Fluoro Ethers Using ''F-TEDA-BF4. Tetrahedron Lett. 1994, 35, 1105–1108. [Google Scholar] [CrossRef]
- Stavber, S.; Sotler-Pečan, T.; Zupan, M. Effect of Reaction Conditions on the Kinetic and Activation Parameters for the Mild Introduction of Fluorine into Phenyl-Substituted Alkenes with AccufluorTM NFTh. Tetrahedron 2000, 56, 1929–1936. [Google Scholar] [CrossRef]
- Stavber, G.; Zupan, M.; Jereb, M.; Stavber, S. Selective and Effective Fluorination of Organic Compounds in Water Using Selectfluor F-TEDA-BF4. Org. Lett. 2004, 6, 4973–4976. [Google Scholar] [CrossRef]
- Stavber, S.; Jereb, M.; Zupan, M. Solvent directing immediate fluorination of aromatic ketones using 1-fluoro-4-hydroxy-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate). Chem. Commun. 2000, 1323–1324. [Google Scholar] [CrossRef]
- Banks, R.E.; Besheesh, M.K.; Górski, R.W.; Lawrence, N.J.; Taylor, A.J. N-Halogeno compounds. Part 20. Vicarious electrophilic fluorodemethylation of 1,3,5-trimethoxy benzene and 2,4,6-trimethoxy toluene with 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis (tetrafluoroborate) (SelectfluorTM reagent F-TEDA-BF4). J. Fluor. Chem. 1999, 96, 129–133. [Google Scholar] [CrossRef]
- Singh, S.; DesMarteau, D.D.; Zuberi, S.S.; Witz, M.; Huang, H.N. N-Fluoroperfluoroalkylsulfonimides. Remarkable new fluorination reagents. J. Am. Chem. Soc. 1987, 109, 7194–7196. [Google Scholar] [CrossRef]
- Misaki, S. Direct Fluorination of Phenol and Cresols. J. Fluor. Chem. 1981, 17, 159–171. [Google Scholar] [CrossRef]
- Zupan, M.; Iskra, J.; Stavber, S. Chemistry of Organo Halogenic Molecules. 140. Role of the Reagent Structure on the Transformations of Hydroxy Substituted Organic Molecules with the N-Fluoro Class of Fluorinating Reagents. Bull. Chem. Soc. Jpn. 1995, 68, 1655–1660. [Google Scholar] [CrossRef]
- Stavber, S.; Zupan, M. The Effect of Heteroatoms on the Reactions of Organic Molecules with Caesium Fluoroxysulphate. Tetrahedron 1992, 48, 5875–5882. [Google Scholar] [CrossRef]
- Fukuhara, T.; Akiyama, Y.; Yoneda, N.; Tada, T.; Hara, S. Effective synthesis of difluorocyclohexadienones by electrochemical oxidation of phenols. Tetrahedron Lett. 2002, 43, 6583–6585. [Google Scholar] [CrossRef]
- Tashiro, M.; Yoshiya, H.; Fukata, G. Selective Preparation.31. Oxidative Coupling of 2-Halo-4,6-di-tert-butylphenols with Potassium Hexacyanoferrate (III) in Benzene. J. Org. Chem. 1981, 46, 3784–37890. [Google Scholar] [CrossRef]
- Pallagi, I.; Toró, A.; Farkas, Ö. Mechanism of the Gibbs Reaction. 3. Indophenol Formation via Radical Electrophilic Aromatic Substitution (SREAr) on Phenols. J. Org. Chem. 1994, 59, 6543–6557. [Google Scholar] [CrossRef]
- Vollert, U. Gas-chromatographische Untersuchungen von Alkylierungsprodukten aus 2-Methoxyphenol und Isobuten. Z. Chem. 1975, 15, 359–360. [Google Scholar]
- Omura, K. Dienone Tautomers of 4-Alkoxy-2,6-di-tert-butylphenols. J. Org. Chem. 1996, 61, 7156–7161. [Google Scholar] [CrossRef]
- Stavber, S.; Zupan, M. Fluorination with Caesium Fluoroxysulphate. Room Temperature Fluorination of Phenyl Substituted Olefins. Tetrahedron 1986, 42, 5035–5043. [Google Scholar]
- Asakura, N.; Usuki, Y.; Iio, H. A new synthesis of α-fluorovinylsulfones utilizing the Peterson olefination methodology. J. Fluor. Chem. 2003, 124, 81–88. [Google Scholar] [CrossRef]
- Stegmann, H.B.; Deuschle, G.; Schuler, P. Synthesis and EPR Investigations of Fluorocatecholamines. J. Chem. Soc. Perkin Trans. 2 1994, 3, 547–555. [Google Scholar] [CrossRef]
- Belov, V.N.; Dudkin, V. Yu.; Urusova, E.A.; Starova, G.L.; Selivanov, S.I.; Nikolaev, S.V.; Eshchenko, N.D.; Morozkina, S.N.; Shavva, A.G. Synthesis, Structure, and Biological Properties of Some 8α Analogues of Steroid Estrogens with Fluorine in Position 2. Russ. J. Bioorg. Chem. 2007, 33, 293–301. [Google Scholar] [CrossRef]
- Lerman, O.; Tor, Y.; Hebel, D.; Rozen, S. A Novel Electrophilic Fluorination of Activated Aromatic Rings Using Acetyl Hypofluorite, Suitable also for Introducing 18F into Benzene Nuclei. J. Org. Chem. 1984, 49, 806–813. [Google Scholar] [CrossRef]
- Lawrence, N.J.; Hepworth, L.A.; Rennison, D.; McGown, A.T.; Hadfield, J.A. Synthesis and anticancer activity of fluorinated analogues of combretastatin A-4. J. Fluor. Chem. 2003, 123, 101–108. [Google Scholar] [CrossRef]
- Sun, W.C.; Gee, K.R.; Klaubert, D.H.; Haugland, R.P. Synthesis of Fluorinated Fluoresceins. J. Org. Chem. 1997, 62, 6469–6475. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pavlinac, J.; Zupan, M.; Stavber, S. Transformations of Organic Molecules with F-TEDA-BF4 in Ionic Liquid Media. Molecules 2009, 14, 2394-2409. https://doi.org/10.3390/molecules14072394
Pavlinac J, Zupan M, Stavber S. Transformations of Organic Molecules with F-TEDA-BF4 in Ionic Liquid Media. Molecules. 2009; 14(7):2394-2409. https://doi.org/10.3390/molecules14072394
Chicago/Turabian StylePavlinac, Jasminka, Marko Zupan, and Stojan Stavber. 2009. "Transformations of Organic Molecules with F-TEDA-BF4 in Ionic Liquid Media" Molecules 14, no. 7: 2394-2409. https://doi.org/10.3390/molecules14072394