Extraction of Oil from Wheat Germ by Supercritical CO2
Abstract
:1. Introduction
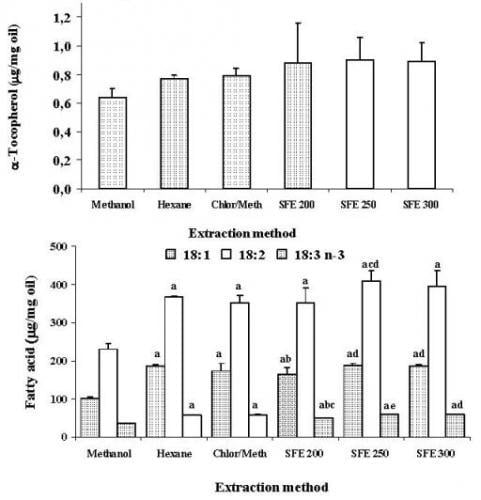
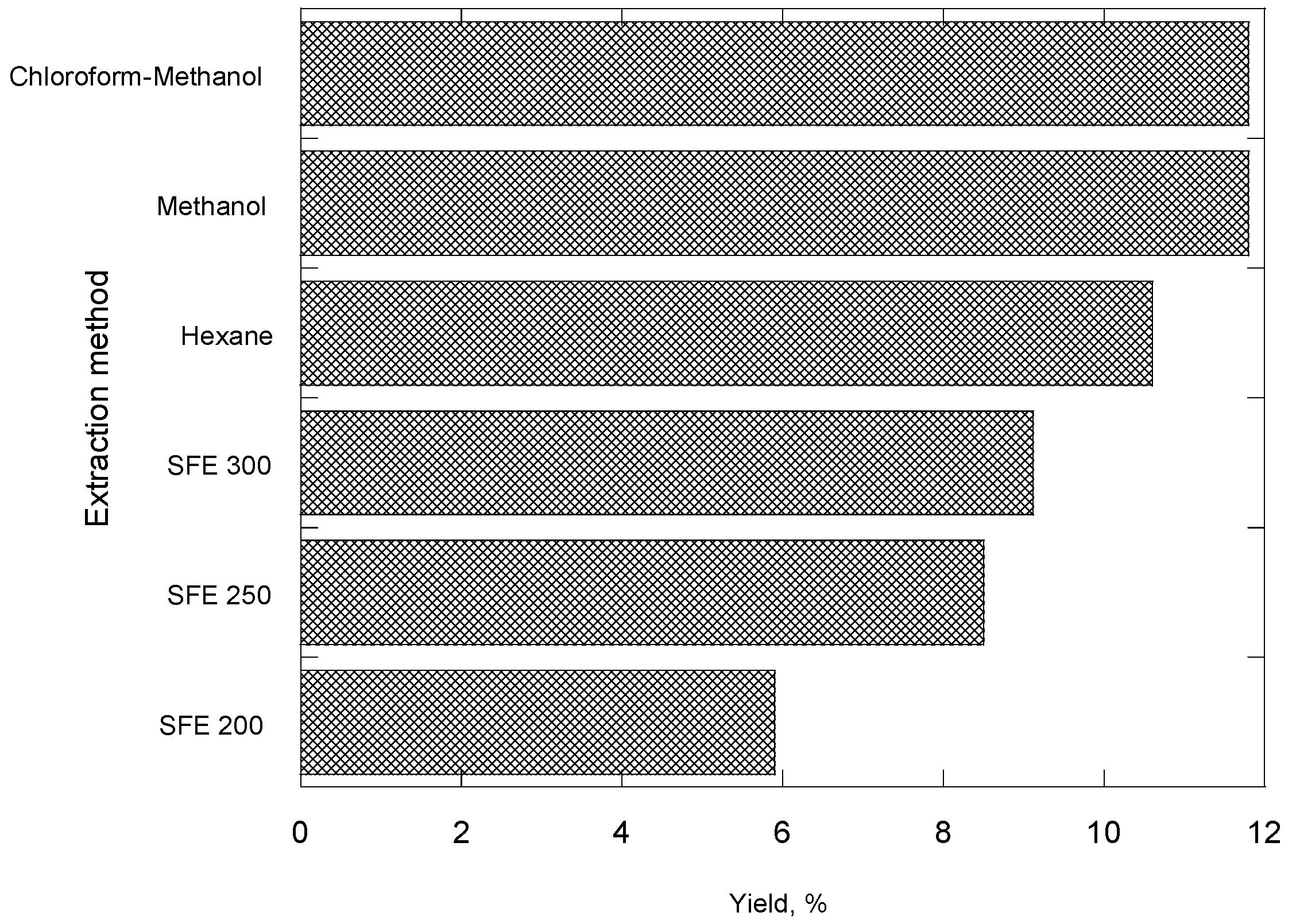
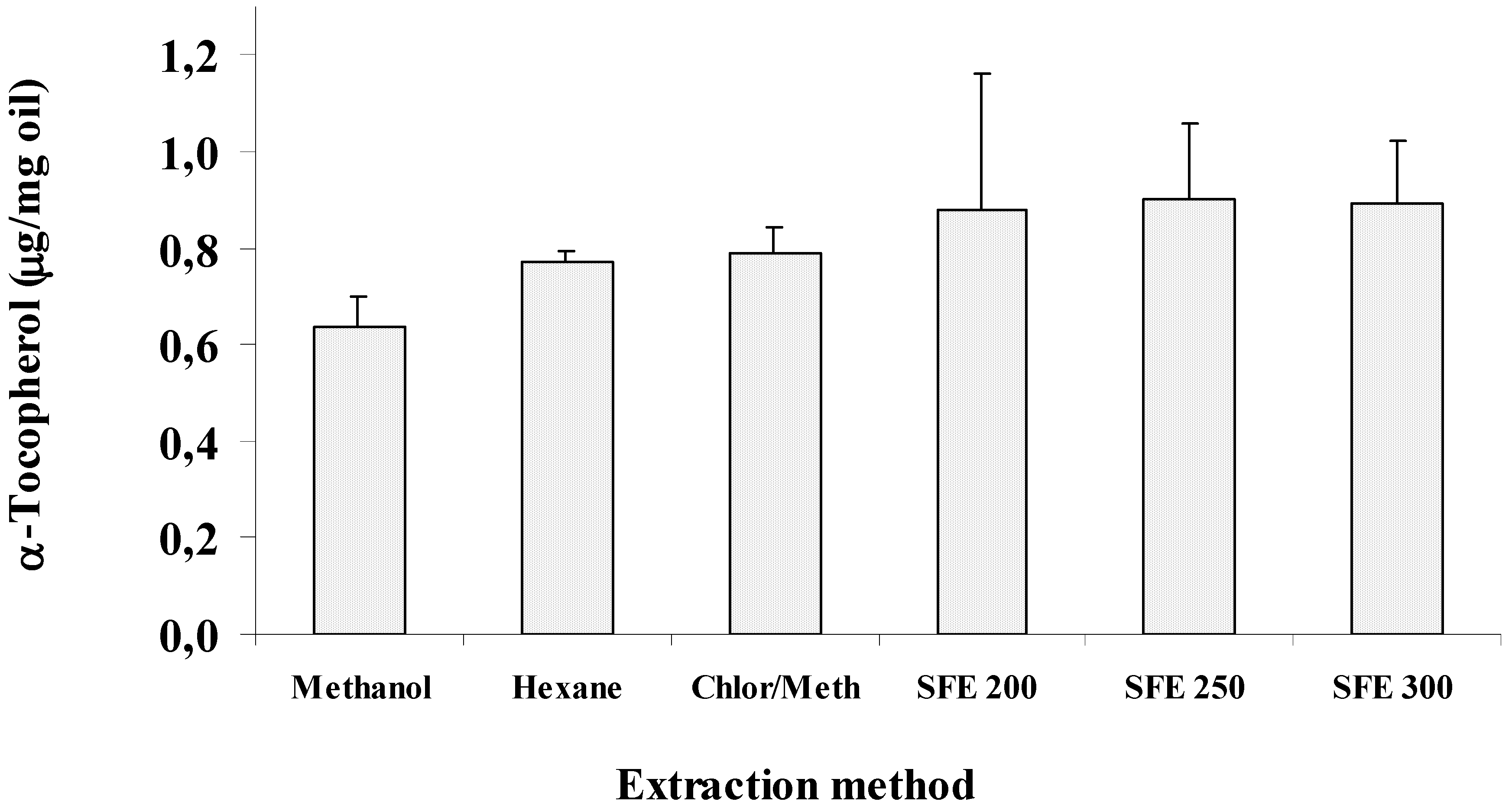
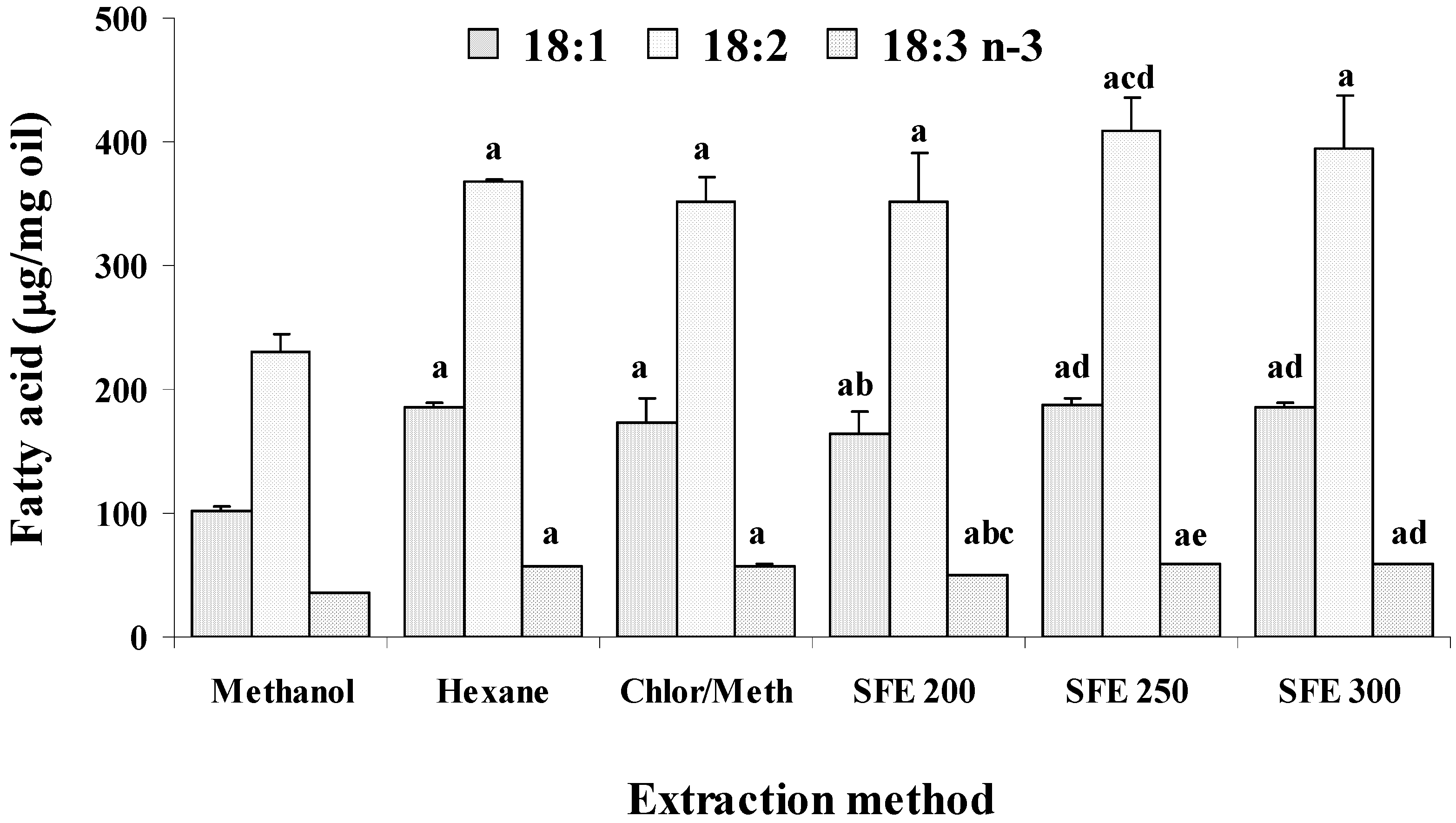
2. Results and Discussion


3. Experimental
3.1. Materials
3.2. Solvent Extraction
3.3. Apparatus for SFE
3.4. Preparation of α-tocopherol and fatty acids
3.5. HPLC analysis
3.6. GC analysis
3.7. Statistical analyses
References
- Shuler, P. Natural antioxidants exploited commercially. In Food Antioxidants; Hudson, B.J.F, Ed.; Elsevier Applied Science: London, UK, 1990; Chapter 4. [Google Scholar]
- Wang, T.; Johnson, L. Refining High-free Fatty Acid Wheat Germ Oil. JAOCS 2001, 78, 71–76. [Google Scholar]
- Coultate, T. Food, the chemistry of its components; The Royal Society of Chemistry: London, UK, 1989; Chapter 4. [Google Scholar]
- Salinas, R. Alimentos Grasos y Nutrición, Bromatología Aplicada a la Salud; El Ateneo: Buenos Aires, Argentina, 1993; Chapter 7. [Google Scholar]
- Krings, U.; Berger, R.G. Antioxidant activity of some roasted foods. Food Chem. 2001, 72, 223–229. [Google Scholar] [CrossRef]
- Dunford, N.T.; Zhang, M.Q. Pressurized solvent extraction of wheat germ oil. Food Res. Int. 2003, 36, 905–909. [Google Scholar] [CrossRef]
- Kahlon, T.S. Nutritional implications and uses of wheat and oat kernel oil. Cereal Food Worls. 1989, 34, 872–875. [Google Scholar]
- Krings, U.; El-Saharty, Y.S.; El-Zeany, B.A.; Pabel, B.; Berger, R.G. Antioxidant activity of extracts from roasted wheat germ. Food Chem. 2000, 71, 91–95. [Google Scholar] [CrossRef]
- Reverchon, E.; Marrone, C. Modeling and simulation of the supercritical CO2 extraction of vegetable oils. J. Supercrit. Fluid. 2001, 19, 161–175. [Google Scholar] [CrossRef]
- Lang, Q.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies-a pratical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Hautal, W.H. Advances with supercritical fluids-review. Chemosphere 2001, 43, 123–135. [Google Scholar] [CrossRef]
- Panfili, G.; Cinquanta, L.; Fratianni, A.; Cubadda, R. Extraction of wheat germ oil by supercritical CO2: oil and defatted cake characterization. JAOCS 2003, 80, 157–161. [Google Scholar]
- Taniguchi, M.; Tsuji, T.; Shibata, M.; Kobayashi, T. Extraction of oils from wheat germ with supercritical carbon dioxide. Agric. Biol. Chem. 1985, 49, 2367–2372. [Google Scholar] [CrossRef]
- Molero Gomez, A.; Martinez de la Ossa, E. Quality of wheat germ oil extracted by liquid and supercritical carbon dioxide. JAOCS 2002, 77, 969–974. [Google Scholar]
- Shao, P.; Sun, P.; Ying, Y. Response surface optimization of wheat germ oil yield by supercritical carbon dioxide extraction. Food Bioprod. Process. 2008, 86, 227–231. [Google Scholar] [CrossRef]
- Eisenmenger, M.; Dunford, N.T. Bioactive components of commercial and supercritical carbon dioxide processed wheat germ oil. JAOCS 2008, 85, 55–61. [Google Scholar]
- Ge, Y.; Yan, H.; Hui, B.; Ni, Y.; Wang, S.; Cai, T. Extraction of natural vitamin E from wheat germ by supercritical carbon dioxide. J. Agric. Food Chem. 2002, 50, 685–689. [Google Scholar] [CrossRef]
- Zacchi, P.; Daghero, J.; Jaeger, P.; Eggers, R. Extraction/fractionation and deacidification of wheat germ oil using supercritical carbon dioxide. Braz. J. Chem. Eng. 2006, 3, 105–110. [Google Scholar]
- Dessì, M.A.; Deiana, M.; Day, B.W.; Rosa, A.; Banni, S.; Corongiu, F.P. Oxidative stability of plyunsaturated fatty acids: effect of squalene. Eur. J. Lipid Sci. Technol. 2002, 104, 506–512. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipid from animals tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Chiang, S.D.; Gessert, C.F.; Lowry, O.H. Colorimetric determination of extracted lipids. An adaptation for microgram amounts of lipids obtained from cerumen. Curr. List Med. Lit. Res. Rep. 1957, 33, 56–113. [Google Scholar]
- Crabas, N.; Marongiu, B.; Piras, A.; Pivetta, T.; Porcedda, S. Extraction, separation and isolation of volatiles and dyes from Calendula officinalis L. and Aloysia triphilla (L’Her) Britton by supercritical CO2. J. Essent. Oil Res. 2003, 15, 272–277. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Porcedda, S. Comparative analysis of the oil and supercritical CO2 extract of Elettaria cardamomum (L) Maton. J. Agric. Food Chem. 2004, 52, 6278–6282. [Google Scholar] [CrossRef]
- Rosa, A.; Deiana, M.; Corona, G.; Atzeri, A.; Incani, A.; Appendino, G.; Dessì, M.A. Protective effect of capsinoid on lipid peroxidation in rat tissues induced by Fe-NTA. Free Radic. Res. 2005, 39, 1155–1162. [Google Scholar] [CrossRef]
- Christie, W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. In Advantage in Lipid Methodology – Two; Christie, W.W., Ed.; The Oily Press: Dundee, UK, 1993; pp. 69–111. [Google Scholar]
- Rosa, A.; Scano, P.; Melis, M.P.; Deiana, M.; Atzeri, A.; Dessì, M.A. Oxidative stability of lipid components of mullet (Mugil cephalus) roe and its product “bottarga”. Food Chem. 2009, 115, 891–896. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the wheat germ oil, extracted by SFE and by solvent extraction, are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Piras, A.; Rosa, A.; Falconieri, D.; Porcedda, S.; Dessì, M.A.; Marongiu, B. Extraction of Oil from Wheat Germ by Supercritical CO2. Molecules 2009, 14, 2573-2581. https://doi.org/10.3390/molecules14072573
Piras A, Rosa A, Falconieri D, Porcedda S, Dessì MA, Marongiu B. Extraction of Oil from Wheat Germ by Supercritical CO2. Molecules. 2009; 14(7):2573-2581. https://doi.org/10.3390/molecules14072573
Chicago/Turabian StylePiras, Alessandra, Antonella Rosa, Danilo Falconieri, Silvia Porcedda, Maria. A. Dessì, and Bruno Marongiu. 2009. "Extraction of Oil from Wheat Germ by Supercritical CO2" Molecules 14, no. 7: 2573-2581. https://doi.org/10.3390/molecules14072573