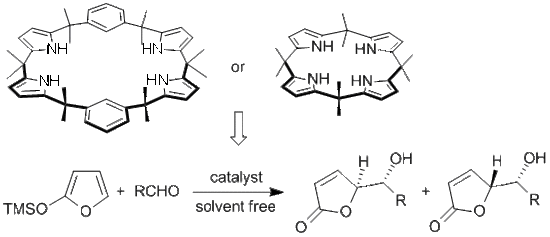
Calixpyrrole Derivatives: “Multi Hydrogen Bond” Catalysts for γ-Butenolide Synthesis
Abstract
:Introduction
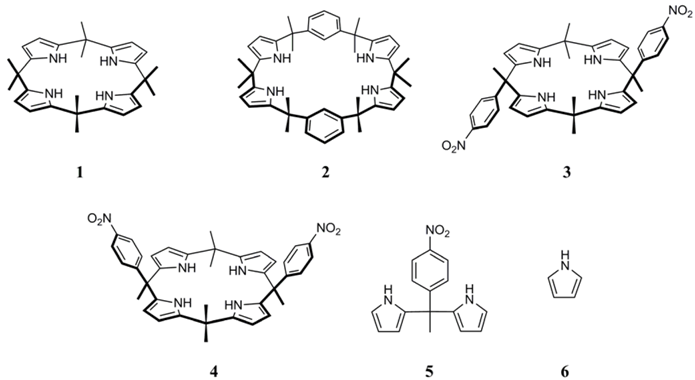
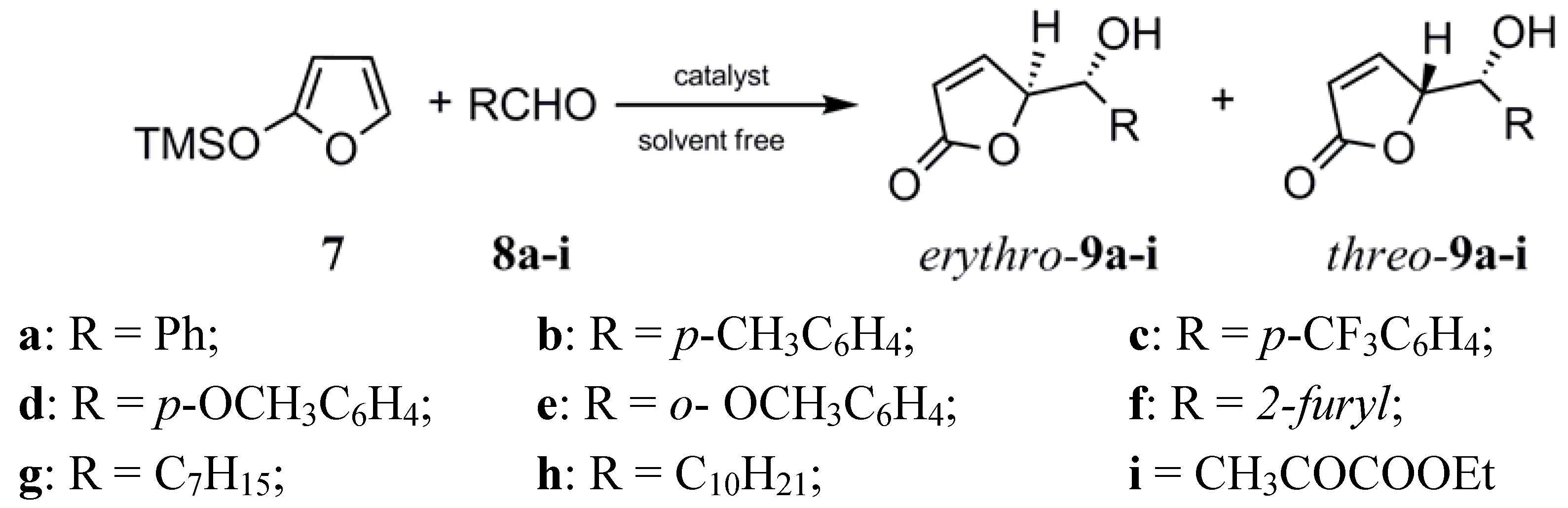
Results and Discussion
| Entry | Catalyst | Yield (%) [b] | erythro/threo [c] |
|---|---|---|---|
| 1 | - | 0 | - |
| 2 | 1 | 45 | 70/30 |
| 3 | 2 | 63 | 80/20 |
| 4 | 3 | 12 | 70/30 |
| 5 | 4 | 30 | 70/30 |
| 6 | 5 | 16 | 60/40 |
| 7 | 6 | 25 | 70/30 |
| 8 | 6 | 0 | - |
| Entry | Catalyst (mol%) | °C/h | Yield (%) [b] | erythro/threo [c] |
|---|---|---|---|---|
| 1[a] | 1 (20) | 25°/48 | 82 | 70/30 |
| 2[a] | 1 (20) | 25°/72 | 18 | 70/30 |
| 3[a] | 1 (20) | 40°/24 | 7 | 70/30 |
| 4[a] | 2 (20) | 25°/48 | 74 | 70/30 |
| 5[a] | 2 (20) | 40°/48 | 34 | 78/22 |
| Entry | Substrate | Yield (%)[b] with 1 | Yield (%)[b] with 2 | erythro/threo[c] with 1 | erythro/threo[c] with 2 |
|---|---|---|---|---|---|
| 1 | p-CH3-C6H4CHO (8b) | 40 | 40 | 80/20 | 80/20 |
| 2 | p-CF3-C6H4 CHO (8c) | 58 | 50 | 70/30 | 70/30 |
| 3 | p-OCH3-C6H4 CHO (8d) | 25 | 4 | 80/20 | - |
| 4 | o-OCH3-C6H4 CHO (8e) | 15 | 0 | 70/30 | - |
| 5 |  (8f) (8f) | 70 | 56 | 70/30 | 70/30 |
| 6 | C7H15 CHO (8g) | 30 | 40 | 70/30 | 70/30 |
| 7 | C10H21 CHO (8h) | 16 | 30 | 70/30 | 70/30 |
| 8 | CH3COCOOEt (8i) | 63 | 63 | 70/30 | 70/30 |
Experimental
General
General Procedure for the Organocatalytic Addition of TMSOF to Aldehydes
Conclusions
Acknowledgements
References
- Lee, C.H.; Miyaji, H.; Yoon, D.W.; Sessler, J.L. Strapped and other topographically nonplanar calixpyrrole analogues. Improved anion receptors. Chem. Commun. 2008, 24–34. [Google Scholar]
- Gale, P.A.; Anzenbacher, P., Jr.; Sessler, J.L. Calixpyrroles II. Coord. Chem. Rev. 2001, 222, 57–102. [Google Scholar] [CrossRef]
- Gale, P.A.; Sessler, J.L.; Kral, V. Calixpyrroles. Chem. Commun. 1998, 1–8, and references therein. [Google Scholar] [CrossRef]
- Akiyama, T.; Itoh, J.; Fuchibe, K. Recent Progress in Chiral Brønsted Acid Catalysis. Adv. Synth. Catal. 2006, 348, 999–1010, and references threin. [Google Scholar]
- Gondi, V.B.; Gravel, M.; Rawal, V.H. Hydrogen bond catalyzed enantioselective vinylogous Mukaiyama aldol reaction. Org. Lett. 2005, 7, 5657–5660. [Google Scholar] [CrossRef]
- Hou, Z.R.; Wang, J.; Liu, X.H.; Feng, X.M. Highly enantioselective Strecker reaction of ketoimines catalyzed by an organocatalyst from (S)-BINOL and L-prolinamide. Chem. Eur. J. 2008, 14, 4484–4486. [Google Scholar] [CrossRef]
- Wanner, M.J.; Hauwert, P.; Schoemaker, H.E.; de Gelder, R.; van Maarseveen, J.H.; Hiemstra, H. Synthesis of enantiopure (S)-indolyglycine by organocatalyzed Friedel-Crafts alkylation of indole. Eur. J. Org. Chem. 2008, 1, 180–185. [Google Scholar]
- Page, P.C.B.; Farah, M.M.; Buckley, B.R.; Blacker, A.J. New chiral binaphthalene-derived iminium salt organocatalysts for asymmetric epoxidation of alkenes. J. Org. Chem. 2007, 72, 4424–4430. [Google Scholar] [CrossRef]
- Rueping, M.; Ieawsuwan, W.; Antonchick, A.P.; Nachtsheim, B.J. Chiral Bronsted acids in the catalytic asymmetric Nazarov cyclization- The first enantioselective organocatalytic electrocyclic reaction. Angew. Chem., Int. Ed. 2007, 46, 2097–2100. [Google Scholar] [CrossRef]
- Connon, S.J. Asymmetric catalysis with bifunctional cinchona alkaloid-based urea and thiourea organocatalysts. Chem. Comm. 2008, 2499–2510. [Google Scholar]
- Weil, T.; Kotke, M.; Kleiner, C.M.; Schreiner, P.R. Cooperative Bronsted acid-type organocatalysis: alcoholysis of styrene oxides. Org. Lett. 2008, 10, 1513–1516. [Google Scholar] [CrossRef]
- Peschiulli, A.; Gun'ko, Y.; Connon, S.J. Highly enantioselective desymmetrization of meso anhydrides by a bifunctional thiourea-based organocatalysts at low catalyst loadings and room temperature. J. Org. Chem. 2008, 73, 2454–2457. [Google Scholar] [CrossRef]
- Fleming, E.M.; Quigley, C.; Rozas, I.; Connon, S.J. Computational study-led organocatalyst design: a novel, highly active urea-based catalyst for addition reactions to epoxides. J. Org. Chem. 2008, 73, 948–956. [Google Scholar]
- Shi, Y.L.; Shi, M. Chiral thiourea-phosphine organocatalysts in the asymmetric Aza-Morita-Baylis-Hillman reaction. Adv. Synth. Catal. 2007, 349, 2129–2135. [Google Scholar] [CrossRef]
- Connon, S.J. Organocatalysis mediated by (thio)urea derivatives. Chem. Eur. J. 2006, 12, 5418–5427. [Google Scholar] [CrossRef]
- Seayad, J.; List, B. Asymmetric organocatalysis. Org. Biomol. Chem. 2005, 3, 719–724. [Google Scholar] [CrossRef]
- Cafeo, G.; De Rosa, M.; Kohnke, F.H.; Neri, P.; Soriente, A.; Valenti, L. Efficient organocatalysis with a calix[4]pyrrole derivative. Tetrahedron Lett. 2008, 49, 153–155. [Google Scholar]
- Cafeo, G.; Kohnke, F.H.; Valenti, L. Regioselective O-alkylations and acylations of polyphenolic substrates using a calix[4]pyrrole derivative. Tetrahedron Lett. 2009, 50, 4138–4140. [Google Scholar] [CrossRef]
- De Rosa, M.; Citro, L.; Soriente, A. The first organocatalytic addition of 2-trimethylsilyloxyfuran to carbonyl compounds: hydrogen-bond catalysis in γ-butenolides synthesis. Tetrahedron Lett. 2006, 47, 8507–8510. [Google Scholar] [CrossRef]
- Smith, C.J.; Abbanat, D.; Bernan, V.S.; Maiese, W.M.; Greenstein, M.; Jompa, J.; Tahir, A.; Ireland, C.M. Novel Polyketide Metabolites from a Species of Marine Fungi. J. Nat. Prod. 2000, 63, 142–145. [Google Scholar] [CrossRef]
- Schuyler, A.; Caufield, C.E.; Hu, W.; Keeney, D.; Labthavikul, P.; Morris, K.; Naughton, S.M.; Petersen, P.J.; Rasmussen, B.A.; Singh, G.; Yang, Y. Pulvinones as bacterial cell wall biosynthesis inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 176–180. [Google Scholar] [CrossRef]
- Li, R.T.; Han, Q.B.; Zheng, Y.T.; Wang, R.R.; Yang, L.M.; Lu, Y.; Sang, S.Q.; Zheng, Q.T.; Zhao, Q.S.; Sun, H.D. Structure and anti-HIV activity of micrandilactones B and C, new nortriterpenoids possessing a unique skeleton from Schisandra micrantha. Chem. Comm. 2005, 2936–2938. [Google Scholar]
- Dalpiaz, A.; Pavan, B.; Scaglianti, M.; Vitali, F.; Bortolotti, F.; Biondi, C.; Scatturin, A.; Manfredini, S. Vitamin C and 6-amino-vitamin C conjugates of diclofenac: synthesis and evaluation. Int. J. Pharm. 2005, 291, 171–181. [Google Scholar] [CrossRef]
- Pelter, A.; Al-Bayati, R.I.H.; Ayoub, M.T.; Lewis, W.; Pardasani, P.; Hänsel, R. Synthetic Routes to the Piperolides, Fadyenolides, Epoxypiperolides, and Related Compounds. J. Chem. Soc. Perkin Trans. 1 1987, 717–742. [Google Scholar]
- Boukouvalas, J.; Maltais, F. An efficient total synthesis of antibiotic patulin. Tetrahedron Lett. 1995, 36, 7175–7176. [Google Scholar] [CrossRef]
- Boukouvalas, J.; Maltais, F. An Efficient Total Synthesis of Neopatulin. Tetrahedron Lett. 1994, 35, 5769–5770. [Google Scholar] [CrossRef]
- Boukouvalas, J.; Maltais, F.; Lachance, N. Furanolate-Based Strategy for Sequential 2,3,4-Trisubstitution of Butenolide: Total Synthesis of Nostoclides I and II. Tetrahedron Lett. 1994, 35, 7897–7900. [Google Scholar]
- Boukouvalas, J.; Lachance, N.; Ouellet, M.; Trudeau, M. Facile Access to 4-aryl-2(5H)-furanones by Suzuki cross coupling: efficient synthesis of rubrolides C and E. Tetrahedron Lett. 1998, 39, 7665–7668. [Google Scholar] [CrossRef]
- By Xu, D.; Sharpless, K.B. Synthesis and stereochemical assignments for goniobutenolides A and B. Tetrahedron Lett. 1994, 35, 4685–4688. [Google Scholar] [CrossRef]
- Von der Ohe, F.; Brückner, R. Z- or E-configurated γ-alkylidenebutenolides from a 2-(trimethylsiloxy)furan and iodomethacrolein stereoselective synthesis of Z- and E-freelingyne. Tetrahedron Lett. 1998, 39, 1909–1910. [Google Scholar] [CrossRef]
- Brown, S.P.; Goodwin, N.C.; MacMillan, D.W.C. The first enantioselective organocatalytic Mukaiyama-Michael reaction: a direct method for the synthesis of enantioenriched γ-butenolide architecture. J. Am. Chem. Soc. 2003, 125, 1192–1194. [Google Scholar] [CrossRef]
- Cafeo, G.; Kohnke, F.H.; White, A.J.P.; Garozzo, D.; Messina, A. Syntheses, structure, and anion-binding properties of two novel calix[2]benzo[4]pyrroles. Chem. Eur. J 2007, 13, 649–656. [Google Scholar] [CrossRef]
- Bruno, G.; Cafeo, G.; Kohnke, F.H.; Nicolò, F. Tuning the anion binding properties of calixpyrroles by means of p-nitrophenyl substituents at their meso-positions. Tetrahedron 2007, 63, 10003–10010. [Google Scholar] [CrossRef]
- Kruger, J.; Carreira, E.M. Apparent catalytic generation of chiral metal enolates: Enantioselective dienolate additions to aldehydes mediated by Tol-BINAP.Cu(II)fluoride complexes. J. Am. Chem. Soc. 1998, 120, 837–838. [Google Scholar] [CrossRef]
- Szlosek, M.; Figadére, B. Highly enantioselective aldol reaction with 2-trimethylsiloxyfuran: the first catalytic asymmetric autoinductive aldol reaction. Angew. Chem. Int. Ed. 2000, 39, 1799–1801. [Google Scholar] [CrossRef]
- Reinhoudt, D.N.; Crego-Calama, M. Synthesis Beyond the Molecule. Science 2002, 295, 2403–2407. [Google Scholar] [CrossRef]
- Acocella, M.R.; De Rosa, M.; Massa, A.; Palombi, L.; Villano, R.; Scettri, A. Silicon tetrachloride in organic synthesis: new applications for the vinylogous aldol reaction. Tetrahedron 2005, 61, 4091–4097. [Google Scholar] [CrossRef]
- Brown, D.W.; Campbell, M.M.; Taylor, A.P.; Zhang, X.A. Regio- and diastereoselectivity in aldol reactions of cyclopent-2-enone, 2-(5H)furanone and their derived trimethylsilyloxydienes. Tetrahedron Lett. 1987, 28, 985–988. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds of interest are available from the authors.
© 2009 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cafeo, G.; De Rosa, M.; Kohnke, F.H.; Soriente, A.; Talotta, C.; Valenti, L. Calixpyrrole Derivatives: “Multi Hydrogen Bond” Catalysts for γ-Butenolide Synthesis. Molecules 2009, 14, 2594-2601. https://doi.org/10.3390/molecules14072594
Cafeo G, De Rosa M, Kohnke FH, Soriente A, Talotta C, Valenti L. Calixpyrrole Derivatives: “Multi Hydrogen Bond” Catalysts for γ-Butenolide Synthesis. Molecules. 2009; 14(7):2594-2601. https://doi.org/10.3390/molecules14072594
Chicago/Turabian StyleCafeo, Grazia, Margherita De Rosa, Franz H. Kohnke, Annunziata Soriente, Carmen Talotta, and Luca Valenti. 2009. "Calixpyrrole Derivatives: “Multi Hydrogen Bond” Catalysts for γ-Butenolide Synthesis" Molecules 14, no. 7: 2594-2601. https://doi.org/10.3390/molecules14072594