Production of β-Maltooligosaccharides of α- and δ-Tocopherols by Klebsiella pneumoniae and Cyclodextrin Glucanotransferase as Anti-Allergic Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Production of the β-glycosides of α- and δ-tocopherols
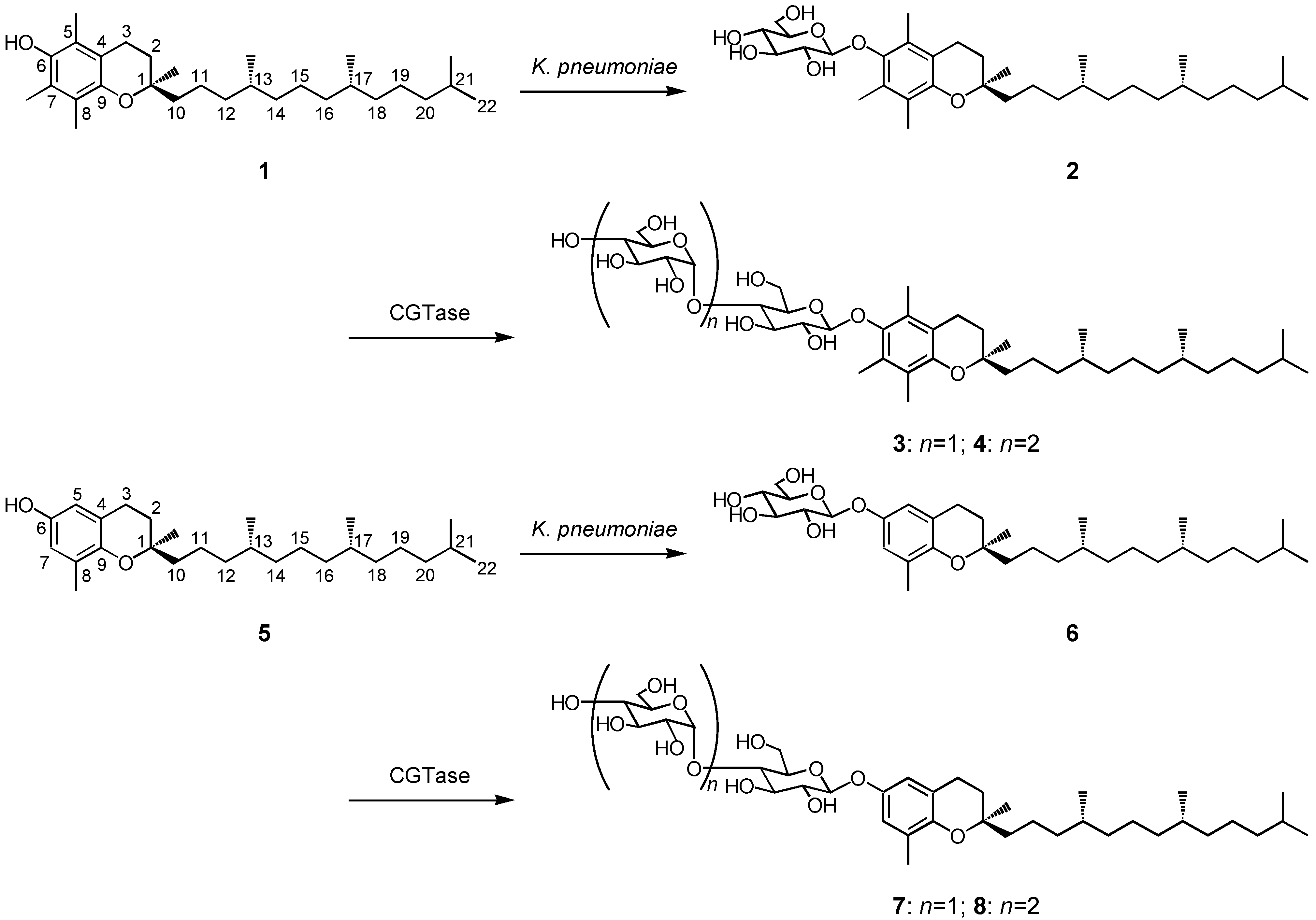
2.2. Anti-allergic activity of β-glycosides of α- and δ-tocopherols
| Compound | IgE levela |
|---|---|
| None | 384.0 ± 128.0 |
| 1 | 146.3 ± 44.8** |
| 2 | 149.3 ± 47.7** |
| 3 | 298.7 ± 95.4 |
| 4 | 320.0 ± 143.1 |
| 5 | 128.0 ± 64.0** |
| 6 | 138.7 ± 57.4** |
| 7 | 170.7 ± 60.3* |
| 8 | 309.3 ± 158.6 |
| Hydrocortisone | 341.3 ± 120.6 |
| Compound | Histamine release (%)a |
|---|---|
| None | 41 |
| 1 | 14 |
| 2 | 17 |
| 3 | 40 |
| 4 | 42 |
| 5 | 10 |
| 6 | 15 |
| 7 | 24 |
| 8 | 39 |
3. Experimental
3.1. General
3.2. Bacterial strain and culture conditions
3.3. Bacterial glucosylation of tocopherols
3.4. Production of tocopheryl β-maltooligosaccharides by CGTase
3.5. Suppressive action on IgE antibody formation
3.6. Inhibitory action on histamine release from rat peritoneal mast cells
4. Conclusions
Acknowledgements
References and Notes
- Evans, H.M.; Bishop, K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56, 650–651. [Google Scholar]
- Packer, L. Vitamin E is nature’s master antioxidant. Sci. Am. Sci. Med. 1994, 1, 54–63. [Google Scholar]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Rimm, E.R.; Stampfer, M.J.; Ascherio, A.; Giovannucci, E.; Colditz, G.A.; Willett, W.C. Vitamin E consumption and the risk of coronary heart disease in men. N. Engl. J. Med. 1993, 328, 1450–1456. [Google Scholar] [CrossRef]
- Stampfer, M.; Hennekens, C.; Manson, J.; Colditz, G.; Rosner, B.; Willett, W. Vitamin E consumption and the risk of coronary disease in women. N. Engl. J. Med. 1993, 328, 1444–1449. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar]
- Engin, K.N. Alpha-tocopherol: looking beyond an antioxidant. Mol. Vis. 2009, 15, 855–860. [Google Scholar]
- Ju, J.; Hao, X.; Lee, M.J.; Lambert, J.D.; Lu, G.; Xiao, H.; Newmark, H.L.; Yang, C.S. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev. Res. 2009, 2, 143–152. [Google Scholar] [CrossRef]
- Rizzo, M.R.; Abbatecola, A.M.; Barbieri, M.; Vietri, M.T.; Cioffi, M.; Grella, R.; Molinari, A.; Forsey, R.; Powell, J.; Paolisso, G. Evidence for anti-inflammatory effects of combined administration of vitamin E and C in older persons with impaired fasting glucose: Impact on insulin action. J. Am. Coll. Nutr. 2008, 27, 505–511. [Google Scholar] [CrossRef]
- Satoh, T.; Miyataka, H.; Yamamoto, K.; Hirano, T. Synthesis and physiological activity of novel tocopheryl glycosides. Chem. Pharm. Bull. 2001, 49, 948–953. [Google Scholar] [CrossRef]
- Uhrig, R.K.; Picard, M.A.; Beyreuther, K.; Wiessler, M. Synthesis of antioxidative and anti-inflammatory drugs glucoconjugates. Carbohydr. Res. 2000, 325, 72–80. [Google Scholar] [CrossRef]
- Sanford, P.A. Exocellular microbial polysaccharides. Adv. Carbohydr. Chem. Biochem. 1979, 36, 265–313. [Google Scholar]
- Welman, A.D.; Maddox, I.S. Fermentation performance of an exopolysaccharide-producing strain of Lactobacillus delbrueckii subsp. bulgaricus. J. Ind. Microbiol. Biotechnol. 2003, 30, 661–668. [Google Scholar] [CrossRef]
- Faria, S.; Vieira, P.A.; Resende, M.M.; Franca, F.P.; Cardose, V.L. A comparison between shaker and bioreactor performance based on the kinetic parameters of xanthan gum production. Appl. Biochem. Biotechnol. 2009, 156, 475–488. [Google Scholar]
- Lahmann, M.; Thiem, J. Synthesis of α-tocopheryl oligosaccharides. Carbohydr. Res. 1997, 299, 23–31. [Google Scholar] [CrossRef]
- Shimoda, K.; Hamada, H.; Hamada, H. Chemo-enzymatic synthesis of ester-linked taxol-oligosaccharide conjugates as potential prodrugs. Tetrahedron Lett. 2008, 49, 601–604. [Google Scholar] [CrossRef]
- Koda, A.; Miura, T.; Inagaki, N.; Sakamoto, O.; Arimura, A.; Nagai, H.; Mori, H. A method for evaluating anti-allergic drugs by simultaneously induced passive cutaneous anaphylaxis and mediator cutaneous reactions. Int. Arch. Allergy. Appl. Immunol. 1990, 92, 209–216. [Google Scholar] [CrossRef]
- Akagi, M.; Katakuse, Y.; Fukuishi, N.; Kan, T.; Akagi, R. Superoxide anion-induced histamine release from rat peritoneal mast cells. Biol. Pharm. Bull. 1994, 17, 732–734. [Google Scholar] [CrossRef]
- Mavon, A.; Raufast, V.; Redoules, D. Skin absorption and metabolism of a new vitamin E prodrug, δ-tocopherol-glucoside: In vitro evaluation in human skin models. J. Controll. Rel. 2004, 100, 221–231. [Google Scholar] [CrossRef]
- Abo, A.; Boyhan, A.; West, I.; Thrasher, A.G.; Segal, A.W. Reconstitution of neutrophil NADPH oxidase activity in the cell-free system by four components: p67-phox, p47-phox, p21rac1, and cytochrome b-245. J. Biol. Chem. 1992, 267, 16767–16770. [Google Scholar]
- Shimoda, K.; Kondo, Y.; Akagi, M.; Abe, K.; Hamada, H.; Hamada, H. Synthesis of α-tocopheryl disaccharides as potential antiallergic agents. Chem. Lett. 2007, 36, 570–571. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shimoda, K.; Akagi, M.; Hamada, H. Production of β-Maltooligosaccharides of α- and δ-Tocopherols by Klebsiella pneumoniae and Cyclodextrin Glucanotransferase as Anti-Allergic Agents. Molecules 2009, 14, 3106-3114. https://doi.org/10.3390/molecules14083106
Shimoda K, Akagi M, Hamada H. Production of β-Maltooligosaccharides of α- and δ-Tocopherols by Klebsiella pneumoniae and Cyclodextrin Glucanotransferase as Anti-Allergic Agents. Molecules. 2009; 14(8):3106-3114. https://doi.org/10.3390/molecules14083106
Chicago/Turabian StyleShimoda, Kei, Masaaki Akagi, and Hiroki Hamada. 2009. "Production of β-Maltooligosaccharides of α- and δ-Tocopherols by Klebsiella pneumoniae and Cyclodextrin Glucanotransferase as Anti-Allergic Agents" Molecules 14, no. 8: 3106-3114. https://doi.org/10.3390/molecules14083106




