Targeted Thiazole Orange Derivative with Folate: Synthesis, Fluorescence and in Vivo Fluorescence Imaging
Abstract
:1. Introduction


2. Results and Discussion
2.1. Synthesis
2.2. Thermal analysis
2.3. NMR spectra
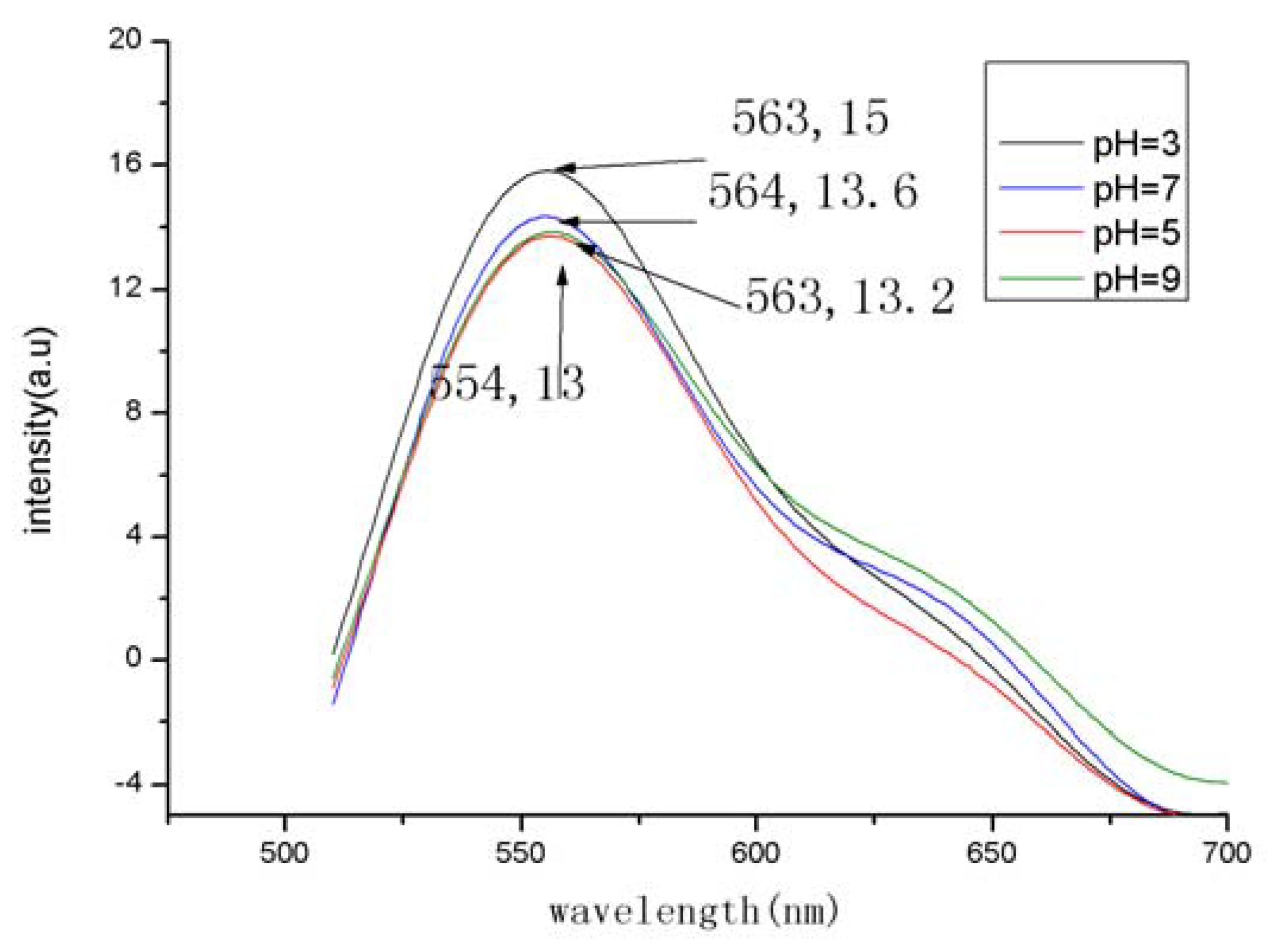
2.4. Fluorescence and ultraviolet properties

2.5. Fluorescence image in vivo

3. Experimental
3.1. General
3.2. Biology
3.2.1. Cell lines
3.2.2. Fluorescent imaging in vivo and biodistribution studies
3.3. Preparation of folate- 1-(3-amidepropyl)-4-methylquinoline bromide (2)
3.4. Preparation of folate-TO (6)
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds 2 and 6 are available from the authors.
References
- Mustroph, H.; Stollenwerk, M.; Bressau, V. Acridizinium-Substituted dendrimers as a new potential rewritable optical data storage material for blu-ray. Angew. Chem., Int. Ed. 2006, 45, 2016–2035. [Google Scholar] [CrossRef]
- Rurack, K.; Kollmannsberger, M.; Daub, J. Molecular switching in the near infrared (NIR) with a functionalized boron-dipyrromethene dye. Angew. Chem. Int. Ed. 2001, 40, 385–387. [Google Scholar] [CrossRef]
- Shah, N.; Cerussi, A.; Eker, C.; Espinoza, J.; Butler, J.; Fishkin, J.; Hornung, R.; Tromberg, B. Noninvasive functional optical spectroscopy of human breast tissue. Proc. Natl. Acad. Sci. USA 2001, 98, 4420–4425. [Google Scholar] [CrossRef]
- Achilefu, S. Lighting up tumors with receptor-specific optical molecular probes. Technol. Cancer. Res. Treat. 2004, 3, 393–409. [Google Scholar]
- Buschmann, V.; Weston, K. D.; Sauer, M. Spectroscopic study and evaluation of red-absorbing fluorescent dyes. Bioconjugate Chem. 2003, 14, 195–204. [Google Scholar] [CrossRef]
- Zen, J. M.; Patonay, G. Near-infrared fluorescence probe for. pH determination. Anal. Chem. 1991, 63, 2934–2938. [Google Scholar] [CrossRef]
- Zhang, Z.; Achilefu, S. Design, synthesis and evaluation of near-infrared fluorescent pH indicators in a physiologically relevant range. Chem. Commun. 2005, 47, 5887–5889. [Google Scholar] [CrossRef]
- Glunde, K.; Guggino, S. E.; Ichikawa, Y.; Bhujwalla, Z. M. A Novel Method of Imaging Lysosomes in Living Human Mammary Epithelial Cells. Mol. Imaging 2003, 2, 24–36. [Google Scholar] [CrossRef]
- Glunde, K.; Foss, C. A.; Takagi, T.; Wildes, F.; Bhujwalla, Z. M. Synthesis of 6'-O-lissamine-rhodamine B-glucosamine as a novel probe for fluorescence imaging of lysosomes in breast tumors. Bioconjugate Chem. 2005, 16, 843–851. [Google Scholar] [CrossRef]
- Nygren, J.; Svanvik, N.; Kubista, M. The interaction between the fluorescent dye thiazole orange and DNA. Biopolymers 1998, 46, 39–51. [Google Scholar] [CrossRef]
- Ross, J. F.; Chaudhuri, P. K.; Ratnam, M. Physiologic and clinical implications. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines: physiologic and clinical implications. Cancer 1994, 73, 2432–2443. [Google Scholar] [CrossRef]
- Toffoli, G.; Cernigoi, C.; Russo, A.; Gallo, A.; Bagnoli, M.; Boiocchi, M. Overexpression of folate binding protein in ovarian cancers. Int. J. Cancer 1997, 74, 193–198. [Google Scholar] [CrossRef]
- Weitman, S. D.; Lark, R. H.; Coney, L. R.; Fort, D.W.; Frasca, V.; Zurawski, V.R., Jr.; Kamen, B.A. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992, 52, 3396–3401. [Google Scholar]
- Singh, P.; Gupta, U.; Asthana, A.; Jain, N. K. Folate and folate-PEG-PAMAM dendrimers: synthesis, characterization, and targeted anticancer drug delivery potential in tumor bearing mice. Bioconjugate Chem. 2008, 19, 2239–2252. [Google Scholar] [CrossRef]
- Garin-Chesa, P.; Campbell, I.; Saigo, P.; Lewis, J.; Old, L.; Rettig, W. Trophoblast and ovarian cancer antigen LK26. Sensitivity and specificity in immunopathology and molecular identification as a folate- binding protein. Am. J. Pathol. 1993, 142, 557–567. [Google Scholar]
- Lu, Y.; Low, P. S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Delivery Rev. 2002, 54, 675–693. [Google Scholar] [CrossRef]
- Kennedy, M. D.; Jallad, K. N.; Thompson, D. H.; Ben-Amotz, D.; Low, P. S. Optical imaging of metastatic tumors using a folate-targeted fluorescent probe . J Biomed. Opt. 2003, 8, 636–641. [Google Scholar] [CrossRef]
- Kim, I. B.; Shin, H.; Garcia, A. J.; Bunz, U. H. F. Use of a folate-PPE conjugate to image cancer cells in vitro. Bioconjugate Chem. 2007, 18, 815–820. [Google Scholar] [CrossRef]
- Fei, X.N.; Yang, S. B.; Zhang, B. L.; Liu, Z. J.; Gu, Y. C. Solid –phase synthesis and modification of Thiazole Orange and its derivatives and their spectral properties. J. Comb. Chem. 2007, 9, 943–950. [Google Scholar] [CrossRef]
- Fei, X.N.; Gu, Y. C.; Liu, Z. J.; Ban, Y.; Zhang, B. L. Thiazole Orange derivatives: Synthesis, fluorescence properties, and labeling cancer cells. Bioorg. Med. Chem. 2009, 17, 585–591. [Google Scholar] [CrossRef]
- Chen, J.; Corbin, I. R.; Li, H.; Cao, W. G.; Glickson, J. D.; Zheng, G. Ligand Conjugated Low-density Lipoprotein Nanoparticles for Enhanced Optical Cancer Imaging in vivo. J. Am. Chem. Soc. 2007, 129, 5798–5799. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fei, X.; Gu, Y.; Wang, Y.; Meng, Q.; Zhang, B. Targeted Thiazole Orange Derivative with Folate: Synthesis, Fluorescence and in Vivo Fluorescence Imaging. Molecules 2010, 15, 6983-6992. https://doi.org/10.3390/molecules15106983
Fei X, Gu Y, Wang Y, Meng Q, Zhang B. Targeted Thiazole Orange Derivative with Folate: Synthesis, Fluorescence and in Vivo Fluorescence Imaging. Molecules. 2010; 15(10):6983-6992. https://doi.org/10.3390/molecules15106983
Chicago/Turabian StyleFei, Xuening, Yingchun Gu, Yiqi Wang, Qingyang Meng, and Baolian Zhang. 2010. "Targeted Thiazole Orange Derivative with Folate: Synthesis, Fluorescence and in Vivo Fluorescence Imaging" Molecules 15, no. 10: 6983-6992. https://doi.org/10.3390/molecules15106983



