One-Pot Synthesis of 2,3,4-Triarylquinolines via Suzuki-Miyaura Cross-Coupling of 2-Aryl-4-chloro-3-iodoquinolines with Arylboronic Acids
Abstract
:1. Introduction
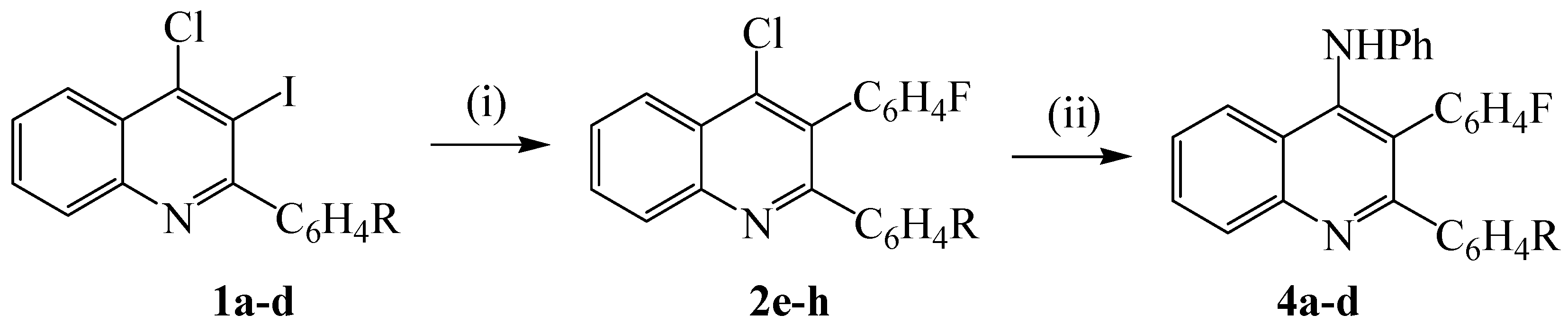
2. Results and Discussion
3. Experimental
3.1. General
3.2. Typical procedure for the one-pot synthesis of 2,3,4-triarylquinolines 2
3.3. Synthesis of 2-aryl-4-chloro-3-(4-fluorophenyl)quinolines 2e-h. typical procedure
3.4. Reaction of 2e-h with aniline. typical procedure
3.5. Hydrolysis of 4 with acetic acid: typical procedure
4. Crystal Structure Solution and Refinement
5. Conclusions
Acknowledgements
References and Notes
- Mphahlele, M.J.; Mtshemla, V. 2-Aryl-4-chloro-3-iodoquinolines as substrates for the synthesis of 2,3-diaryl-4-methoxyquinolines via palladium–catalyzed Suzuki–Miyaura cross–coupling with phenylboronic acid. J. Chem. Res. 2008, 437–440. [Google Scholar] [CrossRef]
- Tsvetkov, A.V.; Latyshev, G.V.; Lukashev, N.V.; Beletskaya, I.P. The successive substitution of halogens in 4-chloro-6-iodoquinoline by aryl groups in cross-coupling reactions with arylboronic acids. Tetrahedron Lett. 2002, 43, 7270. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Tsvetkov, A.V.; Latyshev, G.V.; Lukashev, N.V. Successive replacement of halogen atoms in 4,6-dihaloquinolines in cross-coupling reactions with arylboronic acids catalyzed by palladium and nickel complexes. Russ. J. Org. Chem. 2003, 39, 1660–1667. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Latyshev, A.V.; Tsvetkov, A.V.; Lukashev, N.V. The chemoselective alkynylation of dihaloquinolines by the Sonogashira-Hagihara reaction. Russ. Chem. Bull. 2004, 53, 189–193. [Google Scholar] [CrossRef]
- Reddy, E.A.; Islam, A.; Mukkanti, K.; Bandameedi, V.; Bhowmik, D.R.; Pal, M. Regioselective alkynylation followed by Suzuki coupling of 2,4-dichloroquinoline: synthesis of 2-alkynyl-4-arylquinolines. Beil. J. Org. Chem. 2009, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Grushin, V.V.; Alper, H. Transformations of chloroarenes, catalyzed by transition-metal complexes. Chem. Rev. 1994, 94, 1047–1062. [Google Scholar] [CrossRef]
- Amatore, C.; Jutand, A. Mechanistic and kinetic studies of palladium catalytic systems. J. Organomet. Chem. 1999, 576, 254–278. [Google Scholar] [CrossRef]
- Haman, B.C.; Hartwig, J.F. Sterically hindered chelating alkyl phosphines provide large rate accelerations in palladium-catalyzed amination of aryl iodides, bromides, and chlorides, and the first amination of aryl tosylates. J. Am. Chem. Soc. 1998, 120, 7369–7370. [Google Scholar] [CrossRef]
- Itoh, T. Mase, T. Direct synthesis of hetero-biaryl compounds containing an unprotected NH2 group via Suzuki-Miyaura reaction. Tetrahedron Lett. 2005, 46, 3573–3577. [Google Scholar] [CrossRef]
- Ghodsi, R.; Zarghi, A.; Daraei, B.; Hedayati, M. Design, synthesis and biological evaluation of new 2,3-diarylquinoline derivatives as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. 2010, 18, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.T.; Cohn, S.T.; Manas, E.S.; Harris, W.A.; Mewshaw, R.E. ERβ ligands. Part 4: Synthesis and structure-activity relationships of a series of 2-phenylquinoline derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 4520–4525. [Google Scholar] [CrossRef] [PubMed]
- Rotzoll, S.; Willy, B.; Schönhaber, J.; Rominger, F.; Müller, T.J.J. Regiospecific three-component access to fluorescent 2,4-disubstituted quinolines via one-pot coupling-addition-cyclocondensation-sulfur extrusion sequence. Eur. J. Org. Chem. 2010, 3516–3524. [Google Scholar] [CrossRef]
- CCDC783993 contains the cif file and the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- Li, L.; Wuang, H.K.; Kou, S.C.; Lednicer, D.; Lin, C.M.; Hamel, E.; Lee, K.H. Antitumor agents. 150. 2’,3’,4’,5’,5,6,7-substituted 2-phenyl-4-quinolones and related compounds: Their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J. Med. Chem. 1994, 37, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Hajeri, M.; Peiller, E.; Beney, C.; Deka, D.; Lawson, M.A.; Dumontet, C.; Boumendjel, A. Antimitotic activity of 5-hydroxy-7-methoxy-2-phenyl-4-quinolones. J. Med. Chem. 2004, 47, 4964–4970. [Google Scholar] [CrossRef] [PubMed]
- Raynes, K.J.; Stocks, P.A.; O’Neill, P.M.; Park, B.K.; Ward, S.A. New 4-aminoquinoline Mannich base antimalarials. 1. Effect of an alkyl substituent in the 5’-position of the 4’-hydroxyanilino side chain. J. Med. Chem. 1999, 42, 2747–2751. [Google Scholar] [CrossRef] [PubMed]
- Solomon, R.V.; Puri, S.K.; Srivastava, K.; Katti, S.B. Design and synthesis of new antimalarial agents from 4-aminoquinoline. Bioorg. Med. Chem. 2005, 13, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P.M.; Ward, S.A.; Berry, N.G.; Jeyadevan, J.P.; Biagini, G.A.; Asadollaly, E.; Park, B.K.; Bray, P.G. A medicinal chemistry perspective on 4-aminoquinoline antimalarial drugs. Curr. Top. Med. Chem. 2006, 6, 479–507. [Google Scholar] [PubMed]
- Green, N.; Hu, Y.; Janz, K.; Li, H-Q.; Kaila, N.; Guler, S.; Thomason, J.; Joseph-McCarthy, D.; Tam, S.Y.; Hotchandani, R.; Wu, J.; Huang, A.; Wang, Q.; Leung, L.; Pelker, J.; Marusic, S.; Hsu, S.; Telliez, J-B.; Hall, J.P.; Cuozzo, J.W.; Lin, L-L. Inhibitors of tumor progression loci-2 (Tpl2) kinase and tumor necrosis factor α (TNF-α) production: Selectivity and in vivo antiinflammatory activity of novel 8-substituted-4-anilino-6-aminoquinoline-3-carbonitriles. J. Med. Chem. 2007, 50, 4728–4745. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.C.; Watson, E.J.; Ebetino, F.F.; Lougheed, G.; Stevenson, B.F.; Winterstein, A. ; Bickerton., R.K.; Halliday, R.P.; Pals, D.T. Synthesis and hypotensive properties of new 4-aminoquinolines. J. Med. Chem. 1971, 14, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Pinard, E.; Alanine, A.; Bourson, A.; Bü, *!!! REPLACE !!!*; ttelmann, B.; Heitz, M-P.; Mutel, V.; Gill, R.; Trube, G.; Wyler, R. 4-Aminoquinolines as a novel class of NR1/2B subtype selective NMDA receptor antagonists. Bioorg. Med. Chem. Lett. 2002, 12, 2615–2619. [Google Scholar] [CrossRef]
- Mphahlele, M.J.; Nwamadi, M.S.; Mabeta, P. Synthesis and further studies of chemical transformation of the 2-aryl-3-halogenoquinolin-4(1H)-one derivatives. J. Heterocyclic Chem. 2006, 43, 255–260. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, B. Palladium-catalyzed tandem amination reaction for the synthesis of 4-quinolones. Org. Lett. 2010, 12, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, F-I.; Su, H-J.; Shu, C-F.; Luo, L.; Diau, W-G.; Cheng, C-H.; Duan, J-P.; Lee, G-H. Tuning the emission and morphology of cyclometalated iridium complexes and their applications to organic light-emitting diodes. J. Mater. Chem. 2005, 15, 1035–1042. [Google Scholar] [CrossRef]
- Chen, L.; You, H.; Yang, C.; Zhang, X.; Qin, J.; Ma, D. Tuning the saturated red emission: synthesis, electrochemistry and photophysics of 2-arylquinoline based iridium(III) complexes and their application in OLEDs. J. Mater. Chem. 2006, 16, 3332–3339. [Google Scholar] [CrossRef]
- Kimyonok, A.; Wang, X-Y.; Weck, M. Electroluminescent poly(quinoline)s and metalloquinolates. J. Macromol. Sci. Part C: Polym. Rev. 2006, 46, 47–77. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Lu, L.; Alam, M.M. New conjugated polymers with donor-acceptor architectures: synthesis and photophysics of carbazole-quinoline and phenothiazine-quinoline copolymers and oligomers exhibiting large intramolecular charge transfer. Macromolecules 2001, 34, 7315–7324. [Google Scholar] [CrossRef]
- Kim, M.H.; Jin, J-I.; Lee, C.J.; Kim, N.; Park, K.H. Synthesis and characterization of nonlinear optical polymers having quinoline-based chromophores. Bull. Korean Chem. Soc. 2002, 23, 964–970. [Google Scholar]
- Seo, J.H.; Kim, K.K.; Kim, Y.K. New red electrophosphorescent organic light-emitting devices based on Ir(III) complex of 2,3,4-triphenylquinoline. Mol. Cryst. Liq. Cryst. 2008, 491, 194–202. [Google Scholar] [CrossRef]
- Bruker APEX. Version 2009.1-0. Bruker AXS Inc.: Madison, WI, USA, 2005A. [Google Scholar]
- Bruker SAINT+. Version 7.60A. (includes XPREP and SADABS) Bruker AXS Inc.: Madison, WI, USA, 2005B. [Google Scholar]
- Bruker SHELXTL. Version 5.1. (includes XS, XL, XP, XSHELL) Bruker AXS Inc.: Madison, WI, USA, 1999. [Google Scholar]
- Farrugia, L.J. XRDIFF: simulation of x-ray diffraction patterns. J. Appl. Cryst. 1997, 30, 565–566. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Comp | 4-R | Ar | % Yield (3) |
|---|---|---|---|
| a b c d e f g h | H F Cl OMe H F Cl OMe | -C6H5 -C6H5 -C6H5 -C6H5 p-FC6H4- p-FC6H4- p-FC6H4- p-FC6H4- | 59 55 61 58 72 75 62 62 |

| 4-R | % Yield (2) | % Yield (4) |
|---|---|---|
| H F Cl OMe | 60 (e) 55 (f) 61 (g) 79 (h) | 53 (a) 52 (b) 69 (c) 61 (d) |

| Comp | % 4-R | % Yield (5) |
|---|---|---|
| a b c d | H F Cl OMe | 70 70 65 65 |
| Ring | Torsion angles/deg (molecule A) | Torsion angles/deg (molecule B) | ||
|---|---|---|---|---|
| 2-Ar | N(1)-C(1)-C(22)-C(23) | 42.09° | N(2)-C(28)-C(49)-C(50) | 60.22° |
| C(2)-C(1)-C(22)-C(27) | 45.80° | C(29)-C(28)-C(49)-C(54) | 60.07° | |
| 3-Ar | C(1)-C(2)-C(10)-C(11) | 68.03° | C(30)-C(29)-C(37)-C(42) | 68.93° |
| C(3)-C(2)-C(10)-C(15) | 67.27° | C(28)-C(29)-C(37)-C(38) | 66.95° | |
| 4-Ar | C(2)-C(3)-C(16)-C(17) | 68.08° | C(31)-C(30)-C(43)-C(48) | 74.75° |
| C(4)-C(3)-C(16)-C(21) | 68.29° | C(29)-C(30)-C(43)-C(44) | 71.34° | |
| Empirical formula Formula weight Temperature Wavelength Crystal system Space group Unit cell dimensions Volume Z Density (calculated) Absorption coefficient F(000) Crystal size Theta range for data collection Index ranges Reflections collected Independent reflections Completeness to theta = 27.00° Absorption correction Max. and min. transmission Refinement method Data / restraints / parameters Goodness-of-fit on F2 Final R indices [I>2sigma(I)] R indices (all data) Largest diff. peak and hole | C56H38F6N2O 868.88 173(2) K 0.71073 Å Triclinic P-1 a = 10.2571(2) Å α = 103.2890(10)°. b = 13.2887(2) Å β = 99.4540(10)°. c = 16.7681(3) Å γ = 96.9390(10)°. 2164.00(7) Å3 2 1.333 Mg/m3 0.097 mm−1 900 0.44 × 0.37 × 0.37 mm3 1.27 to 27.00°. -13<=h<=13, -16<=k<=16, -21<=l<=21 40665 9440 [R(int) = 0.0484] 100.0 % None 0.9650 and 0.9586 Full-matrix least-squares on F2 9440 / 0 / 588 1.055 R1 = 0.0424, wR2 = 0.1057 R1 = 0.0640, wR2 = 0.1158 0.218 and -0.379 e.Å−3 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mphahlele, M.J.; Mphahlele, M.M. One-Pot Synthesis of 2,3,4-Triarylquinolines via Suzuki-Miyaura Cross-Coupling of 2-Aryl-4-chloro-3-iodoquinolines with Arylboronic Acids. Molecules 2010, 15, 7423-7437. https://doi.org/10.3390/molecules15107423
Mphahlele MJ, Mphahlele MM. One-Pot Synthesis of 2,3,4-Triarylquinolines via Suzuki-Miyaura Cross-Coupling of 2-Aryl-4-chloro-3-iodoquinolines with Arylboronic Acids. Molecules. 2010; 15(10):7423-7437. https://doi.org/10.3390/molecules15107423
Chicago/Turabian StyleMphahlele, Malose Jack, and Mamasegare Mabel Mphahlele. 2010. "One-Pot Synthesis of 2,3,4-Triarylquinolines via Suzuki-Miyaura Cross-Coupling of 2-Aryl-4-chloro-3-iodoquinolines with Arylboronic Acids" Molecules 15, no. 10: 7423-7437. https://doi.org/10.3390/molecules15107423





