Synthesis of Benzofuran Derivatives via Rearrangement and Their Inhibitory Activity on Acetylcholinesterase
Abstract
:1. Introduction

2. Results and Discussion
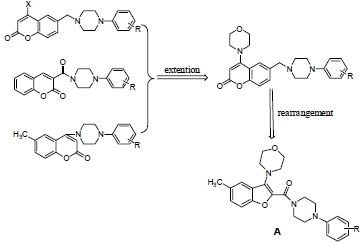
2.1. Chemistry



2.2. Anti-AChE testing
| Compound | AChE inhibition (IC50, μmol/L) a | Compound | AChE inhibition (IC50, μmol/L) a |
|---|---|---|---|
| A1 | 45 ± 0. 3 | A4 | 11 ± 0.2 |
| A2 | 32 ± 0.1 | A5 | 21 ± 0.1 |
| A3 | 73 ± 0. 2 | donepezil | 0.11 ± 0.01 |
3. Conclusions
4. Experimental
4.1. General
4.2. 4-Chloro-6-methylcoumarin (C)
4.3. 6-Methyl-4-morpholinocoumarin (D)
4.4. 3-Bromo-6-methyl-4-morpholinocoumarin (E)
4.5. General synthetic procedure for target compounds A
4.6. In vitro AChE inhibition assay
Acknowledgements
- Samples Availability: Contact the authors.
References and Notes
- Rizzo, S.; Riviere, C.; Piazzi, L.; Bisi, A.; Gobbi, S.; Bartolini, M.; Andrisano, V.; Morroni, F.; Tarozzi, A.; Monti, J. F.; Rampa, A. Benzofuran-based hybrid compounds for the inhibition of cholinesterase activity, beta amyloid aggregation, and abeta neurotoxicity. J. Med. Chem. 2008, 51, 2883–2886. [Google Scholar] [CrossRef]
- Kirilmis, C.; Ahmedzade, M.; Servi, S.; Koca, M.; Kizirgil, A.; Kazaz, C. Synthesis and antimicro- bial activity of some novel derivatives of benzofuran: Part 2. The synthesis and antimicrobial activity of some novel 1-(1-benzofuran-2-yl)-2-mesitylethanone derivatives. Eur. J. Med. Chem. 2008, 43, 300–308. [Google Scholar] [CrossRef]
- Filzen, G.F.; Bratton, L.; Cheng, X.M.; Erasga, N.; Geyer, A.; Lee, C.; Lu, G.; Pulaski, J.; Sorenson, R.J.; Unangst, P.C.; Trivedi, B.K.; Xu, X. Synthesis and SAR of selective benzothiophene, benzofuran, and indole-based peroxisome proliferator-activated receptor δ agonists. Bioorg. Med. Chem. Lett. 2007, 17, 3630–3635. [Google Scholar]
- Dixit, M.; Tripathi, B.K.; Tamrakar, A.K.; Srivastava, A.K.; Kumar, B.; Goel, A. Synthesis of benzofuran scaffold-based potential PTP-1B inhibitors. Bioorg. Med. Chem. 2007, 15, 727–734. [Google Scholar] [CrossRef]
- Nakamura, I.; Mizushima, Y.; Yamagishi, U.; Yamamoto, Y. Synthesis of 2,3-disubstituted benzofurans and indoles by π-Lewis acidic transition metal-catalyzed cyclization of ortho-alkynylphenyl O,O- and N,O-acetals. Tetrahedron 2007, 63, 8670–8676. [Google Scholar] [CrossRef]
- Sakai, N.; Uchida, N.; Konakahara, T. Facile and efficient synthesis of polyfunctionalized benzo-furans: Three-component coupling reactions from an alkynylsilane, an o-hydroxybenzaldehyde derivative, and a secondary amine by a Cu(I)–Cu(II) cooperative catalytic system. Tetrahedron Lett. 2008, 49, 3437–3440. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhang, Y.; Zhang, Y.S.; Herndon, J.W. Synthesis of benzofurans through coupling of dienylacetylenes with carbene complexes: Total synthesis of egonol. Tetrahedron 2003, 59, 5609–5616. [Google Scholar] [CrossRef]
- Sugimoto, H. Donepezil hydrochloride: A treatment drug for Alzheimer's disease. Chem. Rec. 2001, 1, 63–73. [Google Scholar] [CrossRef]
- Ono, M.; Kung, M.P.; Hou, C.; Kung, H.F. Benzofuran derivatives as Abeta-aggregate-specific imaging agents for Alzheimer's disease. Nucl. Med. Biol. 2002, 29, 633–642. [Google Scholar] [CrossRef]
- Allsop, D.; Gibson, G.; Martin, I.K.; Moore, S.; Turnbull, S.; Twymanb, L.J. 3-p-Toluoyl-2-[4'-(3-diethylaminopropoxy)-phenyl]-benzofuran and 2-[4'-(3-diethylaminopropoxy)-phenyl]-benzofuran do not act as surfactants or micelles when inhibiting the aggregation of beta-amyloid peptide. Bioorg. Med. Chem.Lett. 2001, 11, 255–257. [Google Scholar] [CrossRef]
- Lorna, P.; Andrea, C.; Francesco, C.; Federica, B.; Manuela, B.; Francesca, M.; Maurizio, R.; Vincenza, A.; Angela, R. Multi-target-directed coumarin derivatives: hAChE and BACE1 inhibitors as potential anti-Alzheimer compounds. Bioorg. Med. Chem. Lett. 2008, 18, 423–426. [Google Scholar]
- Wang, J.Z.; Wang, Z.F. Role of melatonin in Alzheimer-like neurodegeneration. Acta Pharmacol. Sin. 2006, 1, 41–49. [Google Scholar]
- Walsh, D.M.; Selkoe, D. J. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron 2004, 1, 181–193. [Google Scholar]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- De Ferrari, G.V.; Canales, M.A.; Shin, I.; Weiner, L.M.; Silman, I.; Inestrosa, N.C. A structural mo-tif of acetylcholinesterase that promotes amyloid beta-peptide fibril formation. Biochemistry 2001, 40, 10447–10457. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Alvarez, A.; Perez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer's fibrils: Possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Sugimoto, H.; Yamanishi, Y.; Iimura, Y.; Kawakami, Y. Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr. Med. Chem. 2000, 7, 303–339. [Google Scholar] [CrossRef]
- Kryger, G.; Silman, I.; Sussman, J.L. Three-dimensional structure of a complex of E2020 with acetylcholinesterase from Torpedo californica. J. Physiol. Paris 1998, 92, 191–194. [Google Scholar] [CrossRef]
- Bartolucci, C.; Perola, E.; Pilger, C.; Fels, G.; Lamba, D. Three-dimensional structure of a complex of galanthamine (Nivalin) with acetylcholinesterase from Torpedo californica: Implications for the design of new anti-Alzheimer drugs. Proteins 2001, 42, 182–191. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, X.B.; Wang, T.; Kong, L.Y. Design, synthesis, and acetylcholinesterase inhibitory activity of novel coumarin analogues. Bioorg. Med.Chem. 2008, 16, 8011–8021. [Google Scholar] [CrossRef]
- Zhang, C.E.; Yang, S.M.; Liu, H. The study on the synthesis of 1-(2-methoxyphenyl)piperazine. Appl. Chem. Ind. 2002, 31, 32–34. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, B.; Feartherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhou, X.; Li, M.; Wang, X.-B.; Wang, T.; Kong, L.-Y. Synthesis of Benzofuran Derivatives via Rearrangement and Their Inhibitory Activity on Acetylcholinesterase. Molecules 2010, 15, 8593-8601. https://doi.org/10.3390/molecules15128593
Zhou X, Li M, Wang X-B, Wang T, Kong L-Y. Synthesis of Benzofuran Derivatives via Rearrangement and Their Inhibitory Activity on Acetylcholinesterase. Molecules. 2010; 15(12):8593-8601. https://doi.org/10.3390/molecules15128593
Chicago/Turabian StyleZhou, Xiang, Miao Li, Xiao-Bing Wang, Tao Wang, and Ling-Yi Kong. 2010. "Synthesis of Benzofuran Derivatives via Rearrangement and Their Inhibitory Activity on Acetylcholinesterase" Molecules 15, no. 12: 8593-8601. https://doi.org/10.3390/molecules15128593