Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes
Abstract
:1. Introduction
2. Structural Diversity of Anthocyanins in Red Grapes
2.1. Individual Anthocyanins
2.2. Acetylated Anthocyanins

| Compound | Molecular ion M+(m/z) | Fragment ion M(m/z) | Maxima absorptionwavelengths (nm) |
|---|---|---|---|
| Delphinidin-3-O-monoglucoside | 465 | 303 | 280,523 |
| Cyanidin-3-O-monoglucoside | 449 | 287 | 279,515 |
| Petunidin-3-O-monoglucoside | 479 | 317 | 277,526 |
| Peonidin-3-O-monoglucoside | 463 | 301 | 279,515 |
| Malvidin-3-O-monoglucoside | 493 | 331 | 278,530 |
| Pelargonidin-3-O-monoglucoside | 433 | 271 | 505 |
| Delphinidin-3-O-acetylglucoside | 507 | 303,465 | 280,521 |
| Cyanidin-3-O-acetylglucoside | 491 | 287,449 | 279,514 |
| Petunidin-3-O-acetylglucoside | 521 | 317,479 | 280,530 |
| Peonidin-3-O-acetylglucoside | 505 | 301,463 | 280,518 |
| Malvidin-3-O-acetylglucoside | 535 | 331,493 | 280,521 |
| Delphinidin-3-O-coumarylglucoside | 611 | 303,465 | 282,530 |
| Cyanidin-3-O-coumarylglucoside | 595 | 287,449 | 283,522 |
| Petunidin-3-O-coumarylglucoside | 625 | 317,479 | 280,531 |
| Peonidin-3-O-coumarylglucoside | 609 | 301,463 | 279,523 |
| Malvidin-3-O-coumarylglucoside | 639 | 331,493 | 280,521 |
| Peonidin-3-O-caffeoylglucoside | 625 | 301,463 | 283,525 |
| Petunidin-3-O-caffeoylglucoside | 641 | 317,479 | Unknown |
| Malvidin-3-O-caffeoylglucoside | 655 | 331,493 | 283,538 |
| Malvidin-3-O-feurlylglucoside | 669 | 331,493 | 532 |
| Delphinidin-3,5-O-diglucoside | 627 | 303,465 | 282,520 |
| Cyanidin-3,5-O-diglucoside | 611 | 287,449 | 282,516 |
| Petunidin-3,5-O-diglucoside | 641 | 317,479 | 274,523 |
| Peonidin-3,5-O-diglucoside | 625 | 301,463 | 278,513 |
| Malvidin-3,5-O-diglucoside | 655 | 331,493 | 275,524 |
| Pelargonidin-3,5-O-diglucoside | 595 | 433,271 | Unknown |
| Delphinidin-3-O-acetylglucoside-5-O-glucoside | 669 | 303,465,507 | Unknown |
| Cyanidin-3-O-acetylglucoside-5-O-glucoside | 653 | 287,449,611 | 280,516 |
| Petunidin-3-O-acetylglucoside-5-O-glucoside | 683 | 317,479,641 | 280,530 |
| Malvidin-3-O-acetylglucoside-5-O-glucoside | 697 | 331,493,655,535 | 278,530 |
| Delphinidin-3-O-coumarylglucoside-5-O-glucoside | 773 | 303,465,627,611 | 279,530 |
| Cyanidin-3-O-coumarylglucoside-5-O-glucoside | 757 | 287,449,611,595 | 280,524 |
| Petunidin-3-O-coumarylglucoside-5-O-glucoside | 787 | 317,479,641,625 | 280,530 |
| Peonidin-3-O-coumarylglucoside-5-O-glucoside | 771 | 301,463,625,609 | 279,520 |
| Malvidin-3-O-coumarylglucoside-5-O-glucoside | 801 | 331,493,655,639 | 280,530 |
| Delphinidin-3-O-feruloylglucoside-5-O-glucoside | 803 | 303,465 | Unknown |
| Petunidin-3-O-caffeoylglucoside-5-O-glucoside | 803 | 317,479,641 | Unknown |
2.3. Anthocyanin Derived Pigments

3. Biosynthesis of Anthocyanins in Grapes
3.1. The Basic Flavonoid Upstream Pathway of Anthocyanin Biosynthesis

3.2. Specific Pathway for the Anthocyanin Modification
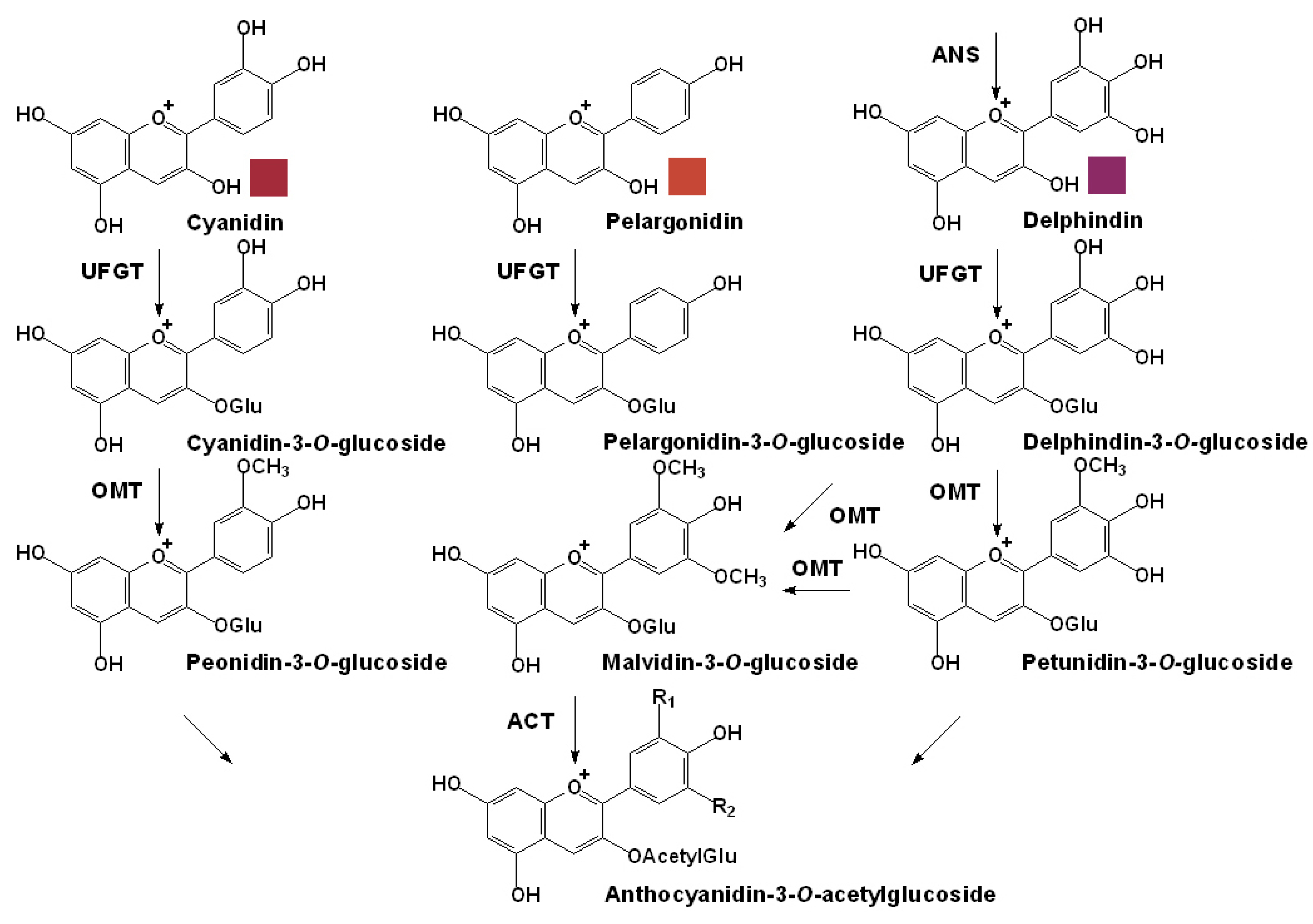
3.3. Transportation and Localization of Anthocyanins
3.4. Degradation of Anthocyanins

4. Regulation of Anthocyanin Biosynthesis in Grapes
4.1. Genetic Regulation of Anthocyanin Biosynthesis
4.2. Influence of Phytohormones on Anthocyanin Accumulation
4.3. Influence of Non-hormone Chemicals of Anthocyanin Biosynthesis
4.4. Influence of Cultivating Conditions on the Anthocyanin Biosynthesis in Grape Cell Suspension
4.5. Environmental Influence on Anthocyanin Biosynthesis of Grapevine
4.6. Influence of Viticulture Practice on Anthocyanin Accumulation of Grapevine
5. Conclusions
Acknowledgements
- Samples Availability: Samples of the compounds in this review are not available from the authors.
References and Notes
- Mazza, G. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis: a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef]
- Holton, T.A.; Cornish, E.C. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar]
- Koes, R.E.; Quattrocchio, F.; Mol, J.N.M. The flavonoid biosynthetic pathway in plants: function and evolution. Bioessays 1994, 16, 123–132. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Noble, A.; Kwiatkowski, M.; Cheynier, V.; Waters, E. Taste and mouth-feel properties of different types of tannin-like polyphenolic compounds and anthocyanins in wine. Anal. Chim. Acta 2004, 513, 57–65. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Dufour, C.; Sauvaitre, I. Interactions between anthocyanins and aroma substances in a model system. Effect on the flavor of grape-derived beverages. J. Agr. Food Chem. 2000, 48, 1784–1788. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Schaefer, H.M.; Schaefer, V.; Levey, D.J. How plant-animal interactions signal new insights in communication. Trends Ecol. Evol. 2004, 19, 577–584. [Google Scholar] [CrossRef]
- Takahama, U. Oxidation of vacuolar and apoplastic phenolic substrates by peroxidases: physiological significance of the oxidation reactions. Phytochem. Rev. 2004, 3, 207–219. [Google Scholar] [CrossRef]
- Bitsch, R.; Netzel, M.; Frank, T.; Strass, G.; Bitsch, I. Bioavailability and biokinetics of anthocyanins from red grape juice and red wine. J. Biomed. Biotechnol. 2004, 293–298. [Google Scholar]
- Lapidot, T.; Harel, S.; Granit, R.; Kanner, J. Bioavailability of red wine anthocyanins as detected in human urine. J. Agr. Food Chem. 1998, 46, 4297–4302. [Google Scholar] [CrossRef]
- Garcia-Alonso, M.; Rimbach, G.; Sasai, M.; Nakahara, M.; Matsugo, S.; Uchida, Y.; Rivas-Gonzalo, J.C.; De Pascual-Teresa, S. Electron spin resonance spectroscopy studies on the free radical scavenging activity of wine anthocyanins and pyranoanthocyanins. Mol. Nutr. Food Res. 2005, 49, 1112–1119. [Google Scholar] [CrossRef]
- Saint-Cricq De Gaulejac, N.; Glories, Y.; Vivas, N. Free radical scavenging effect of anthocyanins in red wines. Food Res. Int. 1999, 32, 327–333. [Google Scholar] [CrossRef]
- Tedesco, I.; Luigi Russo, G.; Nazzaro, F.; Russo, M.; Palumbo, R. Antioxidant effect of red wine anthocyanins in normal and catalase-inactive human erythrocytes. J. Nutr. Biochem. 2001, 12, 505–511. [Google Scholar] [CrossRef]
- Maletić, E.; Kontić, J.K.; Preiner, D.; Jeromel, A.; Patz, C.-D.; Dietrich, H. Anthocyanin profile and antioxidative capacity of some autochthonous Croatian red wines. J. Food Agr. Environ. 2009, 7, 48–51. [Google Scholar]
- Koide, T.; Kamei, H.; Hashimoto, Y.; Kojima, T.; Hasegawa, M. Antitumor effect of hydrolyzed anthocyanin from grape rinds and red rice. Cancer Biother. Radiopharm. 1996, 11, 273–277. [Google Scholar] [CrossRef]
- Jing, P.; Bomser, J.A.; Schwartz, S.J.; He, J.; Magnuson, B.A.; Giusti, M.M. Structure-function relationships of anthocyanins from various anthocyanin-rich extracts on the inhibition of colon cancer cell growth. J. Agr. Food Chem. 2008, 56, 9391–9398. [Google Scholar]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: from plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Mondello, L.; Cotroneo, A.; Errante, G.; Dugo, G.; Dugo, P. Determination of anthocyanins in blood orange juices by HPLC analysis. J. Pharm. Biomed. Anal. 2000, 23, 191–195. [Google Scholar] [CrossRef]
- Camire, M.E.; Chaovanalikit, A.; Dougherty, M.P.; Briggs, J. Blueberry and grape anthocyanins as breakfast cereal colorants. J. Food Sci. 2002, 67, 438–441. [Google Scholar] [CrossRef]
- Goldy, R.G.; Maness, E.P.; Stiles, H.D.; Clark, J.R.; Wilson, M.A. Pigment quantity and quality characteristics of some native Vitis rotundifolia michx. Amer. J. Enol. Viticult. 1989, 40, 253–258. [Google Scholar]
- Lamikanra, O. Anthocyanins of Vitis rotundifolia hybrid grapes. Food Chem. 1989, 33, 225–237. [Google Scholar] [CrossRef]
- Kalbasi, A.; Cisneros-Zevallos, L. Fractionation of monomeric a polymeric anthocyanins from Concord grape (Vitis labrusca L.) juice by membrane ultrafiltration. J. Agr. Food Chem. 2007, 55, 7036–7042. [Google Scholar]
- Choi, J.Y.; Lee, S.J.; Lee, S.J.; Park, S.; Lee, J.H.; Shim, J.-H.; Abd EI-Aty, A.M.; Jin, J.-S.; Jeong, E.-D.; Lee, W.S.; Shin, S.C. Analysis and tentative structure elucidation of new anthocyanins in fruit peel of Vitis coignetiae Pulliat (meoru) using LC-MS/MS: contribution to the overall antioxidant activity. J. Sep. Sci. 2010, 33, 1192–1197. [Google Scholar]
- Kim, M.; Yoon, S.H.; Jung, M.; Choe, E. Stability of meoru (Vitis coignetiea) anthocyanins under photochemically produced singlet oxygen by riboflavin. New Biotechnol. 2010, 27, 435–439. [Google Scholar]
- Zhao, Q.; Duan, C.-Q.; Wang, J. Anthocyanins profile of grape berries of Vitis amurensis, its hybrids and their wines. Int. J. Mol. Sci. 2010, 11, 2212–2228. [Google Scholar] [CrossRef]
- Segade, S.R.; Vázquez, E.S.; Losada, E.D. Influence of ripeness grade on accumulation and extractability of grape skin anthocyanins in different cultivars. J. Food Compos. Anal. 2008, 21, 599–607. [Google Scholar] [CrossRef]
- Conradie, J.D.; Neethling, L.P. A rapid thin-layer chromatographic method for the anthocyanins in grape varieties. J. Chromatogr. A 1968, 34, 419–420. [Google Scholar] [CrossRef]
- Downey, M.O.; Rochfort, S. Simultaneous separation by reversed-phase high-performance liquid chromatography and mass spectral identification of anthocyanins and flavonols in Shiraz grape skin. J. Chromatogr. A 2008, 1201, 43–47. [Google Scholar]
- Hohnová, B.; Šťavíková, L.; Karásek, P. Determination of anthocyanins in red grape skin by pressurised fluid extraction and HPLC. Czech J. Food Sci. 2008, 26, S39–S42. [Google Scholar]
- Degenhardt, A.; Hofmann, S.; Knapp, H.; Winterhalter, P. Preparative isolation of anthocyanins by high-speed countercurrent chromatography and application of the color activity concept to red wine. J. Agr. Food Chem. 2000, 48, 5812–5818. [Google Scholar] [CrossRef]
- Bonerz, D.P.M.; Pour Nikfardjam, M.S.; Creasy, G.L. A new RP-HPLC method for analysis of polyphenols, anthocyanins, and indole-3-acetic acid in wine. Amer. J. Enol. Viticult. 2008, 59, 106–109. [Google Scholar]
- Favretto, D.; Flamini, R. Application of electrospray ionization mass spectrometry to the study of grape anthocyanins. Amer. J. Enol. Viticult. 2000, 51, 55–64. [Google Scholar]
- Grant, D.C.; Helleur, R.J. Rapid screening of anthocyanins in berry samples by surfactant-mediated matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 156–164. [Google Scholar]
- Hayasaka, Y.; Baldock, G.A.; Pollnitz, A.P. Contributions of mass spectrometry in the Australian Wine Research Institute to advances in knowledge of grape and wine constituents. Aust. J. Grape Wine Res. 2005, 11, 188–204. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, Ma.d.L.; Páez-Hernández, Ma.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: a review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Sáenz-López, R.; Fernández-Zurbano, P.; Tena, M.T. Development and validation of a capillary zone electrophoresis method for the quantitative determination of anthocyanins in wine. J. Chromatogr. A. 2003, 990, 247–258. [Google Scholar] [CrossRef]
- Calvo, D.; Sáenz-López, R.; Fernández-Zurbano, P.; Tena, M.T. Migration order of wine anthocyanins in capillary zone electrophoresis. Anal. Chim. Acta 2004, 524, 207–213. [Google Scholar] [CrossRef]
- Janik, L.J.; Cozzolino, D.; Dambergs, R.; Cynkar, W.; Gishen, M. The prediction of total anthocyanin concentration in red-grape homogenates using visible-near-infrared spectroscopy and artificial neural networks. Anal. Chim. Acta 2007, 594, 107–118. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins - a final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar]
- Xie, D.Y.; Dixon, R.A. Proanthocyanidin biosynthesis - still more questions than answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef]
- Tian, L.; Pang, Y.; Dixon, R.A. Biosynthesis and genetic engineering of proanthocyanidins and (iso)flavonoids. Phytochem. Rev. 2007, 1–21. [Google Scholar]
- Bordignon-Luiz, M.T.; Gauche, C.; Gris, E.F.; Falcão, L.D. Colour stability of anthocyanins from Isabel grapes (Vitis labrusca L.) in model systems. Food Sci. Technol. 2007, 40, 594–599. [Google Scholar]
- Dixon, R.A.; Steele, C.L. Flavonoids and isoflavonoids - a gold mine for metabolic engineering. Trends Plant Sci. 1999, 4, 394–400. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science Principle and Applications, 3rd ed; Academic Press: Burlington, VT, USA, 2008; pp. 287–295. [Google Scholar]
- Andersen, Ø.M.; Jordheim, M. The anthocyanins. In Flavonoids: Chemistry, Biochemistry, and Applications; Anderson, Ø.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 471–553. [Google Scholar]
- Saito, N.; Tatsuzawa, F.; Miyoshi, K.; Shigihara, A.; Honda, T. The first isolation of C-glycosylanthocyanin from the flowers of Tricyrtis formosana. Tetrahedron Lett. 2003, 44, 6821–6823. [Google Scholar] [CrossRef]
- Ford, C.M.; Boss, P.K.; Hoj, P.B. Cloning and characterization of Vitis vinifera UDP-glucose:flavonoid 3-O-glucosyltransferase, a homologue of the enzyme encoded by the maize Bronze-1 locus that may primarily serve to glucosylate anthocyanidins in vivo. J Biol. Chem. 1998, 273, 9224–9233. [Google Scholar] [CrossRef]
- Jánváry, L.; Hoffmann, T.; Pfeiffer, J.; Hausmann, L.; Töpfer, R.; Fischer, T.C.; Schwab, W. A double mutation in the anthocyanin 5-O-glucosyltransferase gene disrupts enzymatic activity in Vitis vinifera L. J. Agr. Food Chem. 2009, 57, 3512–3518. [Google Scholar]
- Clifford, M.N. Anthocyanins - nature, occurrence and dietary burden. J. Sci. Food Agr. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Fernández-González, M.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Red-color related phenolic composition of Garnacha Tintorera (Vitis vinifera L.) grapes and red wines. J. Agr. Food Chem. 2009, 57, 7883–7891. [Google Scholar]
- He, F.; He, J.-J.; Pan, Q.-H.; Duan, C.-Q. Mass-spectrometry evidence confirming the presence of pelargonidin-3-O-glucoside in the berry skins of Cabernet Sauvignon and Pinot Noir (Vitis vinifera L.). Aust. J. Grape Wine Res. 2010, 16, 464–468. [Google Scholar] [CrossRef]
- González-Manzano, S.; Santos-Buelga, C.; Dueñas, M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, T. Colour implications of self-association processes of wine anthocyanins. Eur. Food Res. Technol. 2008, 226, 483–490. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: a critical review. Amer. J. Enol. Viticult. 2001, 52, 67–87. [Google Scholar]
- Rentzsch, M.; Schwarz, M.; Winterhalter, P. Pyranoanthocyanins - an overview on structures, occurrence, and pathways of formation. Trends Food Sci. Technol. 2007, 18, 526–534. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Identification of dimeric anthocyanins and new oligomeric pigments in red wine by means of HPLC-DAD-ESI/MSn. J. Mass Spectrom. 2007, 42, 735–748. [Google Scholar] [CrossRef]
- Pati, S.; Losito, I.; Gambacorta, G.; La Notte, E.; Palmisano, F.; Zambonin, P.G. Simultaneous separation and identification of oligomeric procyanidins and anthocyanin-derived pigments in raw red wine by HPLC-UV-ESI-MSn. J. Mass Spectrom. 2006, 41, 861–871. [Google Scholar] [CrossRef]
- García-Beneytez, E.; Cabello, F.; Revilla, E. Analysis of grape and wine anthocyanins by HPLC-MS. J. Agr. Food Chem. 2003, 51, 5622–5629. [Google Scholar] [CrossRef]
- Gueffroy, D.E.; Kepner, R.E.; Webb, A.D. Acylated anthocyanin pigments in Vitis vinifera grapes: Identification of malvidin-3-(6-p-coumaroyl) glucoside. Phytochemistry 1971, 10, 813–819. [Google Scholar]
- Anderson, D.W.; Gueffroy, D.E.; Webba, A.D.; Kepner, R.E. Identification of acetic acid as an acylating agent of anthocyanin pigments in grapes. Phytochemistry 1970, 9, 1579–1583. [Google Scholar]
- Tamura, H.; Hayashi, Y.; Sugisawa, H.; Kondo, T. Structure determination of acylated anthocyanins in Muscat Bailey a grapes by homonuclear Hartmann-Hahn (HOHAHA) spectroscopy and liquid chromatography-mass spectrometry. Phytochem. Anal. 1994, 5, 190–196. [Google Scholar] [CrossRef]
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, phenolics, and color of Cabernet Franc, Merlot, and Pinot Noir wines from British Columbia. J. Agr. Food Chem. 1999, 47, 4009–4017. [Google Scholar]
- Boss, P.K.; Davies, C.; Robinson, S.P. Anthocyanin composition and anthocyanin pathway gene expression in grapevine sports differing in berry skin colour. Aust. J. Grape Wine Res. 1996, 2, 163–170. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, B.; Fan, P.; Yang, C.; Duan, W.; Zheng, X.; Liu, C.; Li, S. Anthocyanin composition and content in grape berry skin in Vitis germplasm. Food Chem. 2008, 111, 837–844. [Google Scholar] [CrossRef]
- Flamini, R.; De Rosso, M.; Smaniotto, A.; Panighel, A.; Vedova, A.D.; Seraglia, R.; Traldi, P. Fast analysis of isobaric grape anthocyanins by Chip-liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 2891–2896. [Google Scholar] [CrossRef]
- Wang, H.; Race, E.J.; Shrikhande, A. Characterization of anthocyanins in grape juices by ion trap liquid chromatography−mass spectrometry. J. Agr. Food Chem. 2003, 51, 1839–1844. [Google Scholar]
- Romero, C.; Bakker, J. Effect of acetaldehyde and several acids on the formation of vitisin A in model wine anthocyanin and colour evolution. Int. J. Food Sci. Technol. 2000, 35, 129–140. [Google Scholar] [CrossRef]
- Morata, A.; Calderón, F.; González, M.C.; Gómez-Cordovés, M.C.; Suárez, J.A. Formation of the highly stable pyranoanthocyanins (vitisins A and B) in red wines by the addition of pyruvic acid and acetaldehyde. Food Chem. 2007, 100, 1141–1152. [Google Scholar]
- Springob, K.; Nakajima, J.; Yamazaki, M.; Saito, K. Recent advances in the biosynthesis and accumulation of anthocyanins. Nat. Prod. Rep. 2003, 20, 288–303. [Google Scholar] [CrossRef]
- Gollop, R.; Farhi, S.; Perl, A. Regulation of the leucoanthocyanidin dioxygenase gene expression in vitis vinifera. Plant Sci. 2001, 161, 579–588. [Google Scholar] [CrossRef]
- Fujita, A.; Soma, N.; Goto-Yamamoto, N.; Shindo, H.; Kakuta, T.; Koizumi, T.; Hashizume, K. Anthocyanidin reductase gene expression and accumulation of flavan-3-ols in grape berry. Amer. J. Enol. Viticult. 2005, 56, 336–342. [Google Scholar]
- Gargouri, M.; Manigand, C.; Mauge´, C.; Granier, T.; Langlois d'Estaintot, B.; Cala, O.; Pianet, I.; Bathany, K.; Chaudiere, J.; Gallois, B. Structure and epimerase activity of anthocyanidin reductase from Vitis vinifera. Acta Crystallogr. D. Biol. Crystallogr. 2009, 65, 989–1000. [Google Scholar] [CrossRef]
- Sparvoli, F.; Martin, C.; Scienza, A.; Gavazzi, G.; Tonelli, C. Cloning and molecular analysis of structural genes involved in flavonoid and stilbene biosynthesis in grape (Vitis vinifera L.). Plant Mol. Biol. 1994, 24, 743–755. [Google Scholar] [CrossRef]
- Boss, P.K.; Davies, C.; Robinson, S.P. Analysis of the expression of anthocyanin pathway genes in developing Vitis vinifera L. cv Shiraz grape berries and the implications for pathway regulation. Plant Physiol. 1996, 111, 1059–1066. [Google Scholar]
- Fung, R.W.M.; Qiu, W.; Su, Y.; Schachtman, D.P.; Huppert, K.; Fekete, C.; Kovács, L.G. Gene expression variation in grapevine species Vitis vinifera L. and Vitis aestivalis Michx. Genet. Resour. Crop Evol. 2007, 54, 1541–1553. [Google Scholar] [CrossRef]
- Ramazzotti, S.; Filippetti, I.; Intrieri, C. Expression of genes associated with anthocyanin synthesis in red-purplish, pink, pinkish-green and green grape berries from mutated 'Sangiovese' biotypes: A case study. Vitis 2008, 47, 147–151. [Google Scholar]
- Durbin, M.L.; McCaig, B.; Clegg, M.T. Molecular evolution of the chalcone synthase multigene family in the morning glory genome. Plant Mol. Biol. 2000, 42, 79–92. [Google Scholar] [CrossRef]
- Tian, L.; Kong, W.F.; Pan, Q.H.; Zhan, J.C.; Wen, P.F.; Chen, J.Y.; Wan, S.B.; Huang, W.D. Expression of the chalcone synthase gene from grape and preparation of an anti-CHS antibody. Protein Express. Purif. 2006, 50, 223–228. [Google Scholar] [CrossRef]
- Goto-Yamamoto, N.; Wan, G.H.; Masaki, K.; Kobayashi, S. Structure and transcription of three chalcone synthase genes of grapevine (Vitis vinifera). Plant Sci. 2002, 162, 867–872. [Google Scholar] [CrossRef]
- Ageorges, A.; Fernandez, L.; Vialet, S.; Merdinoglu, D.; Terrier, N.; Romieu, C. Four specific isogenes of the anthocyanin metabolic pathway are systematically co-expressed with the red colour of grape berries. Plant Sci. 2006, 170, 372–383. [Google Scholar] [CrossRef]
- Jez, J.M.; Noel, J.P. Reaction mechanism of chalcone isomerase. pH dependence, diffusion control, and product binding differences. J. Biol. Chem. 2002, 277, 1361–1369. [Google Scholar]
- da Silva, F.G.; Iandolino, A.; Al-Kayal, F.; Bohlmann, M.C.; Cushman, M.A.; Lim, H.; Ergul, A.; Figueroa, R.; Kabuloglu, E.K.; Osborne, C.; Rowe, J.; Tattersall, E.; Leslie, A.; Xu, J.; Baek, J.; Cramer, G.R.; Cushman, J.C.; Cook, D.R. Characterizing the grape transcriptome. Analysis of expressed sequence tags from multiple Vitis species and development of a compendium of gene expression during berry development. Plant Physiol. 2005, 139, 574–597. [Google Scholar] [CrossRef]
- Waters, D.L.; Holton, T.A.; Ablett, E.M.; Lee, L.S.; Henry, R.J. cDNA microarray analysis of developing grape (Vitis vinifera cv. Shiraz) berry skin. Funct. Integr. Genom. 2005, 5, 40–58. [Google Scholar] [CrossRef]
- Bogs, J.; Ebadi, A.; McDavid, D.; Robinson, S.P. Identification of the flavonoid hydroxylases from grapevine and their regulation during fruit development. Plant Physiol. 2006, 140, 279–291. [Google Scholar]
- Jeong, S.T.; Goto-Yamamoto, N.; Hashizume, K.; Esaka, M. Expression of the flavonoid 3’-hydroxylase and flavonoid 3’,5’-hydroxylase genes and flavonoid composition in grape (Vitis vinifera). Plant Sci. 2006, 170, 61–69. [Google Scholar] [CrossRef]
- De´dalde´champ, F.; Uhel, C. Induction of anthocyanin synthesis in nonpigmented grape cell suspensions by acting on DFR substrate availability or precursors level. Enzyme Microb. Technol. 1999, 25, 316–321. [Google Scholar] [CrossRef]
- Pfeiffer, J.; Kühnel, C.; Brandt, J.; Duy, D.; Punyasiri, P.A.N.; Forkmann, G.; Fischer, T.C. Biosynthesis of flavan 3-ols by leucoanthocyanidin 4-reductases and anthocyanidin reductases in leaves of grape (Vitis vinifera L.), apple (Malus x domestica Borkh.) and other crops. Plant Physiol. Biochem. 2006, 44, 323–334. [Google Scholar] [CrossRef]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef]
- Petit, P.; Granier, T.; d'Estaintot, B.L.; Manigand, C.; Bathany, K.; Schmitter, J.M.; Lauvergeat, V.; Hamdi, S.; Gallois, B. Crystal structure of grape dihydroflavonol 4-reductase, a key enzyme in flavonoid biosynthesis. J. Mol. Biol. 2007, 368, 1345–1357. [Google Scholar] [CrossRef]
- Gollop, R.; Even, S.; Colova-Tsolova, V.; Perl, A. Expression of the grape dihydroflavonol reductase gene and analysis of its promoter region. J. Exp. Bot. 2002, 53, 1397–1409. [Google Scholar] [CrossRef]
- Boss, P.K.; Davies, C.; Robinson, S.P. Expression of anthocyanin biosynthesis pathway genes in red and white grapes. Plant Mol. Biol. 1996, 32, 565–569. [Google Scholar] [CrossRef]
- Do, C.B.; Cormier, F.; Nicolas, Y. Isolation and characterization of a UDP-glucose:cyanidin 3-O-glucosyltransferase from grape cell suspension cultures (Vitis vinifera L.). Plant Sci. 1995, 112, 43–51. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ishimaru, M.; Ding, C.K.; Yakushiji, H.; Goto, N. Comparison of UDP-glucose:flavonoid 3-O-glucosyltransferase (UFGT) gene sequences between white grapes (Vitis vinifera) and their sports with red skin. Plant Sci. 2001, 160, 543–550. [Google Scholar] [CrossRef]
- Offen, W.; Martinez-Fleites, C.; Yang, M.; Kiat-Lim, E.; Davis, B.G.; Tarling, C.A.; Ford, C.M.; Bowles, D.J.; Davies, G.J. Structure of a flavonoid glucosyltransferase reveals the basis for plant natural product modification. EMBO J. 2006, 25, 1396–1405. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; Bruneau, A.; Bantignies, B. Plant O-methyltransferases: molecular analysis, common signature and classification. Plant Mol. Biol. 1998, 36, 1–10. [Google Scholar] [CrossRef]
- Bailly, C.; Cormier, F.; Do, C.B. Characterization and activities of S-adenosyl-l-methionine:cyanidin 3-glucoside 3’-O-methyltransferase in relation to anthocyanin accumulation in Vitis vinifera cell suspension cultures. Plant Sci. 1997, 122, 81–89. [Google Scholar] [CrossRef]
- Hugueney, P.; Provenzano, S.; Verrie` s, C.; Ferrandino, A.; Meudec, E.; Batelli, G.; Merdinoglu, D.; Cheynier, V.; Schubert, A.; Ageorges, A. A novel cation-dependent O-methyltransferase involved in anthocyanin methylation in grapevine. Plant Physiol. 2009, 150, 2057–2070. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Nakayama, T.; Yamazaki, M.; Saito, K. Modification and stabilization of anthocyanins. In Anthocyanins Biosynthesis, Functions, and Applications; Gould, K., Davies, K., Winefield, C., Eds.; Springer Science+Business Media, LLC.: New York, NY, USA, 2009; pp. 169–190. [Google Scholar]
- D'Auria, J.C. Acyltransferases in plants: a good time to be BAHD. Curr. Opin. Plant Biol. 2006, 9, 331–340. [Google Scholar] [CrossRef]
- Agati, G.; Traversi, M.L.; Cerovic, Z.G. Chlorophyll fluorescence imaging for the noninvasive assessment of anthocyanins in whole grape (Vitis vinifera L.) bunches. Photochem. Photobiol. 2008, 84, 1431–1434. [Google Scholar] [CrossRef]
- Saslowsky, D.; Winkel-Shirley, B. Localization of flavonoid enzymes in Arabidopsis roots. Plant J. 2001, 27, 37–48. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wen, P.F.; Kong, W.F.; Pan, Q.H.; Wan, S.B.; Huang, W.D. Changes and subcellular localizations of the enzymes involved in phenylpropanoid metabolism during grape berry development. J. Plant Physiol. 2006, 163, 115–127. [Google Scholar] [CrossRef]
- Barceló, A.R.; Calderón, A.A.; Zapata, J.M.; Muñoz, R. The histochemical localization of anthocyanins in seeded and seedless grapes (Vitis vinifera). Sci. Hort. 1994, 57, 265–268. [Google Scholar] [CrossRef]
- Alfenito, M.R.; Souer, E.; Goodman, C.D.; Buell, R.; Mol, J.; Koes, R.; Walbot, V. Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell 1998, 10, 1135–1149. [Google Scholar]
- Mizuno, H.; Hirano, K.; Okamoto, G. Effect of anthocyanin composition in grape skin on anthocyanic vacuolar inclusion development and skin coloration. Vitis 2006, 45, 173–177. [Google Scholar]
- Kitamura, S.; Shikazono, N.; Tanaka, A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 2004, 37, 104–114. [Google Scholar] [CrossRef]
- Marrs, K.A.; Alfenlto, M.R.; Lloyd, A.M.; Walbot, V. A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene Bronze-2. Nature 1995, 375, 397–400. [Google Scholar]
- Mueller, L.A.; Goodman, C.D.; Silady, R.A.; Walbot, V. AN9, a petunia glutathione S-transferase required for anthocyanin sequestration, is a flavonoid-binding protein. Plant Physiol. 2000, 123, 1561–2000. [Google Scholar] [CrossRef]
- Goodman, C.D.; Casati, P.; Walbot, V. A multidrug resistance–associated protein involved in anthocyanin transport in zea mays. Plant Cell 2004, 16, 1812–1826. [Google Scholar] [CrossRef]
- Conn, S.; Curtin, C.; Bézier, A.; Franco, C.; Zhang, W. Purification, molecular cloning, and characterization of glutathione S-transferases (GSTs) from pigmented Vitis vinifera L. cell suspension cultures as putative anthocyanin transport proteins. J. Exp. Bot. 2008, 59, 3621–3634. [Google Scholar] [CrossRef]
- Marinova, K.; Pourcel, L.; Weder, B.; Schwarz, M.; Barron, D.; Routaboul, J.M.; Debeaujon, I.; Klein, M. The Arabidopsis MATE transporter TT12 acts as a vacuolar Flavonoid/H+-Antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 2007, 19, 2023–2038. [Google Scholar] [CrossRef]
- Gomez, C.; Terrier, N.; Torregrosa, L.; Vialet, S.; Alexandre, F.-L.; Verriès, C.; Souquet, J.-M.; Mazauric, J.-P.; Klein, M.; Cheynier, V.; Ageorges, A. Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters. Plant Physiol. 2009, 150, 402–415. [Google Scholar] [CrossRef]
- Hopp, W.; Seitz, H.U. The uptake of acylated anthocyanin into isolated vacuoles from a cell suspension culture of Daucus carota. Planta 1987, 170, 74–85. [Google Scholar] [CrossRef]
- Xu, W.; Shiorim, H.; Kojima, M.; Nozue, M. Primary structure and expression of a 24-kD vacuolar protein (VP24) precursor in anthocyanin-producing cells of sweet potato in suspension culture. Plant Physiol. 2001, 125, 447–455. [Google Scholar] [CrossRef]
- Braidot, E.; Petrussa, E.; Bertolini, A.; Peresson, C.; Ermacora, P.; Loi, N.; Terdoslavich, M.; Passamonti, S.; Macrì, F.; Vianello, A. Evidence for a putative flavonoid translocator similar to mammalian bilitranslocase in grape berries (Vitis vinifera L.) during ripening. Planta 2008, 228, 203–213. [Google Scholar] [CrossRef]
- Tseng, K.-C.; Chang, H.-M.; Wu, J.S.-B. Degradation kinetics of anthocyanin in ethanolic solutions. J. Food Process. Preserv. 2006, 30, 503–514. [Google Scholar] [CrossRef]
- Lopes, P.; Richard, T.; Saucier, C.; Teissedre, P.-L.; Monti, J.-P.; Glories, Y. Anthocyanone A: a quinone methide derivative resulting from Malvidin 3-o-glucoside degradation. J. Agr. Food Chem. 2007, 55, 2698–2704. [Google Scholar]
- He, F.; Pan, Q.-H.; Shi, Y.; Duan, C.-Q. Biosynthesis and genetic regulation of proanthocyanidins in plants. Molecules 2008, 13, 2674–2703. [Google Scholar] [CrossRef]
- Abrahams, S.; Tanner, G.J.; Larkin, P.J.; Ashton, A.R. Identification and biochemical characterization of mutants in proanthocyanidin pathway in Arabidopsis. Plant Physiol. 2002, 130, 561–576. [Google Scholar] [CrossRef]
- Ramsay, N.A.; Walker, A.R.; Mooney, M.; Gray, J.C. Two basic-helix-loop-helix genes (MYC-146 and GL3) from Arabidopsis can activate anthocyanin biosynthesis in a white-flowered Matthiola incana mutant. Plant Mol. Biol. 2003, 52, 679–688. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef]
- Dubos, C.; Le Gourrierec, J.; Baudry, A.; Huep, G.; Lanet, E.; Debeaujon, I.; Routaboul, J.-M.; Alboresi, A.; Weisshaar, B.; Lepiniec, L. MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. Plant J. 2008, 55, 940–953. [Google Scholar] [CrossRef]
- Jin, H.; Cominelli, E.; Bailey, P.; Parr, A.; Mehrtens, F.; Jones, J.; Tonelli, C.; Weisshaar, B.; Martin, C. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J. 2000, 19, 6150–6161. [Google Scholar]
- Chen, Z.-H.; Nimmo, G.A.; Jenkins, G.I.; Nimmo, H.G. BHLH32 modulates several biochemical and morphological processes that respond to Pi starvation in Arabidopsis. Biochem. J. 2007, 405, 191–198. [Google Scholar]
- Deluc, L.; Barrieu, F.; Marchive, C.; Lauvergeat, V.; Decendit, A.; Richard, T.; Carde, J.-P.; Mérillon, J.-M.; Hamdi, S. Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol. 2006, 140, 499–511. [Google Scholar] [CrossRef]
- Deluc, L.; Bogs, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.-M.; Robinson, S.P.; Barrieu, F. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar] [CrossRef]
- Bogs, J.; Jaffé, F.W.; Takos, A.M.; Walker, A.R.; Robinson, S.P. The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol. 2007, 143, 1347–1361. [Google Scholar] [CrossRef]
- Terrier, N.; Torregrosa, L.; Ageorges, A.; Vialet, S.; Verriès, C.; Cheynier, V.; Romieu, C. Ectopic expression of VvMybPA2 promotes proanthocyanidin biosynthesis in grapevine and suggests additional targets in the pathway. Plant Physiol. 2009, 149, 1028–1041. [Google Scholar]
- Matus, J.T.; Aquea, F.; Arce-Johnson, P. Analysis of the grape MYB R2R3 subfamily reveals expanded wine quality-related clades and conserved gene structure organization across Vitis and Arabidopsis genomes. BMC Plant Biol. 2008, 8. art. no. 83. [Google Scholar]
- Kobayashi, S.; Ishimaru, M.; Hiraoka, K.; Honda, C. Myb-related genes of the Kyoho grape (Vitis labruscana) regulate anthocyanin biosynthesis. Planta 2002, 215, 924–933. [Google Scholar] [CrossRef]
- Cutanda-Perez, M.-C.; Ageorges, A.; Gomez, C.; Vialet, S.; Terrier, N.; Romieu, C.; Torregrosa, L. Ectopic expression of VlmybA1 in grapevine activates a narrow set of genes involved in anthocyanin synthesis and transport. Plant Mol. Biol. 2009, 69, 633–648. [Google Scholar] [CrossRef]
- Koshita, Y.; Kobayashi, S.; Ishimaru, M.; Funamoto, Y.; Shiraishi, M.; Azuma, A.; Yakushiji, H.; Nakayama, M. An anthocyanin regulator from grapes, VlmybA1-2, Produces reddish-purple plants. J. Jpn. Soc. Hort.Sci. 2008, 77, 33–37. [Google Scholar] [CrossRef]
- Kobayashi, S.; Goto-Yamamoto, N.; Hirochika, H. Retrotransposon - induced mutations in grape skin color. Science 2004, 304, 982. [Google Scholar]
- This, P.; Lacombe, T.; Cadle-Davidson, M.; Owens, C.L. Wine grape (Vitis vinifera L.) color associates with allelic variation in the domestication gene VvmybA1. Theor. Appl. Genet. 2007, 114, 723–730. [Google Scholar] [CrossRef]
- Kobayashi, S.; Goto-Yamamoto, N.; Hirochika, H. Association of VvmybA1 gene expression with anthocyanin production in grape (Vitis vinifera) skin -color mutants. J. Jpn. Soc. Hort. Sci. 2005, 74, 196–203. [Google Scholar] [CrossRef]
- Jeong, S.T.; Goto-Yamamoto, N.; Hashizume, K.; Kobayashi, S.; Esaka, M. Expression of VvmybA1 gene and anthocyanin accumulation in various grape organs. Amer. J. Enol. Viticult. 2006, 57, 507–510. [Google Scholar]
- Azuma, A.; Kobayashi, S.; Mitani, N.; Shiraishi, M.; Yamasa, M.; Ueno, T.; Kono, A.; Yakushiji, H.; Koshita, Y. Genomic and genetic analysis of Myb-related genes that regulate anthocyanin biosynthesis in grape berry skin. Theor. Appl. Genet. 2008, 117, 1009–1019. [Google Scholar] [CrossRef]
- Walker, A.R.; Lee, E.; Bogs, J.; Mcdavid, D.A.J.; Thomas, M.R.; Robinson, S.P. White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J. 2007, 49, 772–785. [Google Scholar] [CrossRef]
- Yakushiji, H.; Kobayashi, S.; Goto-Yamamoto, N.; Jeong, S.T.; Sueta, T.; Mitani, N.; Azuma, A. A skin color mutation of grapevine, from black-skinned Pinot Noir to white-skinned Pinot Blanc, is caused by delection of the functional VvmybA1 allele. Biosci. Biotechnol. Biochem. 2006, 70, 1506–1508. [Google Scholar] [CrossRef]
- Fournier-Level, A.; Le Cunff, L.; Gomez, C.; Doligez, A.; Ageorges, A.; Roux, C.; Bertrand, Y.; Souquet, J.-M.; Cheynier, V.; This, P. Quantitative genetic bases of anthocyanin variation in grape (Vitis vinifera L. ssp. sativa) berry: a quantitative trait locus to quantitative trait nucleotide integrated study. Genetics 2009, 183, 1127–1139. [Google Scholar]
- Koepefli, J.B.; Thimann, K.V.; Went, F.W. Phytohormones: structure and physiological. J. Biol. Chem. 1983, 122, 763–780. [Google Scholar]
- Kataoka, I.; Sugiura, A.; Utsunomiya, N.; Tomana, T. Effect of abscisic acid and defoliation on anthocyanin accumulation in Kyoho grapes (Vitis vinifera L. × V. labruscana Baily.). Vitis 1982, 21, 325–332. [Google Scholar]
- Hiratsuka, S.; Onodera, H.; Kawai, Y.; Kubo, T.; Itoh, H.; Wada, R. ABA and sugar effects on anthocyanin formation in grape berry cultured in vitro. Sci. Hort. 2001, 90, 121–130. [Google Scholar] [CrossRef]
- Jeong, S.T.; Goto-Yamamoto, N.; Kobayashi, S.; Esaka, M. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci. 2004, 167, 247–252. [Google Scholar] [CrossRef]
- Ban, T.; Ishimaru, M.; Kobayashi, S.; Shiozaki, S.; Goto-Yamamoto, N.; Horiuchi, S. Abscisic acid and 2,4-dichlorophenoxyacetic acid affect the expression of anthocyanin biosynthetic pathway genes in ‘Kyoho’ grape berries. J. Hortic. Sci. Biotechnol. 2003, 78, 586–589. [Google Scholar]
- Quiroga, A.M.; Berli, F.J.; Moreno, D.; Cavagnaro, J.B.; Bottini, R. Abscisic acid sprays significantly increase yield per plant in vineyard-grown wine grape (Vitis vinifera L.) cv. Cabernet Sauvignon through increased berry set with no negative effects on anthocyanin content and total polyphenol index of both juice and wine. J. Plant Growth Regul. 2009, 28, 28–35. [Google Scholar] [CrossRef]
- El-Kereamy, A.; Chervin, C.; Roustan, J.-P.; Cheynier, V.; Souquet, J.-M.; Moutounet, M.; Raynal, J.; Ford, C.; Latché, A.; Pech, J.-C.; Bouzayen, M. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiol. Plant. 2003, 119, 175–182. [Google Scholar] [CrossRef]
- Bellincontro, A.; Fardelli, A.; De Santis, D.; Botondi, R.; Mencarelli, F. Postharvest ethylene and 1-MCP treatments both affect phenols, anthocyanins, and aromatic quality of Aleatico grapes and wine. Aust. J. Grape Wine Res. 2006, 12, 141–149. [Google Scholar] [CrossRef]
- Zhang, W.; Curtin, C.; Kikuchi, M.; Franco, C. Integration of jasmonic acid and light irradiation for enhancement of anthocyanin biosynthesis in Vitis vinifera suspension cultures. Plant Sci. 2002, 162, 459–468. [Google Scholar] [CrossRef]
- Belhadj, A.; Telef, N.; Saigne, C.; Cluzet, S.; Barrieu, F.; Hamdi, S.; Mérillon, J.-M. Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol. Biochem. 2008, 46, 493–499. [Google Scholar] [CrossRef]
- Wen, P.-F.; Chen, J.-Y.; Kong, W.-F.; Pan, Q.-H.; Wan, S.-B.; Huang, W.-D. Salicylic acid induced the expression of phenylalanine ammonia-lyase gene in grape berry. Plant Sci. 2005, 169, 928–934. [Google Scholar] [CrossRef]
- González-SanJosé, M.L.; Diez, C. Relationship between anthocyanins and sugars during the ripening of grape berries. Food Chem. 1992, 43, 193–197. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, L.; Liu, H.; Pan, Q.; Zhan, J.; Huang, W. Sugars induce anthocyanin accumulation and flavanone 3-hydroxylase expression in grape berries. Plant Growth Regul. 2009, 58, 251–260. [Google Scholar] [CrossRef]
- El Kereamy, A.; Chervin, C.; Souquet, J.-M.; Moutounet, M.; Monje, M.-C.; Nepveu, F.; Mondies, H.; Ford, C.M.; Van Heeswijck, R.; Roustan, J.-P. Ethanol triggers grape gene expression leading to anthocyanin accumulation during berry ripening. Plant Sci. 2002, 163, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Montero, C.; Cristescu, S.M.; Jiménez, J.B.; Orea, J.M.; te Lintel Hekkert, S.; Harren, F.J.M.; González Ureña, A. trans-resveratrol and grape disease resistance. a dynamical study by high-resolution laser-based techniques. Plant Physiol. 2003, 131, 129–138. [Google Scholar] [CrossRef]
- Jeandet, P.; Sbaghi, M.; Bessis, R.; Meunier, P. The potential relationship of stilbene (resveratrol) synthesis to anthocyanin content in grape berry skins. Vitis 1995, 34, 91–94. [Google Scholar]
- Afifi, M.; El-Kereamy, A.; Legrand, V.; Chervin, C.; Monje, M.-C.; Nepveu, F.; Roustan, J.-P. Control of anthocyanin biosynthesis pathway gene expression by eutypine, a toxin from Eutypalata, in grape cell tissue cultures. J. Plant Physiol. 2003, 160, 971–975. [Google Scholar] [CrossRef] [Green Version]
- Deroles, S. Anthocyanin biosynthesis in plant cell cultures: a potential source of natural colourants. In Anthocyanins Biosynthesis, Functions, and Applications; Gould, K., Davies, K., Winefield, C., Eds.; Springer Science+Business Media, LLC.: New York, NY, USA, 2009; pp. 107–168. [Google Scholar]
- Cormier, F.; Crevier, H.A.; Do, C.B. Effects of sucrose concentration on the accumulation of anthocyanins in grape (Vitis vinifera) cell suspension. Can. J. Bot. 1990, 68, 1822–1825. [Google Scholar] [CrossRef]
- Yamakawa, T.; Ohtsuka, H.; Onomichi, K.; Kodama, T.; Minoda, Y. Production of anthocyanin pigment by grape cell culture. In Plant Tissue Culture; Fujiwara, A., Ed.; Japanese Association for Plant Tissue Culture: Tokyo, Japan, 1982; pp. 273–274. [Google Scholar]
- Do, C.B.; Cormier, F. Accumulation of anthocyanins enhanced by a high osmotic potential in grape (Vitis vinifera L.) cell suspensions. Plant Cell Rep. 1990, 9, 143–146. [Google Scholar]
- Bao Do, C.; Cormier, F. Effects of low nitrate and high sugar concentrations on anthocyanin content and composition of grape (Vitis vinifera L.) cell suspension. Plant Cell Rep. 1991, 9, 500–504. [Google Scholar]
- Do, C.B.; Cormier, F. Effects of high ammonium concentrations on growth and anthocyanin formation in grape (Vitis vinifera L.) cell suspension cultured in a production medium. Plant Cell Tissue Org. Cult. 1991, 27, 169–174. [Google Scholar] [CrossRef]
- Dedaldechamp, F.; Uhel, C.; Macheix, J.J. Enhancement of anthocyanin synthesis and dihydroflavonol reductase (DFR) activity in response to phosphate deprivation in grape cell suspensions. Phytochemistry 1995, 40, 1357–1360. [Google Scholar]
- Nagamori, E.; Hiraoka, K.; Honda, H.; Kobayashi, T. Enhancement of anthocyanin production from grape (Vitis vinifera) callus in a viscous additive-supplemented medium. Biochem. Eng. J. 2001, 9, 59–65. [Google Scholar] [CrossRef]
- Hiroyuki, H.; Kousuke, H.; Eiji, N.; Mariko, O.; Yoshihito, K.; Setsuro, H.; Takeshi, K. Enhanced anthocyanin production from grape callus in an air-lift type bioreactor using a viscous additive-supplemented medium. J. Biosci. Bioeng. 2002, 94, 135–139. [Google Scholar]
- Chorti, E.; Guidoni, S.; Ferrandino, A.; Novello, V. Effect of different cluster sunlight exposure levels on ripening and anthocyanin accumulation in Nebbiolo grapes. Amer. J. Enol. Viticult. 2010, 61, 23–30. [Google Scholar]
- Park, J.S.; Choung, M.G.; Kim, J.B.; Hahn, B.S.; Bae, S.C.; Roh, K.H.; Kim, Y.H.; Cheon, C.I.; Sung, M.K.; Cho, K.J. Genes up-regulated during red coloration in UV-B irradiated lettuce leaves. Plant Cell Rep. 2007, 26, 507–516. [Google Scholar] [CrossRef]
- Kataoka, I.; Sugiyama, A.; Beppu, K. Role of ultraviolet radiation in accumulation of anthocyanin in berries of 'Gros Colman' grapes (Vitis vinifera L.). J. Jpn. Soc. Hort. Sci. 2003, 72, 1–6. [Google Scholar] [CrossRef]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry temperature and solar radiation alter acylation, proportion, and concentration of anthocyanin in Merlot grapes. Amer. J. Enol. Viticult. 2008, 59, 235–247. [Google Scholar]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hort. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Yamane, T.; Seok, T.J.; Goto-Yamamoto, N.; Koshita, Y.; Kobayashi, S. Effects of temperature on anthocyanin biosynthesis in grape berry skins. Amer. J. Enol. Viticult. 2006, 57, 54–59. [Google Scholar]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Cohen, S.D.; Tarara, J.M.; Kennedy, J.A. Assessing the impact of temperature on grape phenolic metabolism. Anal. Chim. Acta 2008, 621, 57–67. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Romero-Cascales, I.; López-Roca, J.M.; Ros-García, J.M.; Gómez-Plaza, E. Anthocyanin fingerprint of grapes: environmental and genetic variations. J. Sci. Food Agr. 2006, 86, 1460–1467. [Google Scholar] [CrossRef]
- Sanchez-Ballesta, M.T.; Romero, I.; Jiménez, J.B.; Orea, J.M.; González-Ureña, Á.; Escribano, M.I.; Merodio, C. Involvement of the phenylpropanoid pathway in the response of table grapes to low temperature and high CO2 levels. Postharvest Biol. Technol. 2007, 46, 29–35. [Google Scholar] [CrossRef]
- Romero, I.; Teresa Sanchez-Ballesta, M.; Maldonado, R.; Isabel Escribano, M.; Merodio, C. Anthocyanin, antioxidant activity and stress-induced gene expression in high CO2-treated table grapes stored at low temperature. J. Plant Physiol. 2008, 165, 522–530. [Google Scholar]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; Di Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Esteban, M.A.; Villanueva, M.J.; Lissarrague, J.R. Effect of irrigation on changes in the anthocyanin composition of the skin of cv Tempranillo (Vitis vinifera L) grape berries during ripening. J. Sci. Food Agr. 2001, 81, 409–420. [Google Scholar] [CrossRef]
- Petrie, P.R.; Clingeleffer, P.R. Crop thinning (hand versus mechanical), grape maturity and anthocyanin concentration: outcomes from irrigated Cabernet Sauvignon (Vitis vinifera L.) in a warm climate. Aust. J. Grape Wine Res. 2006, 12, 21–29. [Google Scholar] [CrossRef]
- Peña-Neira, A.; Cáceres, A.; Pastenes, C. Low molecular weight phenolic and anthocyanin composition of grape skins from cv. Syrah (vitis vinifera L.) in the Maipo Valley (Chile): effect of clusters thinning and vineyard yield. Food Sci. Technol. Int. 2007, 13, 153–158. [Google Scholar] [CrossRef]
- Arozarena, I.; Ayestarán, B.; Cantalejo, M.J.; Navarro, M.; Vera, M.; Abril, I.; Casp, A. Anthocyanin composition of Tempranillo, Garnacha and Cabernet Sauvignon grapes from high- and low-quality vineyards over two years. Eur. Food Res. Technol. 2002, 214, 303–309. [Google Scholar] [CrossRef]
- Vian, M.A.; Tomao, V.; Coulomb, P.O.; Lacombe, J.M.; Dangles, O. Comparison of the anthocyanin composition during ripening of Syrah grapes grown using organic or conventional agricultural practices. J. Agr. Food Chem. 2006, 54, 5230–5235. [Google Scholar]
- Yokotsuka, K.; Nagao, A.; Nakazawa, K.; Sato, M. Changes in anthocyanins in berry skins of Merlot and Cabernet Sauvignon grapes grown in two soils modified with limestone or oyster shell versus a native soil over two years. Amer. J. Enol. Viticult. 1999, 50, 1–12. [Google Scholar]
- Cortell, J.M.; Halbleib, M.; Gallagher, A.V.; Righetti, T.L.; Kennedy, J.A. Influence of vine vigor on grape (Vitis vinifera L. cv. Pinot Noir) anthocyanins. 1. anthocyanin concentration and composition in fruit. J. Agr. Food Chem. 2007, 55, 6575–6584. [Google Scholar]
- Cortell, J.M.; Halbleib, M.; Gallagher, A.V.; Righetti, T.L.; Kennedy, J.A. Influence of vine vigor on grape (Vitis vinifera L. cv. Pinot Noir) anthocyanins. 2. anthocyanins and pigmented polymers in wine. J. Agr. Food Chem. 2007, 55, 6585–6595. [Google Scholar]
- Singh Brar, H.; Singh, Z.; Swinny, E.; Cameron, I. Girdling and grapevine leafroll associated viruses affect berry weight, colour development and accumulation of anthocyanins in 'Crimson Seedless' grapes during maturation and ripening. Plant Sci. 2008, 175, 885–897. [Google Scholar] [CrossRef]
- Parrado, J.; Escudero-Gilete, M.L.; Friaza, V.; García-Martínez, A.; Gonza´ lez-Miret, M.L.; Bautista, J.D.; Heredia, F.J. Enzymatic vegetable extract with bioactive components: influence of fertilizer on the colour and anthocyanins of red grapes. J. Sci. Food Agr. 2007, 87, 2310–2318. [Google Scholar]
- Bindon, K.; Dry, P.; Loveys, B. Influence of partial rootzone drying on the composition and accumulation of anthocyanins in grape berries (Vitis vinifera cv. Cabernet Sauvignon). Aust. J. Grape Wine Res. 2008, 14, 91–103. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, F.; Mu, L.; Yan, G.-L.; Liang, N.-N.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057-9091. https://doi.org/10.3390/molecules15129057
He F, Mu L, Yan G-L, Liang N-N, Pan Q-H, Wang J, Reeves MJ, Duan C-Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules. 2010; 15(12):9057-9091. https://doi.org/10.3390/molecules15129057
Chicago/Turabian StyleHe, Fei, Lin Mu, Guo-Liang Yan, Na-Na Liang, Qiu-Hong Pan, Jun Wang, Malcolm J. Reeves, and Chang-Qing Duan. 2010. "Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes" Molecules 15, no. 12: 9057-9091. https://doi.org/10.3390/molecules15129057





