Alkaloids from Stems of Esenbeckia leiocarpa Engl. (Rutaceae) as Potential Treatment for Alzheimer Disease
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of crude extract and hexane and alkaloid fractions on acetylcholinesterase inhibition

2.2. Chromatographic profile of alkaloid fraction

2.3. Isolation and identification of alkaloids from stems of E. leiocarpa

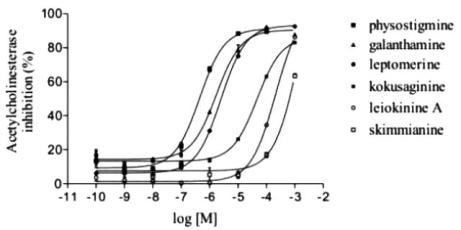
2.4. Anticholinesterasic activity of alkaloids

3. Experimental
3.1. Plant material
3.2. Extraction and isolation
3.3. General methods for compounds identification
3.4. Anticholinesterasic assay
3.5. Statistical analysis of data
4. Conclusions
Acknowledgements
References and Notes
- Barros-Filho, B.A.; Nunes, F.M.; Oliveira, M.C.F.; Andrade-Neto, M.; Mattos, M.C.; Barbosa, F.G.; Mafezoli, J.; Pirani, J.R. Secondary Metabolites of Esenbeckia almawillia Kaastra (Rutaceae). Quím. Nova 2007, 30, 1589–1591. [Google Scholar] [CrossRef]
- Guilhon, G.M.S.P.; Baetas, A.C.S.; Maia, J.G.S.; Conserva, L.M. 2-alkyl-4-quinolone alkaloids and cinnamic acid derivatives from Esenbeckia almawillia. Phytochemistry 1994, 37, 1193. [Google Scholar]
- Nakatsu, T.; Johns, T.; Kubo, I.; Sakai, M., Chatani; Saito, K., Tamagiwa; Kamikawa, T. Isolation, structure, and synthesis of novel 4-quinolinone alkaloids from Esenbeckia leiocarpa. J. Nat. Prod. 1990, 53, 1508–1513. [Google Scholar] [CrossRef]
- Johnstone, R.A.W.; Rose, M.E. A rapid, simple and mild procedure for alkylation of phenols, alcohols, amides and acids. Tetrahedron 1979, 35, 2169–2173. [Google Scholar] [CrossRef]
- Lorenzi, H. Brazilian Trees: A Guide to the Identification and Cultivation of Brazilian Native Trees; Plantarum: Nova Odessa, SP, Brazil, 1992; p. 307. [Google Scholar]
- Monache, F.D.; Monache, G.D.; Souza, M.A.M.; Cavalcanti, M.S., Chiappeta. Isopentenylindole derivatives and other components of Esenbeckia leiocarpa. Gazz. Chim. Ital. 1989, 119, 435–439. [Google Scholar]
- Monache, F.D.; Di Benedetto, R.; Souza, M.A.M.; Sandor, P. Esenbeckia leiocarpa: IIa. Further Componentes. Gazz. Chim. Ital. 1990, 120, 387–389. [Google Scholar]
- Katzman, R.; Jackson, E. Alzheimer disease: basic and clinical advances. J. Amer. Geriat. Soc. 1991, 39, 516–525. [Google Scholar]
- Howes, M.-J.R.; Perry, N.S.L.; Houghton, P.J. Plants with traditional uses and activities, relevant to the management of Alzheimer´s disease and other cognitive disorders. Phytother. Res. 2003, 17, 1. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Medeiros, K.C.P.; Diniz, M.F.; Batista, L.M.; Athayde-Filho, P.F.; Silva, M.S.; Cunha, E.V.L.; Almeida, J.R.G.S.; Quintans-Júnior, L.J. Natural products inhibitors of the enzyme acetylcholinesterase. Braz. J. Pharmacogn. 2006, 16, 258–285. [Google Scholar]
- Houghton, P.J.; Ren, Y.; Howes, M.-J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef]
- Marston, A.; Kissling, J.; Hostettmann, K. A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem. Anal. 2002, 13, 51–54. [Google Scholar] [CrossRef]
- Akhmedzhanova, V.I.; Bessonova, I.A.; Yunusov, S.Y. Alkaloids of Haplophyllum leptomerum. I. Structure of leptomerine. Chem. Nat. Compd. 1986, 22, 78–79. [Google Scholar] [CrossRef]
- Wu, T.-S.; Shi, L.-S.; Wang, J.-J.; Iou, S.-C.; Chang, H.-C.; Chen, Y.-P.; Kuo, Y.-H.; Chang, Y.-L.; Teng, C.-M. Cytotoxic and antiplatelet aggregation principles of Ruta graveolens. J. Chin. Chem. Soc. 2003, 50, 171–178. [Google Scholar]
- Cortez, L.E.R.; Cortez, D.A.G.; Ferreira, A.G.; Vieira, P.C.; Fernandes da Silva, M.F.G.; Fernandes, J.B. Chemical constituents of Almeidea coerulea (Nees and Mart.) A. St.-Hill (Rutaceae). Braz. J. Pharmacogn. 2006, 16, 164–169. [Google Scholar]
- Nunes, F.M.; Barros-Filho, B.A.; Oliveira, M.C.F.; Andrade-Neto, M.; Mattos, M.C.; Mafezoli, J.; Pirani, J.R. 1H and 13C NMR spectra of 3,8-dimethoxyfuro[3,2-g]coumarin and maculine from Esenbeckia grandiflora Martius (Rutaceae). Magn. Reson. Chem. 2005, 43, 864–866. [Google Scholar] [CrossRef]
- Trani, M.; Carbonetti, A.; Monache, G.D.; Monache, F.D. Dihydrochacones and coumarins of Esenbeckia grandiflora subsp. brevipetiolata. Fitoterapia 2004, 75, 99–102. [Google Scholar] [CrossRef]
- Simpson, S.D.; Jacobs, H. Alkaloids and coumarins from Esenbeckia pentaphylla (Rutaceae). Biochem. Syst. Ecol. 2005, 33, 841–844. [Google Scholar] [CrossRef]
- Oliveira, F.M.; Sant´ana, A.E.G.; Conserva, L.M.; Maia, J.G.S.; Guilhon, G.M.P. Alkaloids and coumarins from Esenbeckia species. Phytochemistry 1996, 41, 647–649. [Google Scholar]
- Rios, M.Y.; Delgado, G. Terpenoids and alkaloids from Esenbeckia belizencis. spontaneous oxidation of furoquinoline alkaloids. J. Nat. Prod. 1992, 55, 1307–1309. [Google Scholar] [CrossRef]
- Paulini, H.; Waibel, R.; Schimmer, O. Mutagenicity and structure-mutagenicity relationships of furoquinolines, naturally occurring alkaloids of the Rutaceae. Mutat. Res. Lett. 1989, 227, 179–186. [Google Scholar] [CrossRef]
- Schimmer, O.; Kühne, I. Furoquinoline alkaloids as photosensitizers in Chlamydomonas reinhardtti. Mutat. Res.-Fundam. Mol. Mech. Mut. 1991, 249, 105–110. [Google Scholar] [CrossRef]
- Biavatti, M.W.; Vieira, P.C.; Da Silva, M.F.G.F.; Fernandes, J.B.; Victor, S.R.; Pagnocca, F.C.; Albuquerque, S.; Caracelli, I.; Zukerman-Schpector, J. Biological activity of quinoline alkaloids from Raulinoa echinata and X-ray structure of flindersiamine. J. Braz. Chem. Soc. 2002, 13, 66–70. [Google Scholar] [CrossRef]
- Rhee, I.K.; Meent, M.; Ingkaninan, K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using sílica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar]
- Liu, J.S.; Zhu, Y.L.; Yu, C.M.; Zhou, Y.Z.; Han, Y.Y.; Wu, F.W.; Qi, B.F. The structures of huperzine A and B two new alkaloids exhibiting marked anticholinesterase activity. Can. J. Chem. 1986, 64, 837–839. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1, 2, 3 and 4 are available from the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cardoso-Lopes, E.M.; Maier, J.A.; Silva, M.R.d.; Regasini, L.O.; Simote, S.Y.; Lopes, N.P.; Pirani, J.R.; Bolzani, V.d.S.; Young, M.C.M. Alkaloids from Stems of Esenbeckia leiocarpa Engl. (Rutaceae) as Potential Treatment for Alzheimer Disease. Molecules 2010, 15, 9205-9213. https://doi.org/10.3390/molecules15129205
Cardoso-Lopes EM, Maier JA, Silva MRd, Regasini LO, Simote SY, Lopes NP, Pirani JR, Bolzani VdS, Young MCM. Alkaloids from Stems of Esenbeckia leiocarpa Engl. (Rutaceae) as Potential Treatment for Alzheimer Disease. Molecules. 2010; 15(12):9205-9213. https://doi.org/10.3390/molecules15129205
Chicago/Turabian StyleCardoso-Lopes, Elaine Monteiro, James Andreas Maier, Marcelo Rogério da Silva, Luis Octávio Regasini, Simone Yasue Simote, Norberto Peporine Lopes, José Rubens Pirani, Vanderlan da Silva Bolzani, and Maria Cláudia Marx Young. 2010. "Alkaloids from Stems of Esenbeckia leiocarpa Engl. (Rutaceae) as Potential Treatment for Alzheimer Disease" Molecules 15, no. 12: 9205-9213. https://doi.org/10.3390/molecules15129205