Seeking Polymeric Prodrugs of Norfloxacin. Part 2. Synthesis and Structural Analysis of Polyurethane Conjugates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ring-opening polymerization of cyclic esters

| Run no. | Monomer | Molar ratio* | Yield [%]** | Physical form*** | MnMALDI [Da] | PD MALDI | MnGPC [Da] | PDGPC | MLOH [Da] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CL | 100:1:1 | 63% | ss | - | - | 4,900 | 1.3 | - |
| 2 | CL | 50:1:1 | 72% | ss | - | - | 3,600 | 1.2 | 2,400 |
| 3 | CL | 25:1:1 | 91% | ss | 2,700 | 1.2 | 3,100 | 1.3 | 2,600 |
| 4 | L,L-LA | 100:1:1 | 42% | vl | - | - | 4,800 | 1.3 | - |
| 5 | L,L-LA | 50:1:1 | 51% | vl | 1,400 | 1.2 | 3,100 | 1.2 | 2,000 |
| 6 | L,L-LA | 25:1:1 | 62% | vl | - | - | 2,200 | 1.2 | - |
| 7 | D,L-LA | 100:1:1 | 46% | vl | 2,300 | 1.1 | - | - | - |
| 8 | D,L-LA | 100:1:1 | 46% | vl | 2,300 | 1.1 | - | - | - |
| 9 | D,L-LA | 25:1:1 | 64% | vl | 1,600 | 1.1 | 2,200 | 1.2 | 1,800 |


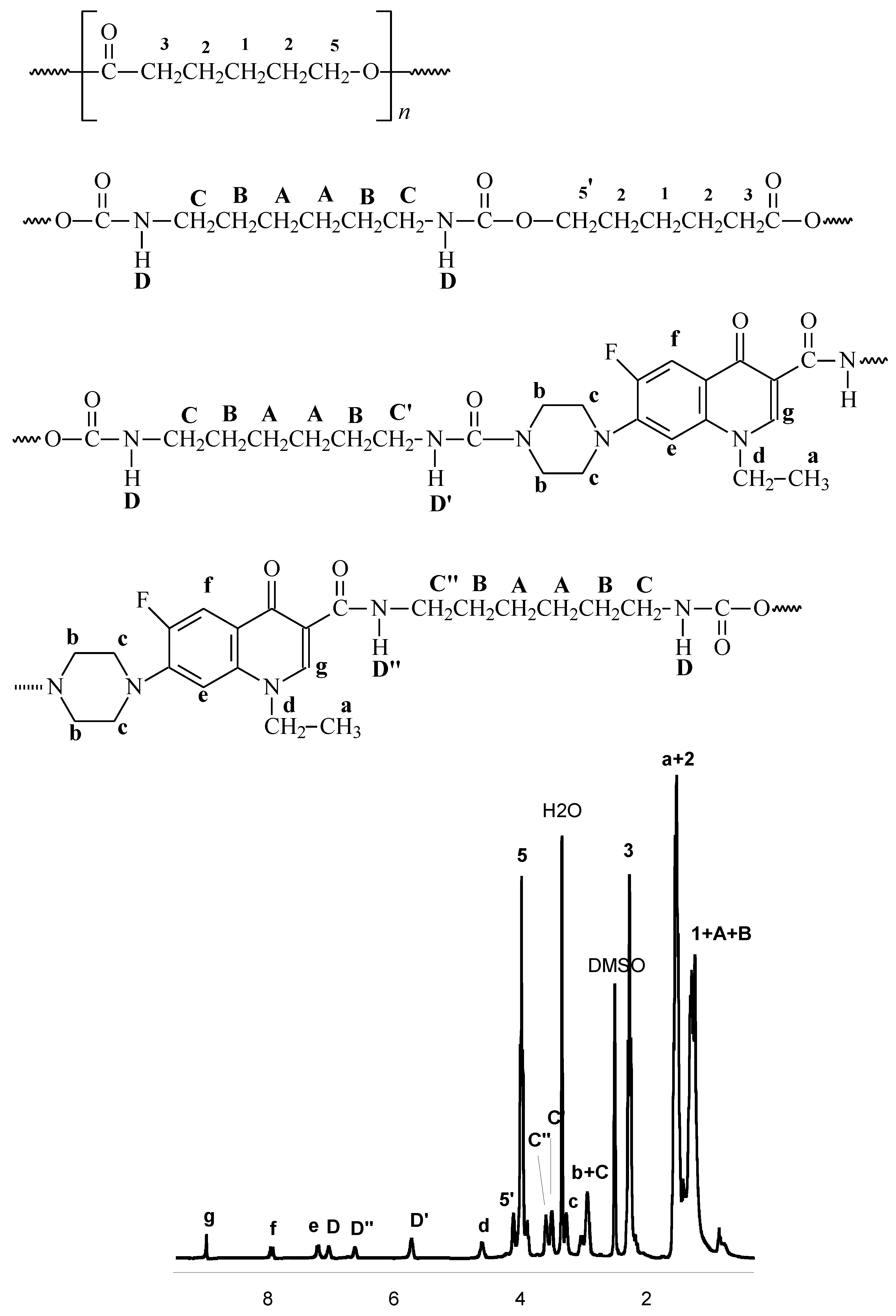
2.2. Synthesis of polyurethane conjugates of norfloxacin

| Run no. | Reagents/catalyst | Synthesis methods* | Yield [%]** | Mv [Da] |
|---|---|---|---|---|
| 1 | HDI-OEAD-NOR DLDBSn | II | ≈ 100 | 23 600 |
| 2 | HDI-OEAD-NOR DLDBSn | I | ≈ 95 | 20 500 |
| 3 | HDI-OEAD-NOR SnOct2 | I | ≈ 92 | 18 900 |
| 4 | HDI-OEAD-NOR SnOct2 | I | ≈ 88 | 14 200 |
| 5 | HDI-OCL-NOR DLDBSn | II | ≈ 96 | 33 300 |
| 6 | HDI-OCL-NOR DLDBSn | I | ≈ 93 | 26 200 |
| 7 | HDI-OCL-NOR SnOct2 | II | ≈ 86 | 30 300 |
| 8 | HDI-OCL-NOR SnOct2 | I | ≈ 79 | 21 400 |
| 9 | HDI-PCL1-NOR DLDBSn | II | ≈ 92 | 40 900 |
| 10 | HDI-PCL1-NOR SnOct2 | II | ≈ 84 | 30 700 |
| 11 | HDI-PCL2-NOR DLDBSn | II | ≈ 94 | 42 800 |
| 12 | HDI-PCL2-NOR SnOct2 | II | ≈ 87 | 33 300 |
| 13 | HDI-PLA1-NOR DLDBSn | II | ≈ 79 | 24 200 |
| 14 | HDI-PLA1-NOR SnOct2 | II | ≈ 65 | 20 200 |
| 15 | HDI-PLA2-NOR DLDBSn | II | ≈ 72 | 18 400 |
| 16 | HDI-PLA2-NOR SnOct2 | II | ≈ 59 | 14 700 |
| Run no. | Reagents/catalyst | C | pH = 1 | pH = 7.4 | ||||
|---|---|---|---|---|---|---|---|---|
| P7 | P14 | P21 | P7 | P14 | P21 | |||
| 1 | HDI-OEAD-NOR DLDBSn | 19 | 5 | 9 | 16 | 3 | 5 | 7 |
| 2 | HDI-PCL1-NOR DLDBSn | 17 | 4 | 6 | 10 | 2 | 3 | 5 |
| 3 | HDI-PLA1-NOR DLDBSn | 18 | 6 | 11 | 18 | 4 | 7 | 10 |
3. Experimental
3.1. Materials
3.2. Instrumentation
3.3. General procedure
3.3.1. Oligoesters synthesis
3.3.2. Macromolecular conjugates synthesis
3.4. Biodegradation of polyurethane conjugates
3.5. IR and NMR data
4. Conclusions
Acknowledgements
- Sample Availability: Contact the authors.
References and Notes
- Jagur-Grodzinski, J. Biomedical application of functional polymers. React. Funct. Polym. 1999, 39, 99–138. [Google Scholar] [CrossRef]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef]
- Veronese, F.M.; Morpurgo, M. Bioconjugation in pharmaceutical chemistry. Farmaco 1999, 54, 497–516. [Google Scholar] [CrossRef]
- Hoste, K.; De Winne, K.; Schacht. Polymeric prodrugs. Int. J. Pharm. 2004, 277, 119–131. [Google Scholar] [CrossRef]
- Ouchi, T.; Ohya, Y. Macromolecular prodrugs. Prog. Polym. Sci. 1995, 20, 211–257. [Google Scholar] [CrossRef]
- Garnett, M.C. Targeted drug conjugates: Principles and progress. Adv. Drug Del. Rev. 2001, 53, 171–216. [Google Scholar] [CrossRef]
- Merkli, A.; Tabatabay, C.; Gurny, R.; Heller, J. Biodegradable polymers for the controlled release of ocular drugs. Prog. Polym. Sci. 1998, 23, 563–580. [Google Scholar] [CrossRef]
- Järvinen, T.; Järvinen, K. Prodrugs for improved ocular delivery. Adv. Drug Del. Rev. 1996, 19, 203–224. [Google Scholar] [CrossRef]
- Khandare, J.; Minko, T. Polymer-drug conjugates: Progress in polymeric prodrugs. Prog. Polym. Sci. 2006, 31, 359–397. [Google Scholar] [CrossRef]
- Sobczak, M.; Olędzka, E.; Kolodziejski, W.; Kuźmicz, R. Pharmaceutical application of polymers. Polimery 2007, 52, 411–420. [Google Scholar]
- Olędzka, E.; Sobczak, M.; Kołodziejski, W.L. Polymers in medicine -review of recent studies. Polimery 2007, 52, 795–803. [Google Scholar]
- Król, P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog. Polym. Sci. 2007, 52, 915–1015. [Google Scholar]
- Oertel, G. Polyurethane Handbook; Hanser Publishers: Munich, Germany, 1994. [Google Scholar]
- Wirpsza, Z. Polyurethanes: Chemistry, Technology and Application; Ellis Horwood/Prentice-Hall: London, UK, 1993. [Google Scholar]
- Kuran, W.; Sobczak, M.; Listoś, T.; Dębek, C.; Florjańczyk, Z. New route to oligocarbonate diols suitable for the synthesis of polyurethane elastomers. Polymer 2000, 41, 8531–8541. [Google Scholar] [CrossRef]
- Rokicki, G.; Piotrowska, A. A new route to polyurethanes from ethylene carbonate, diamines and diols. Polymer 2002, 43, 2927–2935. [Google Scholar] [CrossRef]
- Neffgen, S.; Keul, H.; Höcker, H. Cationic ring-opening polymerization of trimethylene urethane: A mechanistic study. Macromolecules 1997, 30, 1289–1297. [Google Scholar] [CrossRef]
- Kusan, J.; Keul, H.; Höcker, H. Cationic ring-opening polymerization of tetramethylene urethane. Macromolecules 2001, 34, 389–395. [Google Scholar]
- Albertsson, A.C.; Varma, I.V. Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef]
- Florjańczyk, Z.; Plichta, A.; Sobczak, M. Ring opening polymerization initiated by methylaluminoxane/AlMe3 complexes. Polymer 2006, 47, 1081–1090. [Google Scholar] [CrossRef]
- Marcilla, R.; de Geus, M.; Mecerreyes, D.; Duxbury, C.J.; Koning, C.E.; Heise, A. Enzymatic polyester synthesis in ionic liquids. Eur. Polym. J. 2006, 42, 1215–1221. [Google Scholar] [CrossRef]
- He, F.; Li, S.; Garreau, H.; Vert, M.; Zhuo, R. Enzyme-catalyzed polymerization and degradation of copolyesters of ε-caprolactone and γ-butyrolactone. Polymer 2005, 46, 12682–12688. [Google Scholar] [CrossRef]
- Duda, A.; Biela, T.; Kowalski, A.; Lubiszowski, J. Amines as (co)initiators of cyclic esters' polymerization. Polimery 2005, 50, 501–508. [Google Scholar]
- Martin, E.; Dubois, P.; Jerome, R. “In Situ” Formation of Yttrium Alkoxides: A Versatile and Efficient Catalyst for the ROP of ε-Caprolactone. Macromolecules 2003, 36, 5934–5941. [Google Scholar] [CrossRef]
- Storey, R.F.; Sherman, J.W. Kinetics and mechanism of the stannous octoate-catalyzed bulk polymerization of ε-caprolactone. Macromolecules 2002, 35, 1504–1512. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uyama, H.; Kimura, S. Enzymatic polymerization. Chem. Rev. 2001, 101, 3793–3818. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Kinetics and mechanism of cyclic esters polymerization initiated with tin(II) octoate. 3. Polymerization of L,L-dilactide. Macromolecules 2000, 33, 7359–7370. [Google Scholar] [CrossRef]
- Divakar, S. Porcine pancreas lipase catalyzed ring-opening polymerization of ε-Caprolactone. J. Macromol. Sci. Pure Appl. Chem. 2004, A41, 537–546. [Google Scholar] [CrossRef]
- Namekawa, S.; Suda, S.; Uyama, H.; Kobayashi, S. Lipase-catalyzed ring-opening polymerization of lactones to polyesters and its mechanistic aspects. Int. J. Biol. Macromol. 1999, 25, 145–151. [Google Scholar] [CrossRef]
- Sobczak, M.; Kolodziejski, W. Polymerization of cyclic esters initiated by carnitine and tin (II) octoate. Polymerization of cyclic esters initiated by carnitine and tin (II) octoate. Molecules 2009, 14, 621–632. [Google Scholar] [CrossRef]
- Sobczak, M.; Olędzka, E.; Kołodziejski, W.L. Polymerization of cyclic esters using aminoacid initiators. J. Macromol. Sci. A 2008, 45, 872–877. [Google Scholar]
- Wang, C.; Li, H.; Zhao, X. Ring opening polymerization of l-lactide initiated by creatinine. Biomaterials 2004, 25, 5797–5801. [Google Scholar] [CrossRef]
- Hu, W.; Zhou, W.; Xia, C.; Wen, X. Synthesis and anticancer activity of thiosemicarbazones. Bioorg. Med. Chem. Lett. 2213–2218.
- Jeon, H.-J.; Jeong, Y.-I.; Jang, M.-K.; Park, Y.-H.; Nah, J.-W. Effect of solvent on the preparation of surfactant-free poly(DL-lactide-co-glycolide) nanoparticles and norfloxacin release characteristics. Int. J. Pharm. 2000, 207, 99–108. [Google Scholar] [CrossRef]
- Sobczak, M.; Witkowska, E.; Olędzka, E.; Kolodziejski, W. Synthesis and Structural analysis of polyester prodrugs of norfloxacin. Molecules 2008, 13, 96–106. [Google Scholar] [CrossRef]
- Górna, K.; Gogolewski, S. Molecular stability, mechanical properties, surface characteristics and sterility of biodegradable polyurethanes treated with low-temperature plasma. Polym. Degrad. Stabil. 2003, 79, 475–485. [Google Scholar] [CrossRef]
© 2010 by the authors;
Share and Cite
Sobczak, M.; Nurzyńska, K.; Kolodziejski, W. Seeking Polymeric Prodrugs of Norfloxacin. Part 2. Synthesis and Structural Analysis of Polyurethane Conjugates. Molecules 2010, 15, 842-856. https://doi.org/10.3390/molecules15020842
Sobczak M, Nurzyńska K, Kolodziejski W. Seeking Polymeric Prodrugs of Norfloxacin. Part 2. Synthesis and Structural Analysis of Polyurethane Conjugates. Molecules. 2010; 15(2):842-856. https://doi.org/10.3390/molecules15020842
Chicago/Turabian StyleSobczak, Marcin, Katarzyna Nurzyńska, and Waclaw Kolodziejski. 2010. "Seeking Polymeric Prodrugs of Norfloxacin. Part 2. Synthesis and Structural Analysis of Polyurethane Conjugates" Molecules 15, no. 2: 842-856. https://doi.org/10.3390/molecules15020842




