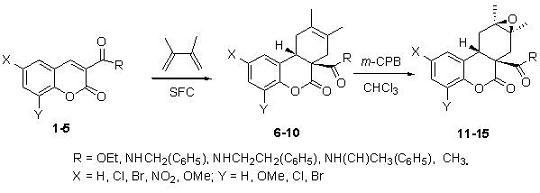
3. Experimental
3.1. General methods
All chemicals and solvents were of reagent grade and used as received. Melting points were measured on an Electrothermal IA 9100 apparatus and were uncorrected. IR spectra were recorded in KBr disks using a Perkin-Elmer 16F PC IR spectrophotometer. Mass spectra were obtained in a GC/MS system (Varian) with an electron ionization mode. Elemental analyses (EA) were performed on a Perkin-Elmer 2400 elemental analyzer.
1H- and
13C-NMR spectra were recorded on a Varian Mercury 300 (
1H, 300.08;
13C, 75.46 MHz) instrument in CDCl
3 solutions, unless otherwise specified, measured with SiMe
4 as the internal reference, δ are in ppm and coupling constants
nJ in Hz.
1H- and
13C-NMR assignments were achieved on the basis of NOE, COSY and HETCOR experiments. Single-crystal X-ray diffraction data for molecules
10b and
15i were collected on a Bruker Apex II area detector diffractometer at 100 and 293 K, respectively, with Mo Kα radiation, λ = 0.71073 Å. A semiempirical absorption correction was applied using SADABS [
18], and the program SAINT [
18] was used for integration of the diffraction profiles. The structures were solved by direct methods using SHELXS97 [
19] program of WinGX package [
20]. The final refinement was performed by full-matrix least-squares methods on
F2 with SHELXL97 [
19] program. H atoms on C, N and O were positioned geometrically and treated as riding atoms, with C―H = 0.93-0.98 Å, and with
Uiso(H) = 1.2
Ueq(C). Mercury was used for visualization, molecular graphics and analysis of crystal structures [
21], software used to prepare material for publication was PLATON [
22]. Crystallographic data (excluding structure factors) for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication CCDC numbers 735605 (
10b) and 721872 (
15i). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (Fax: +44-01223-336033 or E-Mail:
[email protected]). Crystals suitable for X-ray analysis were obtained from saturated CHCl
3 solutions. The program GAUSSIAN98 [
23] was used to perform the
ab initio molecular orbital calculations at RHF-631G** level of theory.
3.2. General synthetic procedure for coumarins 1-5
The starting coumarins 1a-4a were synthesized according with the methodology reported elsewhere [
11,
16,
24]. 6-Substituted acetyl coumarins
5a-i were synthesized by Knoevenagel condensation of ethyl acetoacetate and the corresponding 5-substituted 2-hydroxybenzaldehyde, the spectroscopic data of
5a-d are in agreement with literature [
25].
3-Acetyl-6-methoxy-2H-1-benzopyran-2-one (5e). Prepared from 0.41 mL (3.3 mmol) of 2-hydroxy-5-methoxybenzaldehyde and 0.42 mL (3.3 mmol) of ethyl acetoacetate. Yellow solid 89% yield, mp 180–183 ºC. IR ν(cm-1): 1723 (OC=O), 1677 (C=O), 1226, 1197 (C-O). 1H-NMR: 8.44 (s, 1H, H4), 7.28 (d, 1H, 3J = 9.1, H8), 7.20 (dd, 1H, 3J = 9.1, 4J = 2.9, H7), 7.02 (d, 1H, 4J = 2.6, H5), 3.85 (s, 3H, OCH3), 2.70 (s, 3H, CH3); 13C-NMR: 195.9 (CO), 159.7 (OCO), 156.6 (C6), 150.1 (C10), 147.6 (C4), 124.8 (C3), 123.2 (C7), 117.9 (C5), 118.7 (C9), 111.3 (C8), 56.1 (OCH3), 30.9 (CH3); EA (%) calculated for C12H10O4: 66.05 C, 4.62 H; found: 66.04 C, 4.61 H.
3-Acetyl-8-methoxy-2H-1-benzopyran-2-one (5f). Prepared from 0.5 g (3.3 mmol) of 2-hydroxy-3-methoxybenzaldehyde and 0.42 mL (3.3 mmol) of ethyl acetoacetate. Yellow solid, 88% yield, mp 171–174 °C. IR ν(cm-1): 1727 (OC=O), 1682 (C=O), 1278, 1197 (C-O). 1H-NMR: 8.42 (s, 1H, H4), 7.25 (d, 1H, 4J = 1.1, 3J = 5.7, H7), 7.14 (d, 1H, 4J = 2.0, 3J = 5.7, H5), 7.18 (t, 1H, 3J = 5.5, 4J = 2.0, H6), 3.94 (s, 3H, OCH3), 2.68 (s, 3H, CH3); 13C-NMR: 195.8 (CO), 158.9 (OCO), 145.1 (C10), 147.9 (C4), 147.2 (C8), 125.0 (C5), 124.8 (C3), 121.5 (C6), 116.0 (C7), 118.9 (C9), 56.5 (OCH3), 30.8 (CH3); EA (%) calculated for C12H10O4: 66.05 C, 4.62 H; found: 66.15 C, 4.60 H.
3-Acetyl-6,8-dichloro-2H-1-benzopyran-2-one (5g). Prepared from 0.5 g (2.6 mmol) of 3,5-dichlorosalicylaldehyde and 0.33 mL (2.6 mmol) of ethyl acetoacetate. White solid in 48% yield, mp 172–175 ºC. IR ν(cm-1): 1749 (OC=O), 1676 (C=O), 1217 (C-O), 769 (C-Cl). 1H-NMR: 8.37 (s, 1H, H4), 7.54 (d, 1H, 4J = 2.4, H7), 7.66 (d, 1H, 4J = 2.4, H5), 2.71 (s, 3H, CH3); 13C-NMR 194.8 (CO), 157.7 (OCO), 149.7 (C10), 145.4 (C4), 122.9 (C8), 127.8 (C5), 126.2 (C3), 130.5 (C6), 134.2 (C7), 120.1 (C9), 30.7 (CH3); EA (%) calculated for C11H6O3Cl2: 51.39 C, 2.35 H; found: 51.53 C, 2.42 H.
3-Acetyl-8-bromo-6-chloro-2H-1-benzopyran-2-one (5h). Prepared from 0.5 g (2.1 mmol) of 3-bromo-5-chloro-salicylaldehyde and 0.27 mL of ethyl acetoacetate (2.1 mmol). Yellow solid in 60% yield, mp 192–196 ºC. IR ν(cm-1): 1740 (OC=O), 1675 (C=O), 1202 (C-O), 760 (C-Cl), 556 (C-Br). 1H-NMR (DMSO-d6): 8.55 (s, 1H, H4), 8.05 (d, 1H, 4J = 2.2, H7), 8.12 (d, 1H, 4J = 2.2, H5), 2.56 (s, 3H, CH3); 13C-NMR: 195.4 (CO), 158.0 (OCO), 150.7 (C10), 146.1 (C4), 110.5 (C8), 129.8 (C5), 126.6 (C3), 129.5 (C6), 136.5 (C7), 121.1 (C9), 30.7 (CH3); EA (%) calculated for C11H6O3ClBr: 43.82 C, 2.01 H; found: 43.70 C, 2.12 H.
3-Acetyl-6-bromo-8-methoxy-2H-1-benzopyran-2-one (5i). Prepared from 0.5 g (2.2 mmol) de 5-bromo-2-hydroxy-3-methoxybenzaldehyde and 0.28 mL of ethyl acetoacetate (2.2 mmol). Yellow solid in 89% yield, mp 215–218 ºC. IR ν(cm-1): 1735 (OC=O), 1674 (C=O), 1235, 1128 (C-O), 662 (C-Br). 1H-NMR: 8.36 (s, 1H, H4), 7.24 (d, 1H, 4J = 2.1, H7), 7.34 (d, 1H, 4J = 2.1, H5), 3.96 (s, 3H, OCH3), 2.71 (s, 3H, CH3); 13C-NMR: 195.1 (CO), 158.0 (OCO), 144.0 (C10), 146.3 (C4), 147.6 (C8), 123.1 (C5), 125.5 (C3), 117.1 (C6), 118.8 (C7), 119.7 (C9), 56.5 (OCH3), 30.5 (CH3); EA (%) calculated for C12H9O4Br: 48.5 C, 3.1 H; found: 48.45 C, 2.95.
3.3. General synthetic procedure for DA adducts 6-10
1 mmol (typically 100–300 mg) of the corresponding coumarin
1-5 and the appropriate volume equivalent to 6 mmol of 2,3-dimethyl-1,3-butadiene (typically 0.3–1.0 mL) were placed in a glass ampoule; the sealed ampoule was placed inside a metallic capsule and heated in a sand bath at 160 ºC for a period of 24 hours. The ampoule content was dissolved in CHCl
3 and evaporated to dryness. The resultant solid was washed with hot hexane and the insoluble solid was purified by CC in SiO
2–gel, using CHCl
3 as eluent. Cycloadducts
6a [
5],
7a [
9] are reported elsewhere.
(6aR,10aR)- and (6aS,10aS)-N-[(R)-1-Phenylethyl]-6a,7,10,10a-tetrahydro-8,9-dimethyl-6-oxo-dibenzo[b,d]pyran-6a-carboxamide (8a). White crystalline solid in 77% yield, mp 168.9–171.4 °C. IR/ν (cm-1): 3312 (NH), 1784 (OC=O), 1629 (NC=O), 1532 (C=C), 1222 (N-C), 1146 (C-O). 1H- NMR: 7.30 (m, 1H, H3), 7.26 (t, 2H, 3J = 7.3 and 7.7, Hm), 7.13 (t, 1H, 3J = 7.3, Hp), 7.14 (d, 2H, 3J = 7.3, Ho), 7.07 (t, 1H, 3J = 8.1 and 8.4, H2), 7.00 (d, 1H, 3J = 8.1, H1), 6.83 (dd, 1H, 3J = 5.3, 4J = 2.2, H4), 5.89, 5.82 (d, 1H each, 3J = 7.5, N-H), 4.88 (dq, 1H, 3J = 7.3, CH3), 3.58 (m, 1H, H10a), 2.90 (t, 1H, 3J = 18.3, Heq-7), 2.49 (d, 1H, 3J = 17.4, Hax-7), 2.32 (t, 1H, 3J = 18.7 and 12.3, Heq-10), 2.02 (m, 1H, Hax-10), 1.67, 1.60 (s, 3H each, 2CH3), 1.37, 1.21 (d, 3H, 3J = 7.0, CH3); 13C-NMR: 170.6, 170.5 (NCO), 167.6, 167.4 (OCO), 150.2 (C4a), 142.5, 142.2 (Ci), 129.0 (C3), 128.7, 128.6 (Cm), 128.5, 128.4 (C1), 128.1 (C10b), 128.0, 127.7 (C2), 127.4, 126.1 (Cp), 125.5, 125.7 (Co), 123.5, 123.6 (C9), 123.2, 123.3 (C8), 116.8, 116.7 (C4), 55.0, 54.8 (C6a), 49.5, 49.2 (NCH), 37.0, 36.9 (C7), 36.8, 36.8 (C10), 36.3 (C10a), 21.6, 21.4 (CH3CH), 18.8, 18.8 (CH3); GC/MS m/z (%): 375 (M+, 24), 281 (38), 227 (100), 211 (5), 199 (3), 105 (29), 79 (12), 44 (5).
(6aR,10aR)-rel-N-(2-Phenylethyl)-6a,7,10,10a-tetrahydro-8,9-dimethyl-6-oxodibenzo[b,d]pyran-6a-carboxamide (9a). White crystalline powder in 84% yield, mp 138.6–140.0 °C. IR/ν (cm-1): 3333 (NH), 1771 (OC=O), 1634 (NC=O), 1546 (C=C), 1222 (C–O), 1144 (C–N). 1H-NMR: 7.25 (t, 2H, 3J = 7.3, Hm), 7.27 (d, 1H, 3J = 7.3, H1), 7.21 (dt, 1H, 7.0 Hz, H3), 7.11 (ddt, 1H, 3J = 7.2 and 7.7, 4J = 1.3, H2), 7.05 (dd, 2H, 3J = 7.1, Ho), 7.00 (dd, 1H, 3J = 7.5, H4), 5.79 (t, 1H, 3J = 5.4, NH), 3.58 (dd, 1H, 3J = 11.7 and 11.7, H10a), 3.34 (dt, 2H, 3J = 7.2 and 7.2, 4J = 2.0, NCH2), 2.79 (d, 1H, 3J = 17.1, Heq-7), 2.61 (m, 2H, CH2), 2.30 (d, 1H, 3J = 16.7, Hax-7), 2.26 (d, 1H, Heq-10), 2.00 (dd, 1H, 3J = 15.5 and 12.0, Hax-10), 1.58, 1.65 (s, 3H, 2CH3); 13C-NMR: 170.4 (NC=O), 168.4 (OC=O), 150.0 (C4a), 138.4 (Ci), 129.0 (C10b), 128.9 (Co,m), 128.6 (Cp), 128.4 (C3), 127.9 (C1), 126.8 (C3), 125.4 (C2), 123.7 (C9), 123.1 (C8), 116.8 (C4), 41.3 (NCH2), 37.3 (C7), 36.5 (C10a), 36.3 (C13), 35.5 (CH2), 18.8, 18.7 (CH3); GC/MS m/z (%): 375 (M+, 20), 227 (100), 199 (3), 173 (7); EA (%) calculated for C24H25O3N: 76.77 C, 6.71 H, 3.73 N; found: 76.70C, 6.72 H, 3.82 N.
(6aR,10aR)-rel-6a-Acetyl-6a,7,10,10a-tetrahydro-8,9-dimethyl-6-oxodibenzo[b,d]pyran (10a). White crystalline powder in 83% yield, mp 86–88 ºC. IR/ν(cm-1): 1763 (OC=O), 1702 (C=O), 1159, 1140 (C-O). 1H-NMR: 7.25 (m, 1H, H3), 7.22 (dd, 1H, 3J = 12.1, 4J = 1.8, H1), 7.10 (td, 1H, 3J = 7.3, 4J = 1.2, H2), 7.03 (dd, 1H, 3J = 7.9, 4J = 1.1, H4), 3.47 (dd, 1H, 3J = 11.2 and 11.4, H10a), 2.87 (d, 1H, 3J = 17.3, Heq-7), 2.31 (dd, 1H, 3J = 18.0, Hax-7), 2.24 (d, 1H, 3J = 12.0, Heq-10), 2.12 (s, 3H, CH3), 2.06 (dd, 1H, Hax-10), 1.70, 1.62 (s, 3H, CH3); 13C-NMR 204.1 (CO), 168.6 (OCO), 150.4 (C4a), 128.9 (C3), 127.6 (C1), 127.5 (C10b), 125.1 (C2), 123.5 (C9), 122.8 (C8), 117.2 (C4), 60.6 (C6a), 36.8 (C10a), 35.9 (C10), 34.8 (C7), 26.2 (C14), 18.8, 18.9 (CH3); GC/MS m/z (%): 270 (M+, 4), 227 (100), 199 (8), 185 (7), 173 (10), 43 (22); EA (%) calculated for C17H18O3: 75.53 C, 6.73 H; found: 75.50, 6.70 H.
(6aR,10aR)-rel-6a-Acetyl-2-chloro-6a,7,10,10a-tetrahydro-8,9-dimethyl-6-oxodibenzo[b,d]pyran (10b). Pale yellow solid in 50% yield, mp 102–106 ºC. IR/ν(cm-1): 1781 (OC=O), 1699 (C=O), 1212, 1139 (C-O), 814 (C-Cl). 1H-NMR: 7.27 (dd, 1H, 3J = 8.0, 4J = 1.98, H3), 7.19 (d, 1H, 4J = 2.0, H1), 6.94 (d, 1H, 3J = 8.0 Hz, H4), 3.43 (dd, 1H, 3J = 6.2, 11.3, H10a), 2.86 (d, 1H, 2J = 16.0, Heq-7), 2.28 (dd, 1H, 2J = 18.3, 3J = 6.2, Heq-10), 2.13 (d, 1H, 2J = 16.0, Hax-7), 2.11 (s, 3H, H14), 2.02 (dd, 1H, 2J = 18.3, 3J = 11.3, Hax-10), 1.67 (s, 3H, CH3), 1.59 (s, 3H, CH3); 13C-NMR: 203.5 (CO), 167.9 (OCO), 148.9 (C4a), 130.1 (C10b), 129.3 (C2), 128.8 (C3), 127.6 (C1), 123.8 (C9), 122.9 (C8), 118.5 (C4), 60.2 (C6a), 36.6 (C10a), 35.7 (C10), 34.8 (C7), 26.3 (C14), 18.8, 18.9 (CH3); GC/MSm/z (%): 306 (M+, 1), 261 (100), 233 (11), 219 (11), 207 (10), 165 (5), 70 (7), 43 (26); EA (%) calculated for C17H17O3Cl-0.4H2O: 65.45 C, 5.75 H; found 65.58 C, 5.79 H.
(6aR,10aR)-rel-6a-Acetyl-2-bromo-6a,7,10,10a-tetrahydro-8,9-dimethyl-6-oxodibenzo[b,d]pyran (10c). Pale yellow solid in 55% yield, mp 50–54 °C. IR/ν(cm-1): 1773 (OC=O), 1711 (C=O), 1205, 1138 (C-O), 821 (C-Br). 1H-NMR: 7.30 (dd, 1H, 3J = 7.2, 4J = 1.7, H3), 7.27 (d, 1H, 4J = 1.7 Hz, H1), 6.86 (d, 1H, 3J = 7.2, H4), 3.41 (dd, 1H, 3J = 6.4, 11.4 Hz, H10a), 2.83 (d, 1H, 2J = 16.8, Heq-7), 2.14 (dd, 1H, 2J = 16.9, 3J = 11.4, Heq-10), 2.09 (s, 3H, H14), 2.04 (d, 1H, 2J = 16.9, Hax-7), 1.92 (dd, 1H, 2J = 16.8, 3J = 6.4, Hax-10), 1.57 (s, 3H, CH3), 1.66 (s, 3H, CH3);13C-NMR: 203.6 (CO), 167.9 (OCO), 149.4 (C4a), 130.4 (C1), 131.8 (C3), 129.6 (C2), 123.4 (C9), 122.8 (C8), 118.9 (C4), 117.7 (C10b), 60.3 (C6a), 36.5 (C10a), 35.7 (C10), 34.7 (C7), 26.2 (C14), 18.8, 18.9 (CH3); GC/MS m/z (%): 348 (M+, 1), 305 (100), 277 (5), 251 (6), 198 (10), 165 (10), 43 (58); EA (%) calculated for C17H17O3Br: C 58.47 C, 4.91 H; found: C 58.43 C, 4.82 H.
(6aR,10aR)-rel-6a-Acetyl-6a,7,10,10a-tetrahydro-8,9-dimethyl-2-nitro-6-oxodibenzo[b,d]pyran (10d). Pale yellow powder in 81% yield, mp 142–147 C. IR/ν(cm-1): 1782 (OC=O), 1726 (C=O), 1525 (NO2), 1339, 1230 (C-O). 1H-NMR: 8.16 (d, 1H, 4J = 2.5, H1), 8.14 (dd, 1H, 3J = 9.3, 4J = 2.5, H3), 7.15 (d, 1H, 3J = 9.3, H4), 3.60 (dd,1H, 3J = 6.4, 11.1, H10a), 2.90 (d, 1H, 2J = 17.3, Heq-7), 2.38 (d, 1H, 2J = 17.3, Hax-7), 2.27 (dd, 1H, 2J = 16.7, 3J = 6.2, Heq-10), 2.15 (s, 3H, H14), 2.05 (dd, 1H, 2J = 16.7, 3J = 11.1, Hax-10), 1.62 (s, 3H, CH3), 1.71 (s, 3H, CH3); 13C-NMR: 202.7 (CO), 166.9 (OCO), 154.7 (C4a), 144.6 (C2), 128.9 (C10b), 124.8 (C3), 123.6 (C1), 123.3 (C9), 122.9 (C8), 118.1 (C4), 60.1 (C6a), 36.5 (C10a), 35.6 (C10), 34.6 (C7), 26.1 (C14), 18.8, 18.9 (CH3); GC/MS m/z (%): 315 (M+, 1), 272 (100), 244 (8), 230 (8), 218 (8), 204 (6), 67 (7), 43 (25); EA (%) calculated for C17H17O5N: 64.75 C, 5.43 H, 4.44 N; found 65.10 C, 5.65 H, 4.20 N.
(6aR,10aR)-rel-6a-Acetyl-6a,7,10,10a-tetrahydro-2-methoxy-8,9-dimethyl-6-oxodibenzo[b,d]pyran (10e). Pale yellow solid in 55% yield, m.p 44–46 ºC. IR/ν(cm-1): 1761 (OC=O) 1710 (C=O) 1190, 1147 (C-O). NMR: 1H-NMR: 6.89 (d, 1H, 4J = 3.5, H1), 6.87 (d, 1H, 3J = 6.5, H4), 6.68 (dd, 1H, 3J = 6.5, 4J = 3.2, H3), 3.71 (s, 3H, OCH3), 3.36 (dd,1H, 3J = 6.5, 11.0, H10a), 2.78 (d, 1H, 2J = 16.7, Heq-7), 2.19 (dd, 1H, 2J = 18.2, 3J = 6.5, Heq-10), 2.06 (s, 3H, H14), 2.09 (d, 1H, 2J = 16.7, Hax-7), 2.00 (dd, 1H, 2J = 18.2, 3J = 11.0, Hax-10), 1.59 (s, 3H, CH3), 1.63 (s, 3H, CH3); 13C-NMR: 203.7 (CO), 168.3 (OCO), 156.2 (C2), 143.8 (C4a), 128.0 (C3), 123.0 (C9), 122.4 (C8), 117.5 (C1), 113.2 (C10b), 112.3 (C4), 60.0 (C6a), 55.4 (OCH3), 36.7 (C10a), 35.4 (C10), 34.4 (C7), 25.9 (C14), 18.4, 18.5 (CH3); GC/MS m/z (%): 300 (M+, 1), 218 (80), 190 (5), 175 (15); EA (%) calculated for C18H20O4: 71.98 C, 6.71 H; found: 71.91 C, 6.65 H.
(6aR,10aR)-rel-6a-Acetyl-6a,7,10,10a-tetrahydro-4-methoxy-8,9-dimethyl-6-oxodibenzo[b,d]pyran (10f). Pale yellow powder 85% yield, mp 115–119 ºC. IR ν(cm-1): 1769 (OC=O), 1706 (C=O), 1196, 1095 (C-O). 1H-NMR: 7.03 (dd, 1H, 3J = 8.4, 7.7 H2), 6.82 (d, 1H, 3J = 8.4, Hz, H1), 6.78 (d, 1H, 3J = 7.7, H3), 3.85 (s, 3H, OCH3), 3.44 (dd, 1H, 3J = 6.4, 11.4, H10a), 2.88 (d, 1H, 2J = 16.7, Heq-7), 2.21 (dd, 1H, 2J = 18.2, 3J = 6.4, Heq-10), 2.12 (s, 3H, H14), 2.11 (d, 1H, 2J = 16.7, Hax-7), 2.03 (dd, 1H, 2J = 18.5, 3J = 11.4, Hax-10), 1.68 (s, 3H, CH3),1.59 (s, 3H, CH3); 13C-NMR: 203.9 (CO), 167.9 (OCO), 147.8 (C10), 139.9 (C8), 128.7 (C9), 125.2 (C6), 123.5 (C9), 122.8 (C8), 119.1 (C7), 111.6 (C5), 60.4 (C6a), 56.2 (OCH3), 36.9 (C10a), 35.8 (C10), 34.9 (C7), 26.4 (C14), 18.8, 18.9 (CH3); GC/MS m/z (%): 300 (M+, 1), 257 (100), 229 (7), 203 (8), 105 (2), 105 (2), 91 (4), 77 (4), 43 (17); EA (%) calculated for C18H20O4: 71.98 C, 6.71 H; found: 72.25 C, 6.80 H.
(6aR,10aR)-rel-6a-Acetyl-2,4-dichloro-6a,7,10,10a-tetrahydro-8,9-dimethyl-6-oxodibenzo[b,d]pyran (10g). Pale yellow powder in 71% yield, mp 148–151 ºC. IR/ν(cm-1): 1787 (OC=O), 1703 (C=O), 1233, 1192 (C-O), 810 (C-Cl). 1H-NMR: 7.28 (d, 1H, 4J = 2.4, H3), 7.11 (d, 1H, 4J = 2.4, H1), 3.47 (dd, 1H, 3J = 6.2, 11.5, H10a), 2.89 (d, 1H, 2J = 17.0, Heq-7), 2.28 (dd, 1H, 2J = 17.9, 3J = 6.2, Heq-10), 2.17 (d, 1H, 2J = 18.7, Hax-7), 2.14 (s, 3H, H14), 1.97 (dd, 1H, 2J = 18.9, 3J = 9.3, Hax-10), 1.69 (s, 3H, CH3), 1.59 (s, 3H, CH3); 13C-NMR: 202.8 (CO), 166.8 (OCO), 144.9 (C4a), 130.7 (C4), 130.1 (C2), 129.2 (C3), 126.1 (C1), 123.3 (C9), 122.9 (C8), 60.1 (C6a), 36.9 (C10a), 35.5 (C10), 34.9 (C7), 26.5 (C14), 18.8, 18.9 (CH3); GC/MSm/z (%): 339 (M+, 1), 296 (100), 268 (9), 253 (12), 241 (12), 232 (9), 91 (5), 67 (8), 43 (37); EA (%) calculated for C17H16O3Cl2: 60.19 C, 4.75 H; found 60.15 C, 4.85 H.
(6aR,10aR)-rel-6a-Acetyl-4-bromo-2-chloro-6a,7,10,10a-tetrahydro-8,9-dimethyl-6-oxodibenzo[b,d]pyran (10h). Pale yellow powder in 62% yield, mp 152–155 ºC. IR ν(cm-1): 1785 (OC=O), 1703 (C=O), 1232, 1131 (C-O), 787 (C-Cl), 524 (C-Br). 1H-NMR: 7.45 (d, 1H, 4J = 2.4, H1), 7.15 (d, 1H, 4J = 2.4, H3), 3.46 (dd,1H, 3J = 6.4, 11.4, H10a), 2.89 (d, 1H, 2J = 17.1, Heq-7), 2.31 (dd, 1H, 2J = 17.6, 3J = 6.4, Heq-10), 2.19 (d, 1H, 2J = 16.9, Hax-7), 2.14 (s, 3H, H14), 1.99 (dd, 1H, 2J = 17.6, 3J = 11.4, Hax-10), 1.69 (s, 3H, CH3), 1.60 (s, 3H, CH3); 13C-NMR: 202.8 (CO), 166.9 (OCO), 146.1 (C4a), 132.0 (C3), 130.6 (C2), 130.4 (C4), 126.8 (C1), 123.3 (C9), 122.9 (C8), 111.4 (C10b), 60.1 (C6a), 37.0 (C10a), 35.5 (C10), 34.9 (C7), 26.6 (C14), 18.8, 18.9 (CH3); GC/MSm/z (%): 384 (M+, 1), 341 (100), 325 (5), 313 (6), 287 (10), 232 (15), 67 (16), 43 (27); EA (%) calculated for C17H16O3ClBr: 53.22 C, 4.2 H; found 53.60 C, 4.25 H.
(6aR,10aR)-rel-6a-Acetyl-2-bromo-6a,7,10,10a-tetrahydro-4-methoxy-8,9-dimethyl-6-oxodibenzo-[b,d]pyran (10i). Pale yellow powder in 83% yield, mp 160–164 ºC. IR/ν(cm-1): 1769 (OC=O), 1706 (C=O), 1275, 1196 (C-O), 506 (C-Br). 1H-NMR: 6.94 (s, 1H, H1), 6.94 (s, 1H, H3), 3.84 (s, 3H, OCH3), 3.41(dd, 1H, 3J = 6.2, 11.3, H10a), 2.88 (d, 1H, 2J = 16.9, Heq-7), 2.36 (dd, 1H, 2J = 17.0, 3J = 6.2, Heq-10), 2.18 (d, 1H, 2J = 16.9, Hax-7), 2.13 (s, 3H, H14), 1.97 (dd, 1H, 2J = 17.0, 3J = 11.3, Hax-10), 1.68 (s, 3H, CH3), 1.59 (s, 3H, CH3); 13C-NMR: 203.4 (C=O), 167.4 (OCO), 148.4 (C4a), 138.5 (C4), 130.3 (C2), 123.4 (C9), 122.8 (C8), 121.9 (C3), 117.6 (C10b), 114.9 (C1), 60.2 (C6a), 56.5 (s, 3H, OCH3), 36.7 (C10a), 35.6 (C10), 34.9 (C7), 26.4 (C14), 18.8, 18.8 (CH3); GC/MS m/z (%): 380 (M+, 1), 337 (100), 309 (4), 283 (8), 256 (8), 228 (14), 115 (5), 91 (5), 43 (31); EA (%) calculated for C18H19O4Br: 57.01 C, 5.05 H; found 57.20 C, 5.06 H.
3.4. General synthetic procedure for epoxides 11-15
Prepared from 200 mg of compounds 6-10 and two equivalents of m-CPBA dissolved in CHCl3 (25 mL) and refluxed for 24 h. The CHCl3 solution was extracted with an aqueous saturated NaHCO3 solution. The organic layer was dried with Na2SO4, then filtered and concentrated.
Ethyl (6aR,7aR,8aS,9aR)-rel-6a,7,7a,8a,9,9a-hexahydro-7a,8a-dimethyl-6-oxo-5,8-dioxacyclopropa-[b]phenantrene-6a(6H)-carboxylate (11a). White crystalline solid in 73% yield, mp 79.8–82.5 °C. IR/ν (cm-1): 1772 (OC=O), 1735 (EtOC=O), 1248, 1230, 1150 (C-O). 1H-NMR: 7.19 (ddd, 1H, 3J = 7.7, 7.7, 4J = 1.8 Hz, H3), 7.09 (dd, 1H, 3J = 7.5, 4J = 1.8, H1), 7.03 (dd, 1H, 3J = 7.5, 4J = 1.1, H2), 6.98 (d, 1H, 3J = 8.6, H4), 3.87 (m, 2H, OCH2), 3.36 (dd, 1H, 3J = 12.4 and 5.5, H9a), 2.83 (d, 1H, 3J = 15.2, Heq-7), 2.24 (d, 1H, 3J = 15.4, Hax-7), 2.21 (dd, 1H, 3J = 10.3 and 5.5, Heq-9), 1.60 (dd, 1H, 3J = 15.6 and 12.5, Hax-9), 1.40, 1.24 (s, 3H c/u, 2CH3), 0.83 (t, 3H, CH3-CH2). 13C-NMR: 169.9 (OCO), 167.4 (OCO lactone), 150.9 (C4a), 129.1 (C1), 127.8 (C3), 126.4 (C9b), 125.1 (C2), 117.0 (C4), 62.3 (CH2-O), 61.7 (C8a), 60.3 (C7a, 53.3 (C6a), 35.6 (C9a), 34.5 (C9), 34.4 (C7), 21.0 (CH3-C8a), 19.2 (CH3-C7a), 13.9 (CH3-CH2); GC/MS m/z (%): 316 (M+, 2), 259 (38), 243 (100), 225 (50), 214 (34), 199 (24), 145 (11), 115 (17), 91 (6), 43 (52); EA (%) calculated for C18H20O5: 68.34 C, 6.37 H; found: 68.35 C, 6.32 H.
(6aR,7aR,8aS,9aR)-rel-N-benzyl-6a,7,7a,8a,9,9a-hexahydro-7a,8a-dimethyl-6-oxo-5,8-dioxacyclo-propa[b]phenantrene-6a(6H)-carboxamide (12a). White crystalline solid in 78% yield, mp 205.4–207 °C. IR/ν (cm-1): 3342 (NH), 1773 (OC=O), 1637 (NC= O), 1538 (C=C), 1234 (C-O), 1152 (C-N). 1H- NMR: 7.27 (m, 1H, H3), 7.21 (m, 5H, 5H-C6H5), 7.12 (t, 1H, 3J =7.4, H6), 7.01 (d, 1H, 3J = 8.0, H5), 6.82 (m, 1H, H4), 5.82 (t, 1H, NH), 4.22 (m, 2H, AA’BB’, NCH2), 3.52 (dd, 1H, 2J = 12.5, 3J = 5.3, H9a), 2.94 (d, 1H, 2J = 15.5, Heq-7), 2.32 (dd, 1H, 2J = 15.5, 3J = 5.2, Heq-9), 2.27 (d, 1H, 2J = 15.5, Hax-7), 1.69 (dd, 1H, 2J = 15.6, 3J = 12.7, Hax-9), 1.43 (s, 3H, CH3), 1.30 (s, 3H, CH3); 13C-NMR: 169.4 (NCO), 168.0 (OCO lactone), 150.3 (C4a), 137.4 (Ci), 128.9 (C-m and C1), 128.0 (C3), 127.8 (Cp), 127.5 (Co), 127.0 (C9b), 125.6 (C2), 117.0 (C4), 61.8 (C8a), 60.5 (7a), 54.6 (C6a), 43.9 (NCH2), 36.0 (C9a), 35.7 (C9), 34.7 (C8a), 21.0 (CH3-C8a), 19.1 (CH3-C7a); GC/MS m/z (%): 377 (M+, 2), 318 (10), 243 (40), 227 (15), 225 (30), 211 (32), 199 (8), 173 (8), 150 (100), 131 (10), 91 (95), 43 (30); EA (%) calculated for C23H23O4N: 73.19 C, 6.14 H; found: 73.09 C, 6.14 H.
(6aR,7aR,8aS,9aR)- and (6aS,7aS,8aR,9aS)-6a,7,7a,8a,9,9a-hexahydro-7a,8a-dimethyl-6-oxo-N-[(R)-1-phenylethyl]-5,8-dioxacyclopropa[b]phenantrene-6a(6H)-carboxamide (13a). White crystalline powder in 78% yield, mp 163.9–165.0 °C; IR ν (cm-1): 3256 (NH), 1759 (OC=O), 1638 (NC=O), 1230, 1161, 1151 (C-O); GC/MS m/z (%) 391 (M+, 4), 332 (15), 243 (58), 227 (28), 225 (35), 211 (42), 199 (8), 173 (15), 105 (100), 43 (20). Minor (6aR,7aR,8aS,9aR) isomer 13a: 1H-NMR: 7.3–6.9 (m, 9H, ArH), 5.67 (d, 1H, 3J = 1.5, NH), 3.48 (dd, 1H, 3J = 5.1, 3J = 12.0, H9a), 2.92 (dd, 1H, 2J = 15.4, Heq-7), 2.27 (d, 1H, 2J = 15.6, Hax-7), 2.29 (m, 1H, Heq-9), 1.68 (dd, 1H, 2J = 15.6, 3J = 5.6, Hax-9), 4.84 (dq, 1H, 3J = 6.9, CHCH3), 1.43 and 1.30 (two s, 2 × 3H, 7a- and 8a-CH3), 1.26 (d, 3H, 3J = 7.0, CHCH3); 13C-NMR: 169.6 (NCO), 167.1 (C-6), 150.3 (C-4a), 142.0 (C-1’), 128.8 (C-3’,5’), 128.0 (C-1), no (C-3), 127.6 (C-9b), 127.0 (C-4’), 125.9 (C-2’,6’), 125.5 (C-2), 117.0 (C-4), 61.8 (C-8a), 60.6 (C-7a), 54.6 (C-6a), 49.1 (NCH), 35.9 (C-9a), 34.9 (C-9), 34.8 (C-7), 21.1, 21.0, and 19.1 (3 × CH3). Major (6aS,7aS,8aR,9aS) isomer 13a: 1H-NMR: 7.3–6.9 (m, 9H, ArH), 5.71 (d, 1H, 3J = 1.5, NH), 3.44 (dd, 1H, 3J = 5.1, 3J = 12.0, H-9a), 2.90 (dd, 1H, 2J = 15.4, Heq-7), 2.19 (d, 1H, 2J = 15.6, Hax-7), 2.29 (m, 1H, Heq-9), 1.68 (dd, 1H, 2J = 15.6, 3J = 5.6, Hax-9), 4.83 (dq, 1H, 3J = 6.9, CHCH3), 1.42 and 1.29 (two s, 2 × 3H, 7a- and 8a-CH3), 1.22 (d, 3H, 3J = 7.0, CHCH3); 13C-NMR: 169.3 (NCO), 167.1 (C-6), 150.4 (C-4a), 142.1 (C-1’), 129.0 (C-3’,5’), no (C-1), 127.9 (C-3), 127.7 (C-9b), 126.8 (C-4’), 126.1 (C-2’,6’), 125.5 (C-2), 117.0 (C-4), 61.8 (C-8a), 60.6 (C-7a), 54.5 (C-6a), 49.2 (NCH), 36.0 (C-9a), 35.5 (C-9), 34.8 (C-7), 21.1, 21.0, and 19.1 (3 × CH3).
(6aR,7aR,8aS,9aR)-rel-6a,7,7a,8a,9,9a-Hexahydro-7a,8a-dimethyl-6-oxo-N-(2-phenylethyl)-5,8-dioxacyclopropa[b]phenantrene-6a(6H)-carboxamide (14a). White crystalline powder in 78% yield, mp 189.4–191.6 °C. IR/ν (cm-1): 3322 (NH), 1789 (OC=O), 1640 (CONH), 1146 y 1129 (C-O). 1H- NMR: 7.25(m, 5H, Ph), 7.15 (dd, 1H, 3J = 7.4, 4J = 1.9, H3), 7.10 (dd, 1H, 3J = 7.4, 4J = 1.2, H2), 7.03 (d, 1H, 3J = 6.7, H1), 7.00 (d, 1H, 3J = 8.1, H4), 5.54 (a, 1H, NH), 3.48 (dd, 1H, 3J = 5.2, 3J = 12.6, H9a), 3.29 (q, 2H, 3J = 7.2, NCH2), 2.81 (d, 1H, 3J =5.5, Heq-7), 2.56 (t, 2H, 3J = 7.2, CH2), 2.28 (dd, 1H, 3J = 5.2, 2J = 15.7, Heq-9), 2.11 (d, 1H, 3J = 15.7, Hax-7), 1.65 (dd, 1H, 3J = 5.5 Hz, 3J= 12.6, Hax-9), 1.40, 1.29 (s, 3H c/u, 2CH3); 13C-NMR: 169.5 (NCO), 167.9 (OCO), 150.2 (C4a), 138.3 (Ci), 128.9 (Cm), 128.9 (Co), 128.8 (C1), 127.9 (C3), 126.9 (Cp), 125.7 (C2), 116.9 (C4), 127.1 (C9b), 61.8 (C8a), 60.5 (C7a), 54.4 (C6a), 41.2 (NCH2), 36.0 (CH2), 35.9 (C9a), 35.4 (C9), 34.5 (C7), 21.0 (CH3C8a), 19.1 (CH3C7a); GC/MS m/z (%) 391 (M+, 8), 332 (20), 243 (100), 227 (43), 225 (95), 211 (68), 173 (43); EA (%) calculated for C24H25O4N: 73.64 C, 6.44 H, 3.58 N; found: 73.27 C, 6.24 H, 3.44 N.
(6aR,7aR,8aS,9aR)-rel-6a-Acetyl-6a,7,7a,8a,9,9a-hexahydro-7a,8a-dimethyl-6-oxo-6H-5,8-dioxacyclopropa[b]phenantrene (15a). White crystalline powder in 70% yield, mp 125.6–126.9 °C. IR/ν (cm-1): 1772 (OC=O), 1699 (C=O), 1300, 1258, 1150 (C-O). 1H-NMR: 7.25 (dd, 1H, 3J = 7.6, 7.4, H2), 7.16 (d, 3J = 7.6, H1), 7.09 (dd, 1H, 3J= 7.4, 8.1, H3), 7.02 (d, 1H, 3J = 8.1, H4), 3.45 (dd, 1H, 3J = 12.2, 5.0, H9a), 2.83 (d, 1H, 2J = 15.0, Heq-7), 2.31 (dd, 1H, 2J = 15.4, 3J = 5.0, Heq-9), 2.08 (d, 1H, 2J = 15.0, Hax-7), 2.02 (s, 3H, CH3O), 1.68 (dd, 1H, 2J = 15.4, 3J = 12.2, Hax-9), 1.45, 1.31 (s, 3H c/u, 2CH3); 13C-NMR: 203.5 (COCH3), 170.4 (OCO), 150.6 (C4a), 134.9 (C9b), 130.5 (C1), 128.5 (C2), 125.4 (C3), 117.4 (C4), 62.0 (C8a), 60.4 (C7a), 60.3 (C6a), 35.9 (C9a), 34.3 (C9), 33.2 (C7), 25.5 (CH3CO), 21.0 (CH3C8a), 19.2 (CH3C7a); GC/MS m/z (%): 286 (M+, 1), 243 (16), 225 (28), 211 (100), 184 (14), 114 (17), 91 (9), 43 (60); EA (%) calculated for C17H18O4: 71.31 C, 6.34 H; found: 71.28 C, 6.44 H.
(6aR,7aR,8aS,9aR)-rel-6a-Acetyl-6a,7,7a,8a,9,9a-hexahydro-7a,8a-dimethyl-2-nitro-6-oxo-6H-5,8-dioxacyclopropa[b]phenantrene (15d). White solid in 96% yield, mp 216–220 °C. IR/ν(cm-1): 1786 (OC=O), 1700 (C=O), 1526 (NO2), 1338, 1237, 1142 (C-O). 1H-NMR: 8.18 (dd, 1H, 4J = 2.6, 3J = 8.8, H3), 8.15 (d, 1H, 4J = 2.6, H1), 7.18 (d, 1H, 3J = 8.8, H4), 3.63 (dd, 1H, 3J = 5.2, 12.1, H9a), 2.89 (d, 1H, 2J = 15.5, Heq7), 2.38 (dd, 1H, 2J = 15.5, 3J = 5.2, Heq-9), 2.15 (d, 1H, 2J = 15.5, Hax-7), 2.09 (s, 3H, H14), 1.70 (dd, 1H, 2J = 15.5, 3J = 12.1, Hax-9), 1.35 (s, 3H, CH3), 1.48 (s, 3H, CH3); 13C-NMR: 202.2 (CO), 166.8 (OCO), 154.8 (C4a), 144.7 (C2), 127.4 (C9b), 125.2 (C3), 123.6 (C1), 118.2 (C4), 61.6 (C6a), 60.0 (C8a), 59.9 (C7a), 35.6 (C9), 34.2 (C9a), 32.9 (C7), 25.4 (C14), 20.9, 19.0 (CH3); GC/MS m/z (%): 331 (M+, 1), 288 (19), 256 (100), 230 (26), 115 (5), 91 (4), 43 (78); EA (%) calculated for C17H17O6N: 61.63 C, 5.17 H, 4.23 N; found: 61.44 C, 5.25 H, 4.14 N.
(6aR,7aR,8aS,9aR)-rel-6a-Acetyl-6a,7,7a,8a,9,9a-hexahydro-4-methoxy-7a,8a-dimethyl-6-oxo-6H-5,8-dioxacyclopropa[b]phenantrene (15f). White solid in 92% yield, mp 188–192 °C. IR/ν(cm-1): 1764 (OC=O), 1702 (C=O), 1281, 1153, 1095 (C-O). 1H-NMR: 7.05 (t, 1H, H2, 3J = 7.9), 6.85 (dd, 1H, 3J = 7.9, H1), 6.74 (dd, 1H, 3J = 7.9, H3), 3.85 (s, 3H, OCH3), 3.44 (dd, 1H, 3J = 5.0, 12.3, H9a), 2.85 (d, 1H, 2J = 15.2, Heq-7), 2.31 (dd, 1H, 2J = 15.5, 3J = 5.0, Heq-9), 2.07 (d, 1H, 2J = 15.2, Hax-7), 2.04 (s, 3H, H14), 1.70 (dd, 1H, 2J = 15.5, 3J = 12.3, Hax-9), 1.45 (s, 3H, CH3), 1.31 (s, 3H, CH3); 13C-NMR: 203.4 (CO), 167.8 (OCO), 147.8 (C4a), 139.6 (C4), 127.2 (C9b), 125.5 (C2), 118.9 (C1), 111.9 (C3), 61.9 (C6a), 60.3 (C8a), 60.1 (C7a), 56.3 (OCH3), 35.6 (C9), 34.4 (C9a), 33.2 (C7), 25.6 (C14), 19.2, 21.0 (CH3); GC/MS m/z (%): 316 (M+, 1), 273 (53), 255 (100), 245(4), 241 (60), 211 (11), 115 (13), 91 (8), 43 (47); EA (%) calculated for C18H20O5: 68.34 C, 6.37 H; found: 64.83 C, 6.28 H.
(6aR,7aR,8aS,9aR)-rel-6a-Acetyl-2-bromo-6a,7,7a,8a,9,9a-hexahydro-4-methoxy-7a,8a-dimethyl-6-oxo-6H-5,8-dioxacyclopropa[b]phenantrene (15i). White powder in 95% yield, mp 169–172 °C. IR/ν(cm-1): 1768 (OC=O), 1702 (C=O), 1198, 1154, 1123 (C-O), 512 (C-Br). 1H-NMR: 6.97 (d, 1H, 4J = 2.1, H1), 6.91 (d, 1H, 4J = 2.1, H3), 3.86 (s, 3H, OCH3), 3.42 (dd, 1H, 3J = 5.0, 12.3, H9a), 2.85 (d, 1H, 2J = 15.5, Heq-7), 2.30 (dd, 1H, 2J = 15.5, 3J = 5.0, Heq-9), 2.07 (s, 3H, H14), 2.06 (d, 1H, 2J = 15.5, Hax-7), 1.68 (dd, 1H, 2J = 15.5, 3J = 12.3, Hax-9), 1.45 (s, 3H, CH3), 1.32 (s, 3H, CH3); 13C-NMR: 202.8 (CO), 167.2 (OCO), 148.5 (C4a), 138.7 (C4), 128.8 (C2), 121.7 (C3), 117.8 (C9b), 115.4 (C1), 61.7 (C8a), 60.2 (C7a), 59.9 (C6a), 56.4 (OCH3), 35.4 (C9), 34.3 (C9a), 33.2 (C7), 25.7 (C14), 20.9, 19.2 (CH3); GC/MS m/z (%): 395 (M+, 1), 351 (27), 334 (10), 321 (100), 255 (8), 240 (28), 115 (12), 91 (5), 43 (80); EA (%) calculated for C18H19O5Br: 54.70 C, 4.85 H; found: 54.26 C, 4.74 H.
3.5. Crystal structures
Compound 10b: C17H17C O3, colorless crystals, monoclinic, P21/c, Z = 4, a = 18.809(2) Å, b = 7.1461(9) Å, c = 11.4261(14) Å, α = 90º, β = 105.493(2)º, γ = 90º, V = 1480.0(3) Å3, Dcalcd = 1.368 g/cm3, μ = 0.265 mm-1, 10086 reflections collected, 2603 independent (Rint = 0.025), 2414 observed, R1 = 0.0411, wR2 = 0.1198 (I > 2σ(I)).
Compound 15i: C18H19BrO5, colorless crystals, triclinic, P-1, Z = 2, a = 7.3676(18) Å, b = 10.851(3) Å, c = 12.154(3) Å, α = 108.055(4)º, β = 97.784(4)º, γ = 106.789(4)º, V = 857.0(4) Å3, Dcalcd = 1.532 g/cm3, μ = 2.423 mm-1, 8373 reflections collected, 3017 independent (Rint = 0.046), 2455 observed, R1 = 0.0575, wR2 = 0.1149 (I > 2σ(I)).