Synthesis and Characterization of Organotin Containing Copolymers: Reactivity Ratio Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Organotin Monomers
2.1.1. Dibutyltin Maleate (DBTM, I)
2.1.2. Dibutyltin Citraconate (DBTC, II)
2.2. Copolymerization Method
2.3. Overall Conversion and Structural Characterization
2.4. Reactivity Ratio Determination
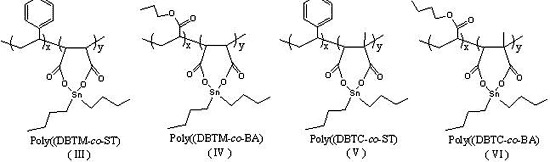
2.4.1. Poly(DBTM-co-ST) (III)
2.4.2. Poly(DBTM-co-BA) (IV)
2.4.3. Poly(DBTC-co-ST) (V)
2.4.4. Poly(DBTC-co-BA) (VI)
3. Experimental
3.1. Materials
3.2. Characterization
3.3. Synthesis of Organotin Monomers
3.3.1. Synthesis of Dibutyltin Maleate (DBTM, I)
3.3.2. Synthesis of Dibutyltin Citraconate (DBTC, II)
3.4. General Procedure for Copolymerization
3.5. Overall Conversion

3.6. Reactivity Ratios Determination

4. Conclusions
References
- Liu, G.; Zhang, L.; Wang, Y.; Zhao, P. Studies on binary copolymerization and glass transition temperatures of methyl methacrylate with ethyl methacrylate and n-butyl methacrylate. J. Appl. Polym. Sci. 2009, 114, 3939–3944. [Google Scholar] [CrossRef]
- Saby-Dubreuil, A.C.; Guerrier, B.; Allain, C. Glass transition induced by solvent desorption for statistical MMA/nBMA copolymers-Influence of copolymer composition. Polymer 2001, 42, 1383–1391. [Google Scholar] [CrossRef]
- Erol, I.; Sen, O.; Dedelioglu, A.; Cifci, C. Synthesis and characterization of novel fluorine-containing methacrylate copolymers: Reactivity ratios, thermal properties, and antimicrobial activity. J. Appl. Polym. Sci. 2009, 114, 3351–3359. [Google Scholar] [CrossRef]
- Hou, C.; Liu, J.; Ji, C.; Ying, L.; Sun, H.; Wang, C. Monomer apparent reactivity ratios for acrylonitrile/methyl vinyl ketone copolymerization system. J. Appl. Polym. Sci. 2006, 102, 4045–4048. [Google Scholar] [CrossRef]
- Habibi, A.; Farahani, E.V.; Semsarzadeh, M.A.; Sadaghiani, K. Monomer reactivity ratios for lauryl methacrylate-isobutyl methacrylate in bulk free radical copolymerization. Polym. Int. 2003, 52, 1434–1443. [Google Scholar] [CrossRef]
- Hou, C.; Ji, C.; Ying, L. Monomer apparent reactivity ratios for acrylonitrile/ammonium itaconate radical copolymerization systems. J. Appl. Polym. Sci. 2007, 103, 3920–3923. [Google Scholar] [CrossRef]
- Miller, A.; Szafko, J.; Turska, E. Reactivity ratios for acrylonitrile-vinyl chloroacetate copolymerization systems. J. Polym. Sci. 1977, 15, 51–63. [Google Scholar] [CrossRef]
- Bajaj, P.; Sen, K.; Hajir, B.S. Solution polymerization of acrylonitrile with vinyl acids in dimethylformamide. J. Appl. Polym. Sci. 1996, 59, 1539–1550. [Google Scholar] [CrossRef]
- Soykan, C.; Coskun, M.; Ahmedzade, M. Synthesis and characterization of phenacyl methacrylate - acrylonitrile copolymers and determination of monomer reactivity ratios. Polym. Int. 2000, 49, 479–484. [Google Scholar] [CrossRef]
- Al-Muaikel, N.S.; Al-Diab, S.S.; Al-Salamah, A.A.; Zaid, A.M.A. Synthesis and characterization of novel organotin monomers and copolymers and their antibacterial activity. J. Appl. Polym. Sci. 2000, 77, 740–745. [Google Scholar] [CrossRef]
- Rehman, S-ur.; Shahid, K.; Ali, S.; Mazhar, M.; Badshah, A.; Eng, G.; Song, X.; Ryczkowski, J. Synthesis, spectroscopic characterization, and in vitro biological activity of organotin(IV) complexes of (E)-3-(4-methoxyphenyl)-2-phenyl-2-propenoic acid. Heter. Chem. 2005, 16, 175–183. [Google Scholar] [CrossRef]
- Garg, B.K.; Corredor, J.; Subramanian, R.V. Copolymerization of Tri-n-butyltin Acrylate and Tri-n-butyltin Methacrylate Monomers with Vinyl Monomers Containing Functional Groups. J. Macromol. Sci. Part A: Pure Appl. Chem. 1977, 11, 1567–1601. [Google Scholar] [CrossRef]
- Al-Diab, S.S. Synthesis of novel organotin copolymers. J. Chem. Res. 1986, (S), 306–307. [Google Scholar]
- Gaina, C.; Gaina, V. Synthesis and characterization of novel organotin carboxylate maleimide monomers and polymers. eXPRESS Polym. Lett. 2009, 3, 352–358. [Google Scholar] [CrossRef]
- Tawfik, S.Y.; Messiha, N.N.; El-Hamouly, S.H. Effect of substitution on the reactivity of some new p-phenylacrylamide derivatives with organotin monomers. J. Polym. Sci. 1993, 31, 427–433. [Google Scholar] [CrossRef]
- Ghanem, N.A.; Messiha, N.N.; Abd-Elmalek, M.M.; Ikladious, N.E.; Shaaban, A.F. J. Coat. Tech. 1981, 53, 57–60.
- Shaaban, A.F.; Hilmym, N.H.; Wakid, A.M.; El-Monairy, O.M.; Mohammed, A.A. Structure-performance relationships in organotin mercaptide stabilizers. Pure Appl. Chem. 1981, 53, 577–582. [Google Scholar]
- Eng, G.; Tierney, E.J.; Bellama, J.M.; Brinckman, F.E. Correlation of molecular total surface area with organotin toxicity for biological and physicochemical applications. Appl. Organomet. Chem. 1988, 2, 171–175. [Google Scholar] [CrossRef]
- Finemann, M.; Ross, S.D. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 1950, 5, 259–262. [Google Scholar] [CrossRef]
- Gilman, H.; Rosenberg, D. Reaction of Triphenyltin Hydride with Methyllithium. J. Am. Chem. Soc. 1953, 75, 3592–3593. [Google Scholar] [CrossRef]
- Shaaban, A.F.; Arief, M.M.; Mahmoud, A.A.; Messiha, N.N. Organotin polymers. XI. Radical copolymerization reactions of di-(tri-n-butyltin) itaconate with 2-chloroethyl acrylate n-butyl acrylate and allyl methacrylate. Acta Polym. 1987, 38, 492–495. [Google Scholar] [CrossRef]
- Pekel, N.; Sahiner, N.; Guven, O.; Rzaev, Z.M.O. Synthesis and characterization of N-vinylimidazole–ethyl methacrylate copolymers and determination of monomer reactivity ratios. Eur. Polym. J. 2001, 37, 2443–2451. [Google Scholar] [CrossRef]
- Minora, Y.; Tadokoro, T.; Susuki, Y. Radical copolymerization of crotonyl compounds with styrene. J. Polym. Sci. Part A-1: Polym. Chem. 1967, 5, 2641–2654. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization, 4th ed.; John Willy & Sons Inc.: New York, NY, USA, 2004; pp. 200–205. [Google Scholar]
- Koton, M.M.; Kiseleva, T.M.; Florinskii, F.S. The effect of chemical structure on the ability of unsaturated organometallic compounds to undergo polymerization. J. Polym. Sci. 1961, 52, 237–244. [Google Scholar] [CrossRef]
- Kreisel, M.; Garbatski, U.; David, H.K. Copolymerization of styrene. I. Copolymerization with styrene derivatives containing nitrile groups in the side-chain. J. Polym. Sci. 1964, 2, 105–121. [Google Scholar] [CrossRef]
- Ghanem, N.A.; Messiha, N.N.; Ikladious, N.E.; Shaaban, A.F. Organotin polymers. IV. Binary and ternary copolymerizations of tributyltin acrylate and methacrylate with styrene, allyl methacrylate, butyl methacrylate, butyl acrylate, and acrylonitrile. J. Appl. Polym. Sci. 1981, 26, 97–106. [Google Scholar] [CrossRef]
- Al-Diab, S.S.; Suh, H.K.; Mark, J.E.; Zimmer, H. Radical copolymerization of 3-tri-n-butylstannylstyrene with several vinyl monomers. J. Polym. Sci. 1990, 28, 299–314. [Google Scholar] [CrossRef]
- Shaaban, A.F.; Arief, M.M.; Mahmoud, A.A.; Messiha, N.N. Organotin polymers: 10. Copolymerization parameters for di-(tri-n-butyltin) itaconate with methyl acrylate, ethyl acrylate, N-vinyl pyrrolidone and acrylonitrile. Polymer 1987, 28, 1423–1425. [Google Scholar] [CrossRef]
- Stanely, R.S.; Dannin, J.; Tsou, K.S. Copolymerization of p-triphenyltinstyrene and p-triphenylleadstyrene with styrene or vinyltoluene. J. Polym. Sci. Part A: Gen. Pap. 1965, 3, 3199–3207. [Google Scholar]
Sample Availability: Samples of the compounds are available from authors. |




| Monomer | Calc. | Found | ||||
|---|---|---|---|---|---|---|
| %C | %H | %Sn | %C | %H | %Sn* | |
| I | 41.54 | 5.81 | 34.21 | 41.85 | 6.01 | 33.11 |
| II | 43.25 | 6.14 | 32.88 | 42.85 | 6.56 | 33.11 |
| Copolymer Ratio | %Sn | |||
|---|---|---|---|---|
| IIIa | IVb | Vc | VId | |
| 80/20 | 15.00 | 7.14 | 15.39 | 7.99 |
| 60/40 | 11.47 | 4.18 | 10.68 | 4.50 |
| 50/50 | 9.00 | 2.88 | 7.66 | 2.93 |
| 40/60 | 6.02 | 2.33 | 5.03 | 2.06 |
| 30/70 | 2.67 | 0.73 | 2.01 | 0.72 |
| Copolymer code | %Sn | Moles of | F a | |||
|---|---|---|---|---|---|---|
| DBTM | DBTC | ST | BA | |||
| III | 3.74 | 0.0314 | - | 0.9011 | - | 0.0367 |
| IV | 1.19 | 0.0100 | - | - | 0.7531 | 0.0133 |
| V | 1.19 | - | 0.0426 | 0.8450 | - | 0.0225 |
| VI | 3.74 | - | 0.0165 | - | 0.7338 | 0.0504 |
| Copolymer Ratio | %Sn | M1a | Fb | m1c | fd | Conversion (wt/wt%)e |
|---|---|---|---|---|---|---|
| 20/80 | 2.67 | 0.2 | 0.2500 | 0.0247 | 0.0253 | 4.45 |
| 40/60 | 6.02 | 0.4 | 0.6667 | 0.0601 | 0.0639 | 3.79 |
| 50/50 | 9.00 | 0.5 | 1.0000 | 0.0964 | 0.1067 | 4.48 |
| 60/40 | 11.47 | 0.6 | 1.5000 | 0.1310 | 0.1507 | 4.60 |
| 70/30 | 15.00 | 0.7 | 2.3330 | 0.1891 | 0.2333 | 3.81 |
| Copolymer Ratio | Monomer Ratio F = M1/M2 | M-Unit Ratio in Copolymer | Parameters of FR Eq. | |
|---|---|---|---|---|
| F2/f | F/f(f-1) | |||
| 20/80 | 0.25 | 0.0253 | 2.4714 | -9.6355 |
| 40/60 | 0.667 | 0.0639 | 6.9520 | -9.7609 |
| 50/50 | 1.0 | 0.1067 | 9.3687 | -8.3687 |
| 60/40 | 1.5 | 0.1507 | 14.9250 | -8.4503 |
| 70/30 | 2.333 | 0.1137 | 23.3340 | -7.6686 |
| Copolymer | r1(k11/k12) a | r2(k21/k22) b | r1r2 |
|---|---|---|---|
| III | 0.0990 | 9.0650 | 0.9807 |
| IV | 0.0248 | 24.4310 | 0.7058 |
| V | 0.3258 | 14.8030 | 4.8228 |
| VI | 0.2727 | 33.6110 | 9.1657 |
| Copolymer Ratio | %Sn | M1a | F b | m1c | F d | Conversion (wt/wt%)f |
|---|---|---|---|---|---|---|
| 20/80 | 0.73 | 0.2 | 0.250 | 0.0080 | 0.0080 | 7.23 |
| 40/60 | 2.33 | 0.4 | 0.6667 | 0.0263 | 0.0270 | 6.49 |
| 50/50 | 2.88 | 0.5 | 1.000 | 0.0327 | 0.0338 | 8.01 |
| 60/40 | 4.18 | 0.6 | 1.500 | 0.0487 | 0.0512 | 7.19 |
| 70/30 | 7.14 | 0.7 | 2.333 | 0.0886 | 0.0972 | 1.84 |
| Copolymer Ratio | Monomer Ratio F = M1/M2 | M-Unit Ratio in Copolymer | Parameters of FR Eq. | |
|---|---|---|---|---|
| F2/f | F/f(f-1) | |||
| 20/80 | 0.250 | 0.0080 | 7.7895 | -30.0908 |
| 40/60 | 0.667 | 0.0027 | 16.4899 | -24.0554 |
| 50/50 | 1.000 | 0.0338 | 29.5598 | -28.5598 |
| 60/40 | 1.500 | 0.0512 | 43.9158 | -27.7772 |
| 70/30 | 2.333 | 0.0972 | 67.7638 | -26.7532 |
| Copolymer Ratio | %Sn | M1a | F b | m1 c | F d | Conversion (wt/wt%)e |
|---|---|---|---|---|---|---|
| 20/80 | 2.01 | 0.2 | 0.250 | 0.0184 | 0.0188 | 3.15 |
| 40/60 | 5.03 | 0.4 | 0.667 | 0.0494 | 0.0520 | 4.09 |
| 50/50 | 7.66 | 0.5 | 1.000 | 0.0803 | 0.0873 | 4.03 |
| 60/40 | 10.68 | 0.6 | 1.500 | 0.1214 | 0.1382 | 3.76 |
| 70/30 | 15.39 | 0.7 | 2.333 | 0.2016 | 0.2525 | 2.15 |
| Copolymer Ratio | Monomer Ratio F = M1/M2 | M-Unit Ratio in Copolymer | Parameters of FR Eq. | |
|---|---|---|---|---|
| F2/f | F/f(f-1) | |||
| 20/80 | 0.250 | 0.0188 | 3.3318 | -13.0773 |
| 40/60 | 0.667 | 0.0520 | 8.5572 | -12.1624 |
| 50/50 | 1.000 | 0.0873 | 11.454 | -10.4544 |
| 60/40 | 1.500 | 0.1382 | 16.276 | -9.3509 |
| 70/30 | 2.333 | 0.2525 | 21.552 | -6.9049 |
| Copolymer Ratio | %Sn | M1a | Fb | m1c | fd | Conversion (wt/wt%)e |
|---|---|---|---|---|---|---|
| 20/80 | 0.72 | 0.2 | 0.250 | 0.0079 | 0.0079 | 13.38 |
| 40/60 | 2.06 | 0.4 | 0.667 | 0.0231 | 0.0237 | 4.49 |
| 50/50 | 2.93 | 0.5 | 1.000 | 0.0343 | 0.0355 | 6.82 |
| 60/40 | 4.50 | 0.6 | 1.500 | 0.0532 | 0.0562 | 3.00 |
| 70/30 | 7.99 | 0.7 | 2.333 | 0.1021 | 0.1137 | 1.56 |
| Copolymer Ratio | Monomer Ratio F = M1/M2 | M-Unit Ratio in Copolymer | Parameters of FR Eq. | |
|---|---|---|---|---|
| F2/f | F/f(f-1) | |||
| 20/80 | 0.250 | 0.0079 | 7.8379 | -31.1018 |
| 40/60 | 0.667 | 0.0237 | 18.795 | -27.5115 |
| 50/50 | 1.000 | 0.0355 | 28.132 | -27.1319 |
| 60/40 | 1.500 | 0.0562 | 40.051 | -25.2009 |
| 70/30 | 2.333 | 0.1137 | 47.888 | -18.1932 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Deyab, S.S.; Al-Hazmi, A.M.; El-Newehy, M.H. Synthesis and Characterization of Organotin Containing Copolymers: Reactivity Ratio Studies. Molecules 2010, 15, 1784-1797. https://doi.org/10.3390/molecules15031784
Al-Deyab SS, Al-Hazmi AM, El-Newehy MH. Synthesis and Characterization of Organotin Containing Copolymers: Reactivity Ratio Studies. Molecules. 2010; 15(3):1784-1797. https://doi.org/10.3390/molecules15031784
Chicago/Turabian StyleAl-Deyab, Salem S., Ali Mohsen Al-Hazmi, and Mohamed H. El-Newehy. 2010. "Synthesis and Characterization of Organotin Containing Copolymers: Reactivity Ratio Studies" Molecules 15, no. 3: 1784-1797. https://doi.org/10.3390/molecules15031784