Triterpenes from Stachyurus himalaicus var. himalaicus Hook. f. et Thoms. ex Benth
Abstract
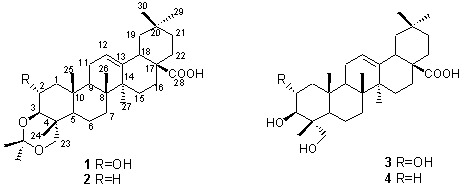
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General
3.2. Plant material
3.3. Extraction and isolation
3.4. Spectral data
3.5. Cytotoxic activity
4. Conclusions
Acknowledgments
References
- Institutum Botanicum Kunmingense Academiae Sinica. Flora Yunnanica, (in Chinese); Science Press: Beijing, China, 1982; Tomus 3; p. 339. [Google Scholar]
- Okuda, T.; Hatano, T.; Yazaki, K. Praecoxin B, C, D and E, novel ellagitannins tannins from Stachyurus praecox. Chem. Pharm. Bull. 1983, 31, 333–336. [Google Scholar] [CrossRef]
- Hatano, T.; Yazaki, K.; Okonogi, A.; Okuda, T. Tannins of Stachyurus species. II. Praecoxins A, B, C and D, four new hydrolysable tannins from Stachyurus praecox leaves. Chem. Pharm. Bull. 1991, 39, 1689–1693. [Google Scholar] [CrossRef]
- Li, H.; Hatano, T.; Okuda, T.; Yoshida, T. Tannins of Stachyurus species. III. Stachyuranins A, B and C, three new complex tannins from Stachyurus praecox leaves. Chem. Pharm. Bull. 1995, 43, 2109–2114. [Google Scholar]
- Yang, J.H.; Wang, Y.S.; Huang, R.; Luo, S.D.; Zhang, H.B.; Li, L. New polyoxgenated triterpenoids from Stachyurus himalaicus var. himalaicus. Helv. Chim. Acta. 2006, 89, 2830–2835. [Google Scholar] [CrossRef]
- Wang, Y.S.; Yang, J.H.; Luo, S.D.; Zhang, H.B.; Li, L. New cytotoxic steroid from Stachyurus himalaicus var. himalaicus. Molecules 2007, 12, 536–542. [Google Scholar] [CrossRef] [PubMed]
- González, A.G.; Alvarenga, N.L.; Bazzocchi, I.L.; Ravelo, A.G.; Moujir, L. A new bioactive norquinone-methide triterpene from Maytenus scutioides. Planta Med. 1998, 64, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Shiojima, K.; Arai, Y.; Masuda, K.; Takase, Y.; Ageta, T.; Ageta, H. Mass spectra of pentacyclic triterpenoids. Chem. Pharm. Bull. 1992, 40, 1683–1690. [Google Scholar] [CrossRef]
- Budzikiewics, H.; Wilson, J.M.; Djerassi, C. Mass spectrometry in structural and stereochemical problems. XXXII. 1 Pentacyclic triterpenes. J. Am. Chem. Soc. 1963, 85, 3688–3699. [Google Scholar] [CrossRef]
- Li, B.Z.; Wang, B.G.; Jia, Z.J. Pentacyclic triterpenoids from Rubus xanthocarpus. Phytochemistry 1998, 49, 2477–2481. [Google Scholar] [CrossRef]
- Higuchi, R.; Kawasaki, T. Pericarp saponins of Akebia quinata Decne. II. Arjunolic and norarjunolic acids and their glycosides. Chem. Pharm. Bull. 1976, 24, 1314–1323. [Google Scholar] [CrossRef]
- Anisimov, M.M.; Shentsova, E.B.; Shcheglov, V.V.; Strigina, L.I.; Chetyrina, N.S.; Aladjina, N.G.; Vecherko, L.P.; Zorina, A.D.; Matyukhina, L.G.; Saltykova, I.A. Toxic effects of certain pentacyclic triterpenoids on early embryogenesis of the sea urchin. Toxicon 1976, 14, 259–265. [Google Scholar] [CrossRef]
- Kizu, H.; Tomimori, T. Studies on the constituents of Clematis species. V. On the saponins of the root of Clematis chinensis OSBECK. (5). Chem. Pharm. Bull. 1982, 30, 3340–3346. [Google Scholar] [CrossRef]
- Javsinghe, L.; Wannigamma, G.P.; Macleod, J.K. Triterpenoids from Anamirta cocculus. Phytochenmistry 1993, 34, 1111–1116. [Google Scholar]
Sample Availability: Available from the authors. |


| Atom No. | 1 | 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 2.05, 0.98 (each 1H, m) | 46.8 (t) | 1.67, 1.01 (each 1H, m) | 38.9 (t) |
| 2 | 3.78 (1H, ddd, 4.5, 4, 9.7) | 65.6 (d) | 1.61, 1.40 (each 1H, m) | 23.4 (t)b |
| 3 | 3.32 (1H, d, 9.6) | 82.4 (d) | 3.49 (1H, dd, 3.6, 9.5) | 77.7 (d) |
| 4 | / | 37.3 (s) | / | 36.9 (s) |
| 5 | 0.88 (1H, m) | 51.8 (d) | 0.78 (1H, m) | 51.6 (d) |
| 6 | 1.40, 0.86 (each1H, m) | 17.9 (t) | 1.38, 1.22 (each 1H, m) | 17.8 (t) |
| 7 | 1.42, 1.26 (each 1H, m) | 32.4 (t) | 1.41, 1.25 (each 1H, m) | 32.3 (t) |
| 8 | / | 39.7 (s) | / | 39.6 (s) |
| 9 | 1.70 (1H, m) | 48.0 (d) | 1.58 (1H, m) | 47.8 (d) |
| 10 | / | 38.5 (s) | / | 37.4 (s) |
| 11 | 1.94, 1.80 (each 1H, m) | 23.2 (t)c | 1.90, 1.78 (each 1H, m) | 23.6 (t)b |
| 12 | 5.28 (1H, s) | 122.6 (d) | 5.27 (1H, s) | 122.6 (d) |
| 13 | / | 143.9 (s) | / | 143.7 (s) |
| 14 | / | 42.0 (s) | / | 41.0 (s) |
| 15 | 1.67, 1.04 (each 1H, m) | 28.0 (t) | 1.71, 1.06 (each 1H, m) | 27.8 (t) |
| 16 | 1.99, 1.61 (each 1H, m) | 23.6 (t)c | 1.98, 1.41 (each 1H, m) | 23.0 (t) |
| 17 | / | 46.6 (s) | / | 46.6 (s) |
| 18 | 2.82 (1H, dd, 3.7, 13.7) | 41.3 (d) | 2.82 (1H, dd, 3.7, 13.3) | 41.7 (d) |
| 19 | 1.58, 1.14 (each 1H, m) | 46.2 (t) | 1.63, 1.16 (each 1H, m) | 46.0 (t) |
| 20 | / | 31.0 (s) | / | 30.8 (s) |
| 21 | 1.34, 1.20 (each 1H, m) | 34.1 (t) | 1.34, 1.22 (each 1H, m) | 33.9 (t) |
| 22 | 1.77, 1.56 (each 1H, m) | 32.8 (t) | 1.76, 1.56 (each 1H, m) | 32.6 (t) |
| 23 | 3.49, 3.46 (each 1H, d, 10.7) | 73.0 (t) | 3.44, 3.52 (each 1H, d, 10.7) | 72.8 (t) |
| 24 | 1.06 (3H, s) | 13.8 (q) | 1.04 (3H, s) | 12.6 (q) |
| 25 | 1.02 (3H, s) | 18.1 (q) | 0.96 (3H, s) | 16.6 (q) |
| 26 | 0.73 (3H, s) | 17.4 (q) | 0.72 (3H, s) | 17.2 (q) |
| 27 | 1.14 (3H, s) | 26.3 (q) | 1.14 (3H, s) | 26.1 (q) |
| 28 | / | 183.5 (s) | / | 184.4 (s) |
| 29 | 0.91 (3H, s) | 33.4 (q) | 0.90 (3H, s) | 33.2 (q) |
| 30 | 0.93 (3H, s) | 23.9 (q) | 0.92 (3H, s) | 23.7 (q) |
 | 1.45, 1.44 (each 3H, s) | 100.0 (s) 19.8 (q) 30.0 (q) | 1.45, 1.42 (each 3H, s) | 99.1 (s) 19.5 (q) 30.0 (q) |
| Compound | IC50 (μM) |
|---|---|
| 1 | > 142 |
| 2 | 29.3 ± 3.7 |
| 3 | 49.8 ± 4.3 |
| 4 | 19.3 ± 6.8 |
| Camptothecine | 0.5 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Y.-S.; Huang, R.; Li, N.-Z.; Xu, H.-Y.; Yang, J.-H. Triterpenes from Stachyurus himalaicus var. himalaicus Hook. f. et Thoms. ex Benth. Molecules 2010, 15, 2096-2102. https://doi.org/10.3390/molecules15042096
Wang Y-S, Huang R, Li N-Z, Xu H-Y, Yang J-H. Triterpenes from Stachyurus himalaicus var. himalaicus Hook. f. et Thoms. ex Benth. Molecules. 2010; 15(4):2096-2102. https://doi.org/10.3390/molecules15042096
Chicago/Turabian StyleWang, Yun-Song, Rong Huang, Ning-Zhong Li, Hong-Yi Xu, and Jing-Hua Yang. 2010. "Triterpenes from Stachyurus himalaicus var. himalaicus Hook. f. et Thoms. ex Benth" Molecules 15, no. 4: 2096-2102. https://doi.org/10.3390/molecules15042096