Boronate Derivatives of Functionally Diverse Catechols: Stability Studies
Abstract
:1. Introduction
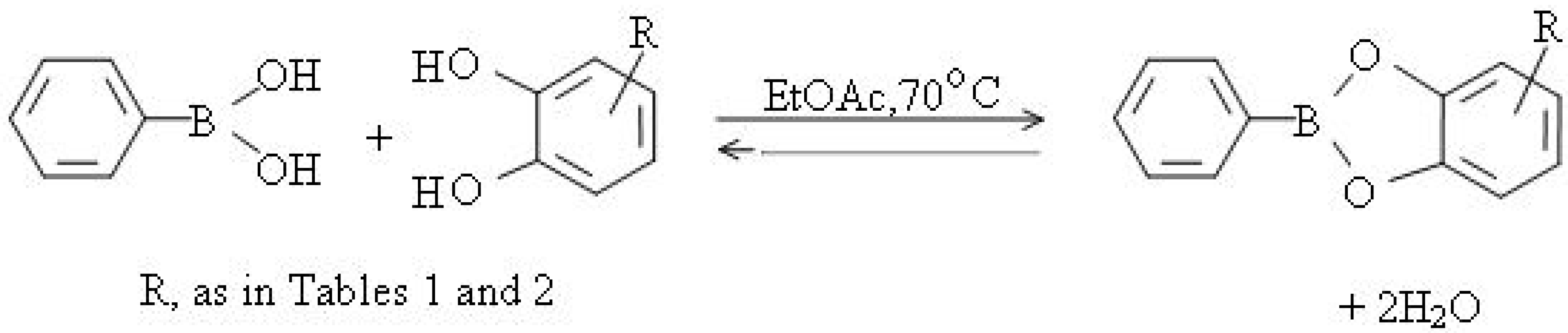
| Parent compound | Compound No | Amount used mg (mmol) | Benzeneboronic acidmg (mmol) | Data corresponding benzeneboronates | |||||
|---|---|---|---|---|---|---|---|---|---|
| Amount recovered mg a(% crude yield) | m.p. °C | Mol Formula (Mol. Wt) | Analysis | MS | |||||
| Found | Calc. | M+ (%) b | |||||||
| Methyl 3,4-dihydroxybenzoate c | 1 | 831 | 629.5 | 1050 | C14H11BO4 | C, 65.90 | C, 66.14 | 254 | |
| Methyl -3,4-dihydroxy-phenylacetate | 2 | 300 | 203 | 400 | C15H11BO4 | C, 67.20 | C, 67.16 | 268 | |
| Methyl 3,4-dihydroxydihydro-cinnamate | 3 | 500 | 317 | 680 | C16H15BO4 | C, 68.24 | C, 68.09 | 282 | |
| Methyl 3,4-dihydroxycinnamate | 4 | 263 | 170 | 349 | C16H13BO4 | C, 68.7 | C, 68.57 | 280 | |
| N-Acetyldopamine | 5 | 977 | 612 | 1405 | C16H16BNO3 | C, 68.0 | C, 68.33 | 281 | |
| Estra-1,3,5(10)-triene-3,4-diol i | 6 | 22 | 10 | 28 | 176-177 j | C24H27BO2 | C, 80.42 | C, 80.45 j | 358 |
| 4-Methyl-7,8-dihydroxy-coumarin k | 7 | 260 | 165 | 370 | 191-193 l | C16H11BO4 | l | 278 | |
- a GLC analysis (GC column as in b) of these crude products showed in each case a single peak corresponding to their benzeneboronate derivatives. Traces of excess benzeneboronic acid (eluted as triphenylboroxine) were also observed.
- b Mass spectral data were recorded at electron energy 20 eV, using an LKB 9000 GC-MS instrument, fitted with a glass column (2 m × 4 mm, i.d.) of 1% OV-1 on Gas Chrom Q (100–120 mesh). The flash heater was at 250 °C, the molecular separator at 270 °C, and ion source at 265 °C. The helium carrier gas flow rate was 30 mL/min. The trap current was 60 mA, filament current 4 A and accelerating voltage 3.5 KV. In Table 1 the abundances for the ion are shown in brackets ( ).
- c The isomeric methyl 2,3-dihydroxybenzoate did not react fully with benzeneboronic acid, as judged by GLC. On recrystallisation of the crude reaction mixture, from acetone-hexane; the recovered material was largely benzeneboronic acid. Vacuum sublimation of the crude product, also failed to yield any cyclic ester.
- d Recrystallisation from acetone-hexane; m.p. 107–108.5 °C; then vacuum sublimation: yield a middle fraction which was collected.
- e Vacuum sublimation at 55 °C/0.01 torr.
- f Vacuum sublimation at 55 °C/0.01 torr, yielded fine white crystals.
- g Recrystallisation from acetone-hexane; m.p. 125–127 °C; then followed by vacuum sublimation; a middle fraction was collected.
- h Vacuum sublimation at 130 °C/torr; m.p. 157–160 °C, a second sublimation yielded fine white crystals.
- i The isomeric 2-hydroxy-17-deoxoestrone benzeneboronate vacuum sublimation at 140 °C/0.01 torr; yielded a viscous gum, which failed to crystallize from acetone, EtOAc or hexane. Trituration in hexane at low temperature yielded a semi-solid product, which crystallized on standing m.p. 244–247 °C. GC showed traces of benzeneboronic acid. GC-MS, M+358 (100%), a satisfactory micro-analysis or HRMS was not adequate for this compound. This is due to partial hydrolysis on storage. The 1H-NMR showed satisfactory results.
- j Recrystallisation from acetone; then vacuum sublimation at 160 °C/0.01 torr. The sublimed material was recrystallised twice from acetone. High resolution MS, C24H27BO2, requires 358.2810; found 358.2104.
- k The isomeric 4-methyl-6,7-dihydroxycoumarin benzeneboronate was formed and showed GC-MS peak with M+ 278 (100%) but attempts to isolate pure crystals were unsuccessful.
- l Two recrystallisation from CHCl3-hexane, then vacuum sublimation at 140 °C/0.01 torr, gave a middle fraction which was recrystallised from EtOAc-hexane. GLC analysis for this product showed the presence of ca. 0.1% of benzeneboronic acid: the micro-analysis was not satisfactory for carbon. High resolution MS, C16H11BO4, requires 278.0672; found 278.0750.
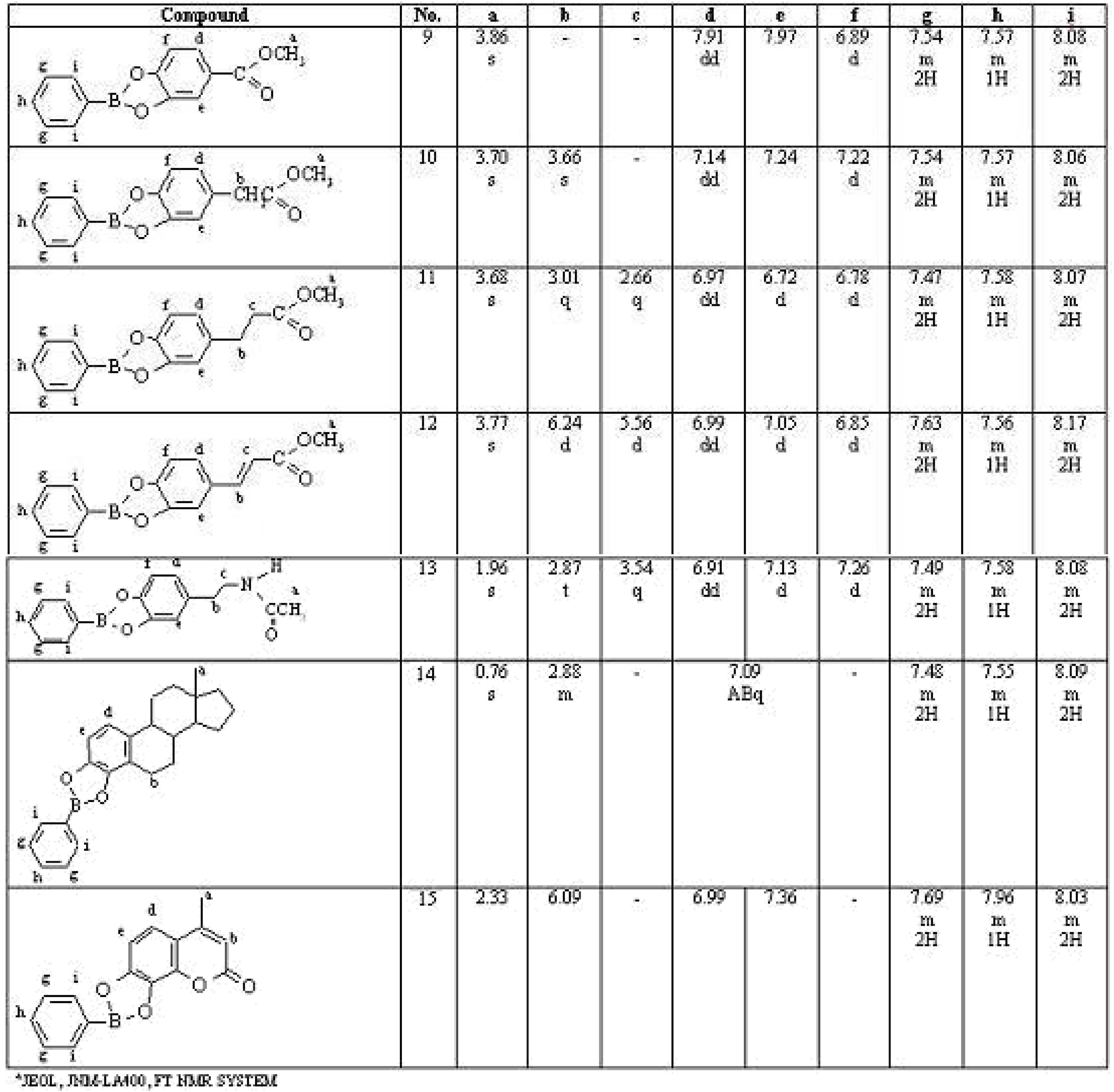
2. Results and Discussion
3. Experimental
General
4. Conclusions
Acknowledgements
References
- Denmark, S.E. Organic Reactions; John Wiley and Sons: Hoboken, NJ, USA, 2009; Volume 73. [Google Scholar]
- Hall, D.G. Boronic Acids, Preparation and Application in Organic Synthesis and Medicine; Wiley-VOH Verlag Gmbh & Co. KGaA: Weinheim, German, 2005. [Google Scholar]
- Kuivila, H.G.; Keough, A.H.; Obo czenski, E.J. Areneboronates from Diols and Polyols. J. Org. Chem. 1954, 19, 780–783. [Google Scholar] [CrossRef]
- Wolfrom, M.L; Solms, J. Phenylboronates of Pentoses and 6-Deoxyhexoses. J. Org. Chem. 1956, 21, 815–816. [Google Scholar] [CrossRef]
- Ferrier, R.J. The Interaction of Phenylboronic Acid with Hexosides. J. Chem. Soc. 1961, 2325–2330. [Google Scholar] [CrossRef]
- Ferrier, R.J.; Blattner, R.; Field, R.A.; Furneaux, R.H.; Gardiner, J.M.; Hoberg, J.O.; Kartha, K.P.R.; Tilbrook, D.M.C.; Tyler, P.C.; Wightman, R.H. Separatory and Analytical Methods for Sugars. Carbohyd. Chem. 2002, 33, 365–374. [Google Scholar]
- Ferrier, R.J.; Prasad, D.; Rudowski, A. Boronic Acid Derivatives as Reagents in Carbohydrate Chemistry. J. Chem. Soc. 1965, 858–863. [Google Scholar]
- Yurkevich, A.M.; Dolodkina, I.I.; Varshavskaya, L.S.; Borodulina-shvetz, V.I.; Rudakova, I.P.; Preobrazhenski, N.A. The Reaction of Phenylboronic acid with Nucleosides and Mononucleotides. Tetrahedron 1969, 25, 477–484. [Google Scholar] [CrossRef]
- Perun, T.J.; Martin, J.R.; Egan, R.S. Cyclic Phenylboronates as Hydroxyl Protecting Groups in the Synthesis of Monoesters of Macrolide Aglycones. J. Org. Chem. 1974, 39, 1490–1493. [Google Scholar] [CrossRef]
- Hungerford, N.L.; McKinney, A.R.; Stenhouse, A.M.; McLeod, M.D. Selective Manipulation of Steroid Hydroxyl Groups with Boronate Esters: Efficient access to Antigenic C-3 linked Steroid-Protein Conjugates and Steroid Sulfate Standards for Drug Detection. Org. Biomol. Chem. 2006, 4, 3951–3959. [Google Scholar] [CrossRef]
- Di Luccio, M.; Smith, B.D.; Kida, T.; Borges, C.P.; Alves, T.L.M. Separation of Fructose from a Mixture of Sugars using supported Liquid Membranes. J. Membr. Sci. 2000, 174, 217–224. [Google Scholar] [CrossRef]
- Peng, D.; Liang, L.; Jiangang, H.; Zhen, L.; Hong-Yuan, C. Boronate Functionalized Magnetic Nanoparticles and off-line Hyphenation with Capillary Electrophoresis for Specific Extraction and Analysis of Biomolecules Containing cis-Diols. J. Chromatogr.A 2009, 1216, 7558–7563. [Google Scholar]
- Yurkevich, A.M.; Kolodkina, I.I.; Varshavskaya, L.S.; Borodulina-shvetz, V.I.; Rudakova, I.P.; Preobrazhenski, N.A. The Reaction of Phenylboronic Acid with Nucleosides and Mononucleotides. Tetrahedron 1969, 25, 477–484. [Google Scholar] [CrossRef]
- Liu, X.C.; Scouten, W.H. Studies on Oriented and Reversible immobilization of Glycoprotein using Novel Boronate Affinity Gel. J. Mol. Recognit. 1996, 9, 462–467. [Google Scholar] [CrossRef]
- Ren, L.; Liu, Z.; Dong, M.; Ye, M.; Zou, H. Synthesis and Characterization of a Newboronate Affinity Monolithic Capillary for Specific Capture of cis-diol-Containing Compounds. J. Chromatogr. A 2009, 1216, 4768–4774. [Google Scholar]
- Gamoh, K.; Ketuly, K. A.; Cole, W.J.; Brooks, C.J.W. Chromatographic and Mass-Spectrometric Studies of Cyclic 2-(N,N-Dimethylaminomethyl)ferroceneboronates and Related Esters. Anal. Sci. 1994, 10, 705–711. [Google Scholar] [CrossRef]
- Kiplinger, J.P.; Crowder, C.A.; Catherine, A.; Sorensen, D.N.; Bartmess, J.E. Gas-Phase Ion-Molecule Chemistry of Borate and Boronate Esters. J. Am. Soc. Mass Spectrom. 1994, 5, 169–176. [Google Scholar] [CrossRef]
- Smith, A.G.; Brooks, C.J. Gas Chromatography Chemical Ionization Mass Spectrometry of Prostaglandin F Alpha Cyclic Boronate Derivatives. Biomed. Mass Spectrum. 1977, 4, 258–64. [Google Scholar] [CrossRef]
- Bullinger, D.; Fux, R.; Nicholson, G.; Plontke, S.; Belka, C.; Laufer, S.; Gleiter, C.H.; Kammerer, B. Identification of Urinary Modified Nucleosides and Ribosylated Metabolites in Humans Via Combined ESI-FTICR MS and ESI-IT MS Analysis. J. Am. Soc. Mass Spectrom. 2008, 19, 1500–1513. [Google Scholar] [CrossRef]
- Longstaff, C.; Rose, M.E. Derivatization and Mass Spectrometric Investigations of Substituted Benzeneboronic Acids. The use of linked Scanning during Gas Chromatography Mass Spectrometry. Org. Mass Spectrom. 1982, 17, 508–518. [Google Scholar] [CrossRef]
- Brooks, C.J.W.; Middleditch, B.S.; Harvey, D.J. The Mass Spectra of some Corticosteroid Boronates. Org. Mass Specrom. 1971, 5, 1429–1453. [Google Scholar] [CrossRef]
- Harrisson, P.; Morris, J.; Marder, T.B.; Steel, P.G. Microwave-Accelerated Iridium-Catalyzed Borylation of Aromatic C−H Bonds. Org. Lett. 2009, 11, 3586–3589. [Google Scholar] [CrossRef]
- Simov, B.P.; Wuggenig, F.; Mereiter, K.; Andres, H.; France, J.; Schnelli, P.; Hammerschmidt, F. Direct Chemical Synthesis of Chiral Methanol of 98% ee and Its Conversion to [2H1,3H]Methyl Tosylate and [2H1,3H-Methyl]Methionine. J. Am. Chem. Soc. 2005, 127, 13934–13940. [Google Scholar]
- Inglis, S.R.; Woon, E.C.Y.; Thompson, A.L.; Schofield, C.J. Observations on the Deprotection of Pinanediol and Pinacol Boronate Esters via Fluorinated Intermediates. J. Org. Chem. 2010, 75, 468–471. [Google Scholar] [CrossRef]
- Resnick, S. M.; Torok, D. S.; Gibson, D.T. Chemoenzymic Synthesis of Chiral Boronates for the Determination of the Absolute Configuration and Enantiomeric Excess of Bacterial and Synthetic cis-Dienediols. J. Org. Chem. 1995, 60, 3546–3549. [Google Scholar] [CrossRef]
- van den Berg, R.; Peters, J. A.; van Bekkum, H. The structure and local stability constants of borate esters of mono- and di-sacchrides as studied by 11B and 13C NMR Spectroscopy. Carbohydr. Res. 1994, 253, 1–12. [Google Scholar] [CrossRef]
- Ruman, T.; Dlugopolska, K.; Kusnierz, A.; Rode, W. Synthesis and NMR properties of derivatives of 5,6-dihydroborauracil and 5,6-dihydroborathymine. Bioorg. Chem. 2009, 37, 180–184. [Google Scholar] [CrossRef]
- Meiland, M.; Heinze, T.; Guenther, W.; Liebert, T. Seven-Membered Ring Boronates at trans-Diol moieties of Carbohydrates. Tetrahedron Lett. 2009, 50, 469–472. [Google Scholar]
- Yeste, S.L.; Powell, M.E.; Bull, S.D.; James, T.D. Simple Chiral Derivatization Protocols for 1H NMR and 19F NMRSpectroscopic Analysis of the Enantiopurity of Chiral Diols. J. Org. Chem. 2009, 74, 427–430. [Google Scholar]
- Shimanouchi, H.; Saito, N.; Sasada, Y. Crystal and Molecular Structure of N-(p-bromophenyl)-α-D-Ribopyranosylamine 2,4-Cyclic Benzeneboronate. Bull. Chem. Soc. Jap. 1969, 42, 1239–1247. [Google Scholar] [CrossRef]
- Wang, A.H.J.; Paul, I.C. Structure of a Derivative of Streptovaricin C Triacetate. Crystal and Molecular Structure of the Atropisomer of the Cyclic p-Bromobenzeneboronate Ester of Streptovaricin C Triacetate: Methylene Dichloride 1:1 Solvate. J. Am. Chem. Soc. 1976, 98, 4612–4619. [Google Scholar] [CrossRef]
- Barker, S.A.; Hatt, B.W.; Somers, P.J. Effect of Areneboronic Acids on the Alkaline Conversion of D-glucose into D-fructose. Carbohyd. Res. 1973, 26, 41–53. [Google Scholar] [CrossRef]
- Soloway, A.H.; Tjarks, W.; Barnum, B. A.; Rong, F.; Barth, R.F.; Codogni, I.M.; Wilson, J. G. The Chemistry of Neutron Capture Therapy. Chem. Rev. 1998, 98, 1515–1562. [Google Scholar] [CrossRef]
- Wiecko, J.; Sherman, W. R. Mass Spectral Study of Cyclic Alkaneboronates of Sugar Phosphates. Org. Mass Spectrom. 2005, 10, 1007–1020. [Google Scholar] [CrossRef]
- Butcher, F.K.; Gerrard, W.; Howarthy, M.; Mooney, E.F.; Willis, H.A. The Infrared Spectra of The Aryl Boronate Esters Derived from Catechol and 2:3 Dihydroxynaphthalene. Spectrochimica Acta 1964, 20, 79–95. [Google Scholar] [CrossRef]
- Stacey, B.E.; Tierney, B. The use of Boric Acid and Benzeneboronic Acids in the Partial Acetonation of Monosaccharides. Carbohyd. Res. 1976, 49, 129–140. [Google Scholar] [CrossRef]
- Gray, C.W.; Walker, B.T.; Foley, R.A.; Houston, T.A. Boronate derivatives of bioactive amines. Tetrahedron Lett. 2003, 44, 3309–3312. [Google Scholar]
- Pouchert, C.J.; Campbell, J.R. The Aldrich Library of NMR Sepctra; Adrich Chemical Co.: St. Louis, MO, USA, Vol. IV-VII, K.X., 162.
- Nakagawa, T.; Mishima, Y. Title. Kyoto Daigaku genshiro Jikkensho (Tech. Rep.) 1980, KURRI-IR-195, 44–48. [Google Scholar]
- Mills, R.R.; Lake, C.R.; Alworth, W.L. Biosynthesis of N-acetyl dopamine by the American cockroach. J. Insect Physiol. 1967, 13, 1539–1546. [Google Scholar] [CrossRef]
- Katz, H.E. 18-Anthracenediethynylbis(catechol boronate): A Bidentate Lewis Acid on A Novel Framework. J. Org. Chem. 1989, 54, 2179–2183. [Google Scholar] [CrossRef]
- Huang-Minlon, Reaction of Steroid Ketones and other Carbonyl Compounds by Modified Wolff-Kishner Method. J. Am. Chem. Soc. 1949, 71, 3301–3303. [CrossRef]
- Gelbke, H.P.; Haupt, O.; Knuppen, R. A Simple Chemical Method of the Synthesis of Catechol Estrogens. Steroids 1972, 21, 205–212. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ketuly, K.A.; Hadi, A.H.A. Boronate Derivatives of Functionally Diverse Catechols: Stability Studies. Molecules 2010, 15, 2347-2356. https://doi.org/10.3390/molecules15042347
Ketuly KA, Hadi AHA. Boronate Derivatives of Functionally Diverse Catechols: Stability Studies. Molecules. 2010; 15(4):2347-2356. https://doi.org/10.3390/molecules15042347
Chicago/Turabian StyleKetuly, Kamal Aziz, and A. Hamid A. Hadi. 2010. "Boronate Derivatives of Functionally Diverse Catechols: Stability Studies" Molecules 15, no. 4: 2347-2356. https://doi.org/10.3390/molecules15042347




