Synthesis and Physico-Chemical Properties of New Tetraethylammonium-Based Amino Acid Chiral Ionic Liquids
Abstract
:Abbreviations
| [N2222] | tetraethylammonium |
| [ser] | serinate |
| [pro] | prolinate |
| [thr] | threoninate |
| [ile] | isoleucinate |
| [asn] | asparaginate |
| [gln] | glutaminate |
| [glu] | glutamate |
| [met] | methioninate |
| [his] | histidinate |
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
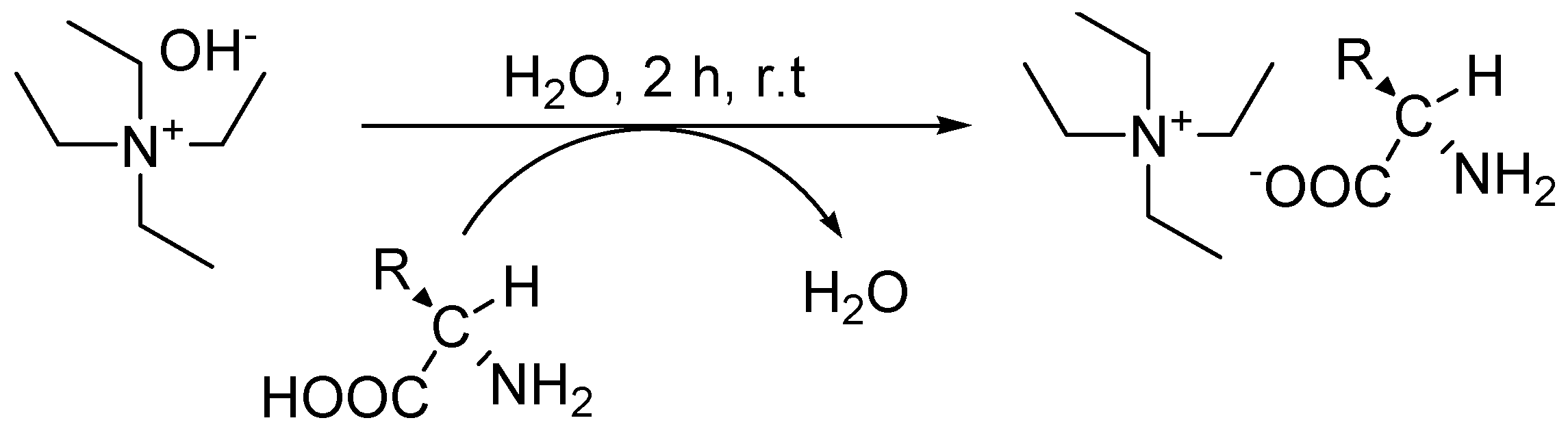
2.2. Thermal Stability and Physico-Chemical Properties

| No | CILs | Tm (°C) | Tonset (°C) | η (cP) | κ (mS cm-1) |
|---|---|---|---|---|---|
| 1 | [N2222][ser] | ND | 187 | 1763 | 0.16 |
| 2 | [N2222][pro] | ND | 172 | 438 | 0.46 |
| 3 | [N2222][thr] | ND | 188 | 1002 | 0.24 |
| 4 | [N2222][ile] | ND | 168 | 526 | 0.35 |
| 5 | [N2222][asn] | 58 ± 1.0 | 191 | a | a |
| 6 | [N2222][gln] | ND | 210 | a | a |
| 7 | [N2222][glu] | ND | 210 | a | a |
| 8 | [N2222][met] | ND | 176 | 462 | 0.45 |
| 9 | [N2222][his] | 54 ± 1.0 | 174 | a | a |

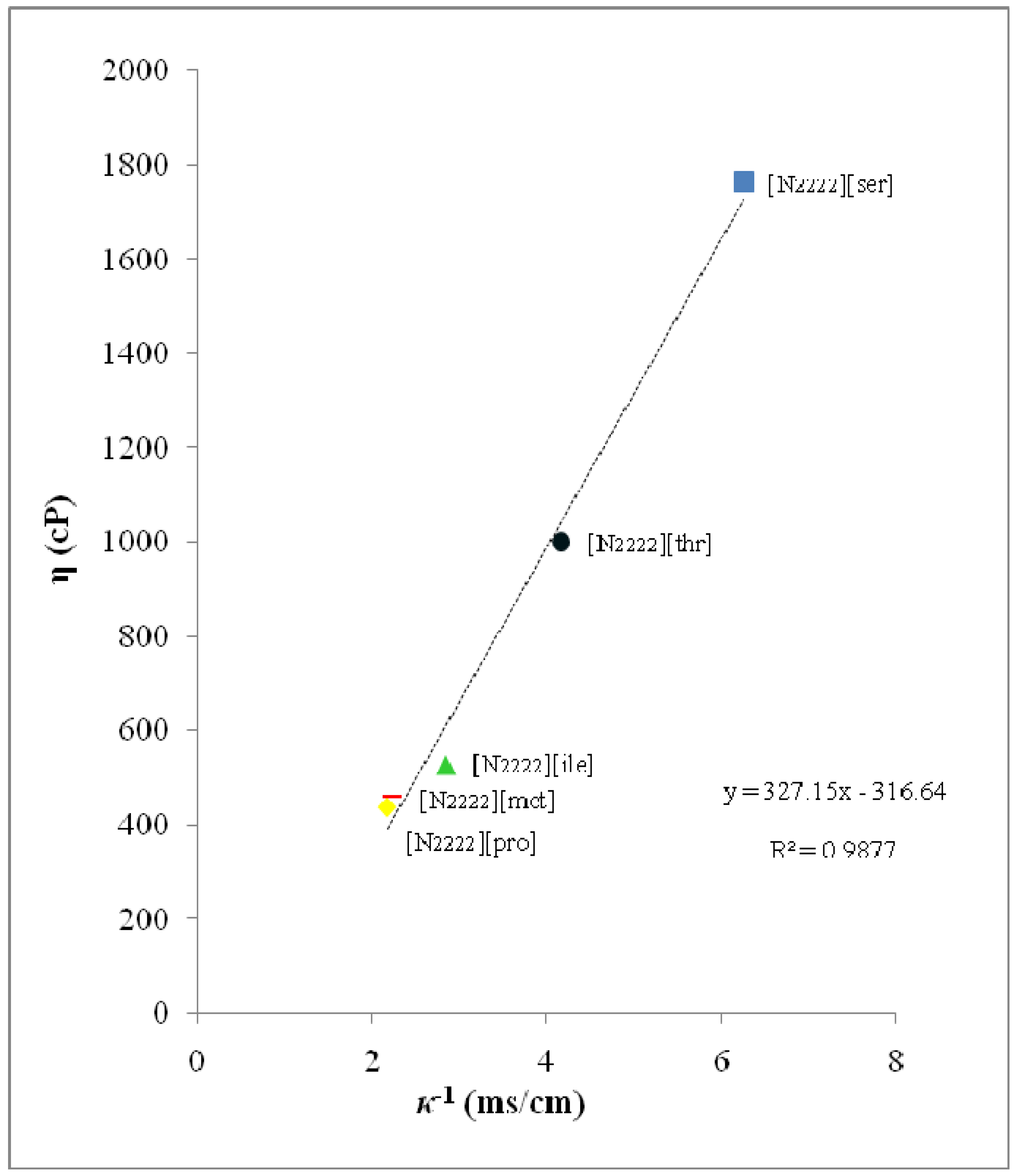
2.3. Viscosity (η) and Ionic Conductivity (κ)
3. Experimental
3.1. General

3.2. Typical Synthetic Procedure for Tetraethylammonium-Based Amino Acids
4. Conclusions
Acknowledgements
References
- Ohno, H.; Fukumoto, K. Amino acid ionic liquids. Acc. Chem. Res. 2007, 40, 1122–1129. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef]
- Van Rantwijk, F.; Lau, R.M.; Sheldon, R.A. Biocatalytic transformations in ionic liquids. Trends Biotech. 2003, 21, 131–138. [Google Scholar] [CrossRef]
- Obliosca, J.M.; Arco, S.D.; Huang, M.H. Synthesis and optical properties of 1-alkyl-3-methylimidazolium lauryl sulfate ionic liquids. J. Fluoresc. 2007, 17, 613–618. [Google Scholar] [CrossRef]
- Baudequin, C.; Baudoux, J.; Levillain, J.; Cahard, D.; Gaumont, A.-C.; Plaquevent, J.-C. Ionic liquids and chirality: Opportunities and challenges. Tetrahedron Asymmetry 2003, 14, 3081–3093. [Google Scholar] [CrossRef]
- Ding, J.; Armstrong, D.W. Chiral ionic liquids: Synthesis and applications. Chirality 2005, 17, 281–292. [Google Scholar] [CrossRef]
- Baudequin, C.; Brégeon, D.; Levillain, J.; Guillen, F.; Plaquevent, J.-C.; Gaumont, A.-C. Chiral ionic liquids, a renewal for the chemistry of chiral solvents? Design, synthesis and applications for chiral recognition and asymmetric synthesis. Tetrahedron Asymmetry 2005, 16, 3921–3945. [Google Scholar] [CrossRef]
- Abdul Rahman, M.B.; Jumbri, K.; Sirat, K.; Kia, R.; Fun, H.-K. Tetraethylammonium L-malate 1.36-hydrate. Acta Cryts. 2009, E65, 49–50. [Google Scholar]
- Abdul Rahman, M.B.; Jumbri, K.; Sirat, K.; Kia, R.; Fun, H.-K. Tetraethylammonium L-tartarate dihydrate. Acta. Cryts. 2008, E64, 2343. [Google Scholar]
- Bao, W.; Wang, Z.; Li, Y. Synthesis of chiral ionic liquids from natural amino acids. J. Org. Chem. 2003, 68, 591–593. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Bösmann, A.; Bolm, C. Synthesis and properties of ionic liquids derived from the 'chiral pool'. Chem. Commun. 2002, 200–201. [Google Scholar]
- Clavier, H.; Boulanger, L.; Audic, N.; Toupet, L.; Mauduit, M.; Guillemin, J.-C. Design and synthesis of imidazolinium salts derived from (L)-valine, Investigation of their potential in chiral molecular recognition. Chem. Commun. 2004, 1224–1225. [Google Scholar]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef]
- Tao, G.-H.; He, L.; Sun, N.; Kou, Y. New generation ionic liquids: Cations derived from amino acids. Chem. Commun. 2005, 3562–3564. [Google Scholar]
- Allen, C.R.; Richard, P.L.; Ward, A.J.; van de Water, L.G.A.; Masters, A.F.; Maschmeyer, T. Facile synthesis of ionic liquids possessing chiral carboxylates. Tetrahedron Lett. 2006, 47, 7367–7370. [Google Scholar]
- Jiang, Y.-Y.; Wang, G.-N.; Zhou, Z.; Wu, Y.-T.; Geng, J.; Zhang, Z.-B. Tetraalkylammonium amino acids as functionalized ionic liquids of low viscosity. Chem. Commun. 2008, 505–507. [Google Scholar]
- Shirota, H.; Castner, E.W., Jr. Why are viscosities lower for ionic liquids with - CH2Si(CH3)3 vs -CH2C(CH3)3 substitutions on the imidazolium cations? J. Phys. Chem. B 2005, 109, 21576–21585. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A.; Torres, M.J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2775–2287. [Google Scholar]
- Kagimoto, J.; Fukumoto, K.; Ohno, H. Effect of tetrabutylphosphonium cation on the physico-chemical properties of amino-acid ionic liquids. Chem. Commun. 2006, 2254–2256. [Google Scholar]
- MacFarlane, D.R.; Pringle, J.M.; Johansson, K.M.; Forsyth, S.A.; Forsyth, M. Lewis base ionic liquids. Chem. Commun. 2006, 1905–1917. [Google Scholar]
- McFarlane, D.R.; Sun, J.; Golding, J.; Meakin, P.; Forsyth, M. High conductivity molten salts based on the imide ion. Electrochim. Acta 2000, 45, 1271–1278. [Google Scholar] [CrossRef]
- Matz, R.A.; Trulove, P.C. Ionic Liquids in Synthesis; Wasserscheid, P., Welton, T., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2003; Volume 3, p. 56. [Google Scholar]
- Ohno, H. Functional design of ionic liquids. Bull Chem. Soc. Jpn. 2006, 79, 1665–1680. [Google Scholar] [CrossRef]
- Sample Availability: All samples are available from the authors.
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rahman, M.B.A.; Jumbri, K.; Basri, M.; Abdulmalek, E.; Sirat, K.; Salleh, A.B. Synthesis and Physico-Chemical Properties of New Tetraethylammonium-Based Amino Acid Chiral Ionic Liquids. Molecules 2010, 15, 2388-2397. https://doi.org/10.3390/molecules15042388
Rahman MBA, Jumbri K, Basri M, Abdulmalek E, Sirat K, Salleh AB. Synthesis and Physico-Chemical Properties of New Tetraethylammonium-Based Amino Acid Chiral Ionic Liquids. Molecules. 2010; 15(4):2388-2397. https://doi.org/10.3390/molecules15042388
Chicago/Turabian StyleRahman, Mohd Basyaruddin Abdul, Khairulazhar Jumbri, Mahiran Basri, Emilia Abdulmalek, Kamaliah Sirat, and Abu Bakar Salleh. 2010. "Synthesis and Physico-Chemical Properties of New Tetraethylammonium-Based Amino Acid Chiral Ionic Liquids" Molecules 15, no. 4: 2388-2397. https://doi.org/10.3390/molecules15042388





