Synthesis and Antimicrobial Activity of Novel Ag-N-Hetero-cyclic Carbene Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of silver-carbene complexes 1a-f

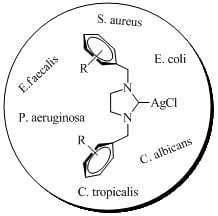
2.2. Antimicrobial properties of silver-NHC complexes
| Ag-NHC | E. coli | S. aureus | E. faecalis | P. aerug. | C. albicans | C. tropicalls |
|---|---|---|---|---|---|---|
| 1a | 100 | 100 | 100 | 100 | 50 | 12.5 |
| 1b | 200 | 200 | 200 | 200 | 100 | 50 |
| 1c | 200 | 200 | 200 | 200 | 6.25 | 6.25 |
| 1d | 200 | 100 | 100 | 200 | 100 | 100 |
| 1e | 100 | 100 | 100 | 100 | 25 | 25 |
| 1f | 100 | 100 | 100 | 100 | 50 | 6.25 |
| Ampicillin | 3.12 | 3.12 | 1.56 | - | - | - |
| Ciprofloxacin | 1.56 | 0.39 | 0.78 | 3.12 | - | - |
| Fluconazole | - | - | - | - | 3.12 | 3.12 |
3. Experimental
3.1. General
3.2. General method for the preparation of silver NHC complexes
3.3. Antimicrobial activities of silver NHC complexes
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Mascini, M.; Bagni, G.; Pietro, M.L.D.; Ravera, M.; Baracco, S.; Osella, D. Electrochemical biosensor evaluation of the interaction between DNA and metallo-drugs. BioMetals 2006, 19, 409–418. [Google Scholar] [CrossRef]
- Kostova, I. Platinum complexes as anticancer agents. Recent Pat. Anti-Cancer Drug Discover. 2006, 1, 1–22. [Google Scholar] [CrossRef]
- Guo, Z.; Sadler, P.J. Advances in Inorganic Chemistry; Academic Press: San Diego, CA, USA, 2000; Volume 1, pp. 1–22. [Google Scholar]
- Farrell, N. Inorganic Complexes as Drugs and Chemotherapeutic Agents. In Comprehensive Coordination Chemistry, 2nd; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier Pergamon: Oxford, UK, 2004; Volume 9, pp. 809–840. [Google Scholar]
- Clement, J.L.; Jarret, P.S. Antibacterial Silver. Metal-Based Drugs 1994, 1, 467–482. [Google Scholar] [CrossRef]
- Tambe, S.M.; Sampath, L.; Modak, S.M. In-vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. J. Antimicrob. Chemother. 2001, 47, 589–598. [Google Scholar] [CrossRef]
- Jakupec, M.A.; Unfried, P.; Keppler, B.K. In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. Rev. Physiol. Biochem. Pharmacol. 2005, 153, 101–111. [Google Scholar]
- Nomiya, K.; Takahashi, S.; Noguchi, R.; Nemoto, S.; Takayama, T.; Oda, M. Synthesis and Characterization of Water-Soluble Silver(I) Complexes with l-Histidine (H2his) and (S)-(−)-2-Pyrrolidone-5-carboxylic Acid (H2pyrrld) Showing a Wide Spectrum of Effective Antibacterial and Antifungal Activities. Crystal Structures of Chiral Helical Polymers [Ag(Hhis)]n and {[Ag(Hpyrrld)]2}n in the Solid State. Inorg. Chem. 2000, 39, 3301–3311. [Google Scholar]
- Kasuga, N.C.; Sugie, A.; Nomiya, K. Syntheses, structures and antimicrobial activities of water-soluble silver(I)-oxygen bonding complexes with chiral and racemic camphanic acid (Hca) ligands. Dalton Trans. 2004, 3732–3740. [Google Scholar]
- Nomiya, K.; Tsuda, K.; Sudoh, T.; Oda, M. Ag(I)-N bond-containing compound showing wide spectra in effective antimicrobial activities: Polymeric silver(I) imidazolate. J. Inorg. Biochem. 1997, 68, 39–44. [Google Scholar] [CrossRef]
- Nomiya, K.; Noguchi, R.; Oda, M. Synthesis and crystal structure of coinage metal(I) complexes with tetrazole (Htetz) and triphenylphosphine ligands, and their antimicrobial activities. A helical polymer of silver(I) complex [Ag(tetz)(PPh3)2]n and a monomeric gold(I) complex [Au(tetz)(PPh3)]. Inorg. Chim. Acta. 2000, 298, 24–32. [Google Scholar] [CrossRef]
- Liu, J.J.; Galettis, P.; Farr, A.; Maharaj, L.; Samarasinha, H.; McGechan, A.C.; Baguley, B.C.; Bowen, R.J.; Berners-Price, S.J.; McKeage, M.J. In vitro antitumour and hepatotoxicity profiles of Au(I) and Ag(I) bidentate pyridyl phosphine complexes and relationships to cellular uptake. J. Inorg. Biochem. 2008, 102, 303–310. [Google Scholar] [CrossRef]
- Thati, B.; Noble, A.; Creaven, B.S.; Walsh, M.; McCann, M.; Kavanagh, K.; Devereux, M.; Egan, D.A. In vitro anti-tumour and cyto-selective effects of coumarin-3-carboxylic acid and three of its hydroxylated derivatives, along with their silver-based complexes, using human epithelial carcinoma cell lines. Cancer Lett. 2007, 248, 321–331. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Melaiye, A.; Hindi, K.; Durmus, S.; Panzner, M.J.; Hogue, L.A.; Mallett, R.J.; Hovis, C.E.; Coughenour, M.; Crosby, S.D.; Milsted, A.; Ely, D.L.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. Synthesis from Caffeine of a Mixed N-Heterocyclic Carbene−Silver Acetate Complex Active against Resistant Respiratory Pathogens. J. Med. Chem. 2006, 49, 6811–6818. [Google Scholar]
- Melaiye, A.; Sun, Z.; Hindi, K.; Milsted, A.; Ely, D.; Reneker, D.H.; Tessier, C.A.; Youngs, W.J. Silver(I)−imidazole cyclophane gem-diol complexes encapsulated by electrospun tecophilic nanofibers: Formation of nanosilver particles and antimicrobial activity. J. Am. Chem. Soc. 2005, 127, 2285–2291. [Google Scholar]
- Garrison, J.C.; Tessier, C.A.; Youngs, W.J. Synthesis and crystallographic characterization of multi-donor N-heterocyclic carbene chelating ligands and their silver complexes: Potential use in pharmaceuticals. J. Organomet. Chem. 2005, 690, 6008–6020. [Google Scholar] [CrossRef]
- Barnard, P.J.; Wedlock, L.E.; Baker, M.V.; Berners-Price, S.J.; Joyce, D.A.; Skelton, B.W.; Steer, J.H. Luminescence studies of the intracellular distribution of a dinuclear gold(I) N-heterocyclic carbene complex. Angew. Chem. Int. Ed. 2006, 45, 5966–5970. [Google Scholar]
- Özdemir, I.; Denizci, A.; Öztürk, H.T.; Çetinkaya, B. Synthetic and antimicrobial studies on new gold(I) complexes of imidazolidin-2-ylidenes. Appl. Organomet. Chem. 2004, 18, 318–322. [Google Scholar]
- Herrmann, W.A. N-Heterocyclic Carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Peris, E.; Crabtree, R.H. Recent homogeneous catalytic applications of chelate and pincer N-heterocyclic carbenes. Coord. Chem. Rev. 2004, 248, 2239–2246. [Google Scholar] [CrossRef]
- Gonzalez, S.D.; Maria, N.; Nolan, S.P. N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef]
- Lin, I.J.B.; Vasam, C.S. Preparation and application of N-heterocyclic carbene complexes of Ag(I). Coord. Chem. Rev. 2007, 251, 642–670. [Google Scholar]
- Hindi, K.M.; Ditto, A.J.; Panzeri, M.J.; Medvetz, D.A.; Han, S.D.; Hovis, C.E.; Hilliard, J.K.; Taylor, J.B.; Yun, Y.H.; Cannon, C.L.; Youngs, W.J. The antimicrobial efficacy of sustained release silver-carbene complex-loaded l-tyrosine polyphosphate nanoparticles: Characterization, in vitro and in vivo studies. Biomaterials 2009, 30, 3771–3779. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. N-Heterocyclic carbene-silver complexes: A new class of antibiotics. Coord. Chem. Rev. 2007, 251, 884–895. [Google Scholar]
- Hindi, K.M.; Siciliano, T.J.; Durmus, S.; Panzner, M.J.; Medvetz, D.A.; Reddy, D.V.; Hogue, L.A.; Hovis, C.E.; Hilliard, J.K.; Mallet, R.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. Synthesis, stability, and antimicrobial studies of electronically tuned Silver acetate N-heterocyclic carbenes. J. Med. Chem. 2008, 51, 1577–1583. [Google Scholar]
- Hindi, K.M.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. The Medicinal applications of imidazolium carbene−metal complexes. Chem. Rev. 2009, 109, 3859–3884. [Google Scholar] [CrossRef]
- Medvetz, D.A.; Hindi, K.M.; Panzner, M.J.; Ditto, A.J.; Yun, Y.H.; Youngs, W.J. Anticancer activity of Ag(I) N-heterocyclic carbene complexes derived from 4,5-dichloro-1H-imidazole. Met. Based Drugs 2008, 384010–384016. [Google Scholar]
- Lin, J.C.Y.; Huang, R.T.W.; Lee, C.S.; Bhattacharyya, A.; Hwang, W.S.; Lin, I.J.B. Coinage metal−N-heterocyclic carbene complexes. Chem. Rew. 2009, 109, 3561–3598. [Google Scholar] [CrossRef]
- Peris, E. Routes to N-Heterocyclic Carbene Complexes. Top. Organomet. Chem. 2007, 21, 83–116. [Google Scholar]
- Lee, C.K.; Vasam, C.S.; Huang, T.W.; Wang, H.M.J.; Yang, R.Y.; Lee, C.S.; Lin, I.J.B. Silver(I) N-heterocyclic carbenes with long N-alkyl chains. Organometallics 2006, 25, 3768–3775. [Google Scholar]
- Garrison, J.C.; Youngs, W.J. Ag(I) N-heterocyclic carbene complexes: Synthesis, structure, and application. Chem. Rev. 2005, 105, 3978–4008. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Dias, H.W.R.; Calabrase, J.C.; Davidson, F. Homoleptic carbene-silver(I) and carbene-copper(I) complexes. Organometallics. 1993, 12, 3405–3409. [Google Scholar] [CrossRef]
- Guerret, O.; Sole´, S.; Gornitzka, H.; Trinquier, G.; Bertrand, G. 1,2,4-Triazole-3,5-diylidene: A building block for organometallic polymer synthesis. J. Am.Chem. Soc. 1997, 119, 6668–6669. [Google Scholar] [CrossRef]
- Wang, H.M.J.; Lin, I.J.B. Facile Synthesis of silver(I)−carbene complexes. Useful carbene transfer agents. Organometallics 1998, 17, 972–975. [Google Scholar]
- Tulloch, A.A.D.; Danopoulos, A.A.; Winston, S.; Kleinhenz, S.; Estham, G. N-Functionalised heterocyclic carbene complexes of silver. J. Chem. Soc. Dalton Trans. 2000, 4499–4506. [Google Scholar]
- Vasam, C.S.; Lin, I.J.B. Preparation and application of N-heterocyclic carbene complexes of Ag(I). Coord. Chem. Rev. 2007, 251, 642–670. [Google Scholar]
- Lin, I.J.B.; Vasam, C.S. Silver(I) N-heterocyclic carbenes. Comment. Inorg. Chem. 2004, 25, 75–129. [Google Scholar] [CrossRef]
- Wang, H.M.J.; Chen, C.Y.L.; Lin, I.J.B. Synthesis, structure, and spectroscopic properties of gold(I)−carbene complexes. Organometallics 1999, 18, 1216–1223. [Google Scholar]
- Lee, C.K.; Lee, K.M.; Lin, I.J.B. Inorganic−organic hybrid lamella of di- and tetranuclear silver−carbene complexes. Organometallics 2002, 21, 10–12. [Google Scholar]
- Gürbüz, N.; Özdemir, I.; Demir, S.; Çetinkaya, B. Improved palladium-catalyzed coupling reactions of aryl halides using saturated N-heterocarbene ligands. J. Mol. Catal. A Chem. 2004, 209, 23–28. [Google Scholar] [CrossRef]
- Liu, F.; Chen, W.; Wang, D. Synthesis and structural characterization of one- and two-dimensional coordination polymers based on platinum-silver metallic backbones. Dalton Trans. 2006, 3015–3024. [Google Scholar]
- Chen, W.; Liu, F.; Xu, D.; Matsumoto, K.; Kishi, S.; Kato, M. Luminescent amidate-Bridged one-dimensional platinum(II)−thallium(I) coordination polymers assembled via metallophilic attraction. Inorg. Chem. 2006, 45, 5552–5560. [Google Scholar] [CrossRef]
- Nielsen, D.J.; Cavell, K.J.; Skelton, B.W. Tetrafluoroborate anion B-F bond activation-unusual formation of a nucleophilic heterocyclic carbene:BF3 adduct. Inorg. Chim. Acta. 2003, 352, 143–148. [Google Scholar] [CrossRef]
- Pytkowicz, J.; Roland, S.; Mangeney, P. Synthesis of chiral silver(I) diaminocarbene complexes from (R,R)-4,5-di-tert-butylimidazoline. J. Organomet. Chem. 2001, 631, 157. [Google Scholar]
- Lee, H.M.; Chiu, P.L.; Hu, C.H.; Lai, C.L.; Chou, Y.C. Synthesis and structural characterization of metal complexes based on pyrazole/imidazolium chlorides. J. Organomet. Chem. 2005, 690, 403–414. [Google Scholar]
- Pernak, J.; Skrzypczak, A. 3-Alkylthiomethyl-1-ethylimidazolium chlorides. Correlation between critical micelle concentrations and minimum inhibitory concentrations. Eur. J. Med. Chem. 1996, 31, 901–903. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Instıtute, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard-Seventh Edition; CLSI Document M7-A7, Clinical and Laboratory Standards Instıtute: Wayne, PA, USA, 2003.
- Clinical and Laboratory Standards Instıtute, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard-Second Edition; NCCLS document M27-A2 (ISBN 1-56238-469-4). NCCLS: Wayne, PA, USA, 2002.
- Hindler, J.; Hochstein, L.; Howell, A. Preparation of Routine Media and Reagents Used in Antimicrobial Susceptibility Testing. Part 1. McFarland Standards. In Clinical Microbiology Procedures Handbook; Isenberg, H.D., Ed.; American Society for Microbiology: Washington, DC, USA; Volume 1, pp. 5.19.1–5.19.6.
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Özdemir, İ.; Özcan, E.Ö.; Günal, S.; Gürbüz, N. Synthesis and Antimicrobial Activity of Novel Ag-N-Hetero-cyclic Carbene Complexes. Molecules 2010, 15, 2499-2508. https://doi.org/10.3390/molecules15042499
Özdemir İ, Özcan EÖ, Günal S, Gürbüz N. Synthesis and Antimicrobial Activity of Novel Ag-N-Hetero-cyclic Carbene Complexes. Molecules. 2010; 15(4):2499-2508. https://doi.org/10.3390/molecules15042499
Chicago/Turabian StyleÖzdemir, İlknur, Emine Özge Özcan, Selami Günal, and Nevin Gürbüz. 2010. "Synthesis and Antimicrobial Activity of Novel Ag-N-Hetero-cyclic Carbene Complexes" Molecules 15, no. 4: 2499-2508. https://doi.org/10.3390/molecules15042499