Preparation, Characterization and Adsorption Performance of a Novel Anionic Starch Microsphere
Abstract
:1. Introduction
2. Results and Discussion
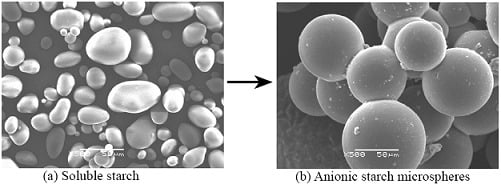
2.1. Morphology of ASMs
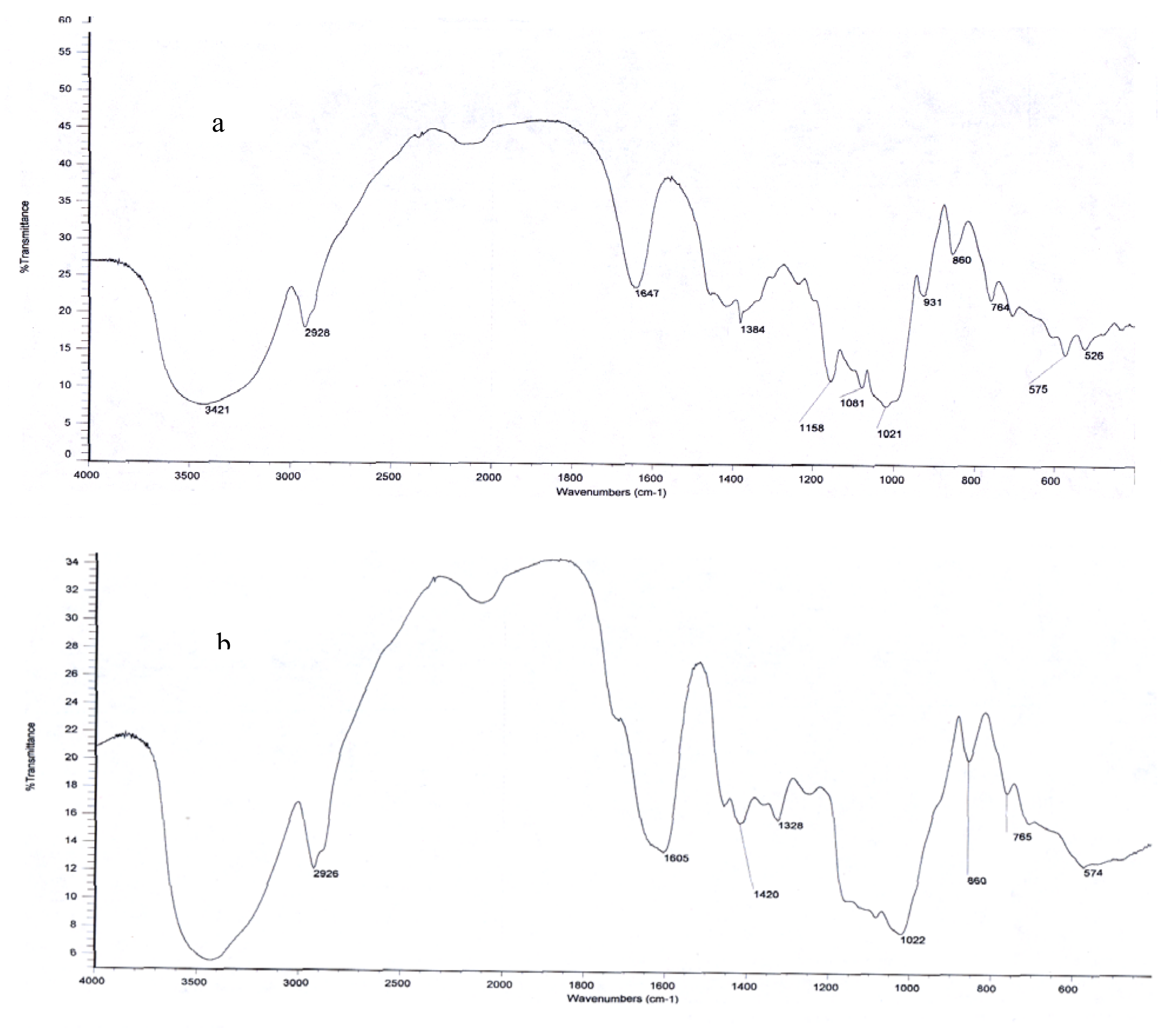
2.2. Structural Organization by FTIR
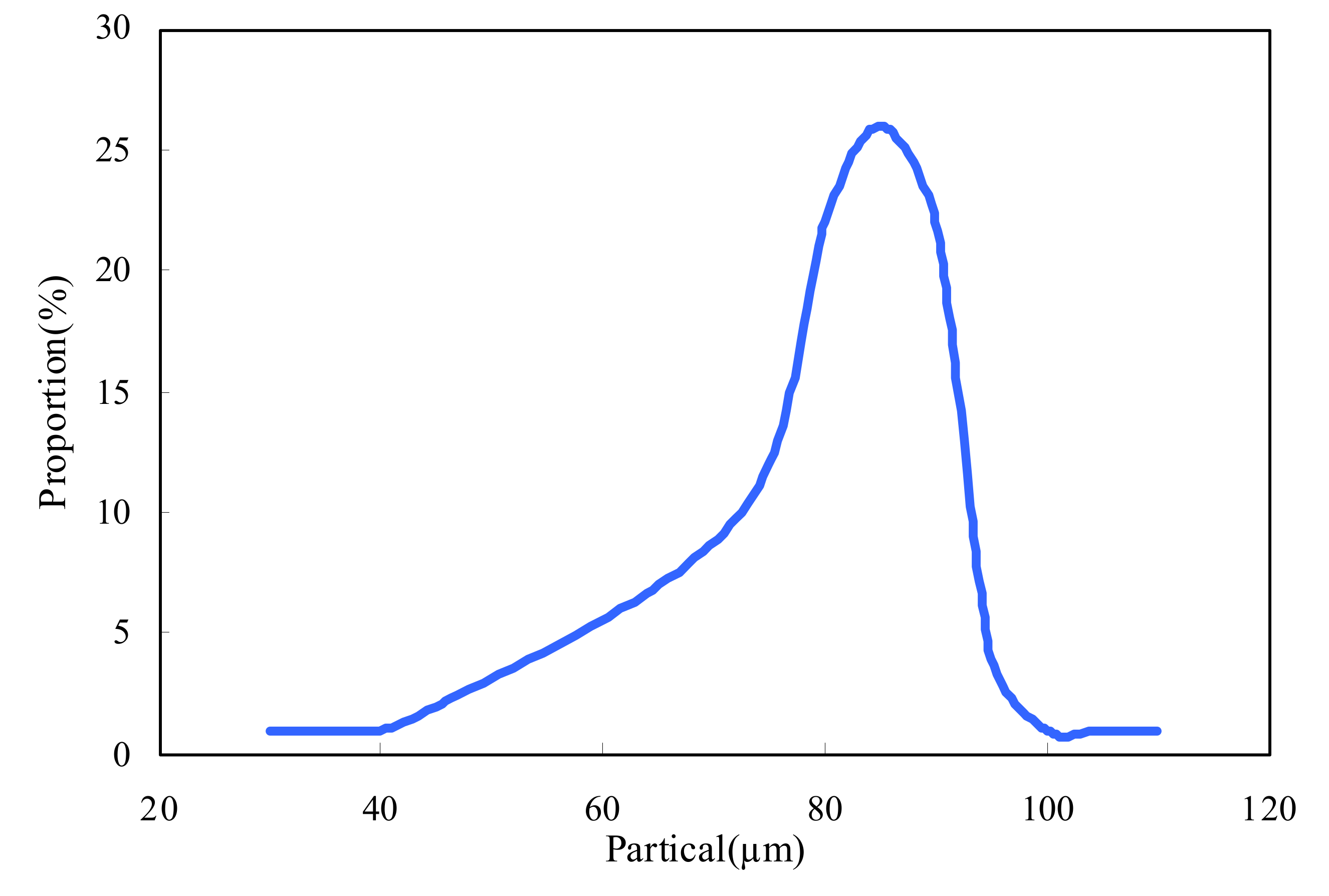
2.3. Particle Size of ASMs
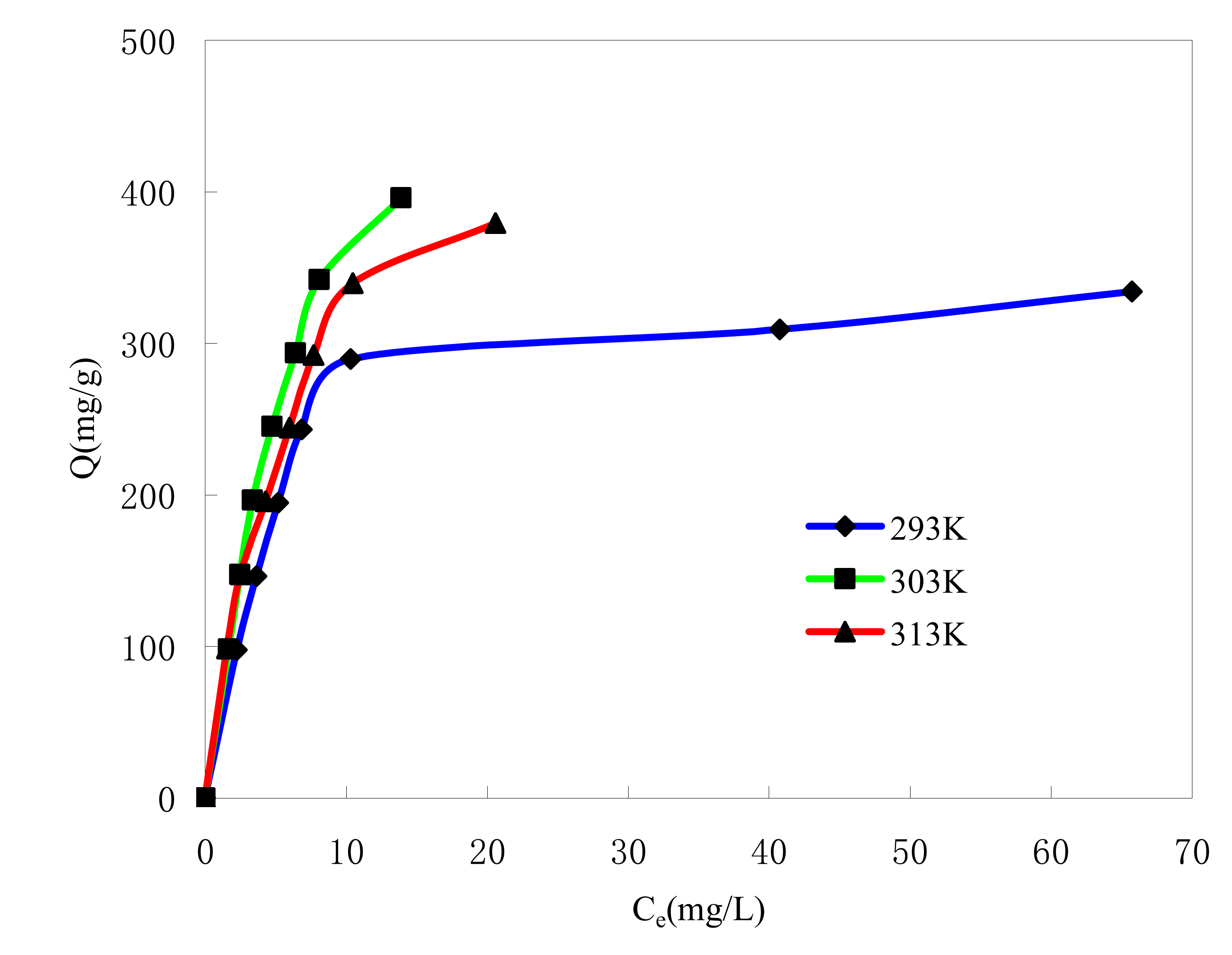
2.4. The Adsorption Isotherm and Kinetic Characteristics of MB Adsorption on Microspheres
2.4.1. Effect of Initial MB Concentration
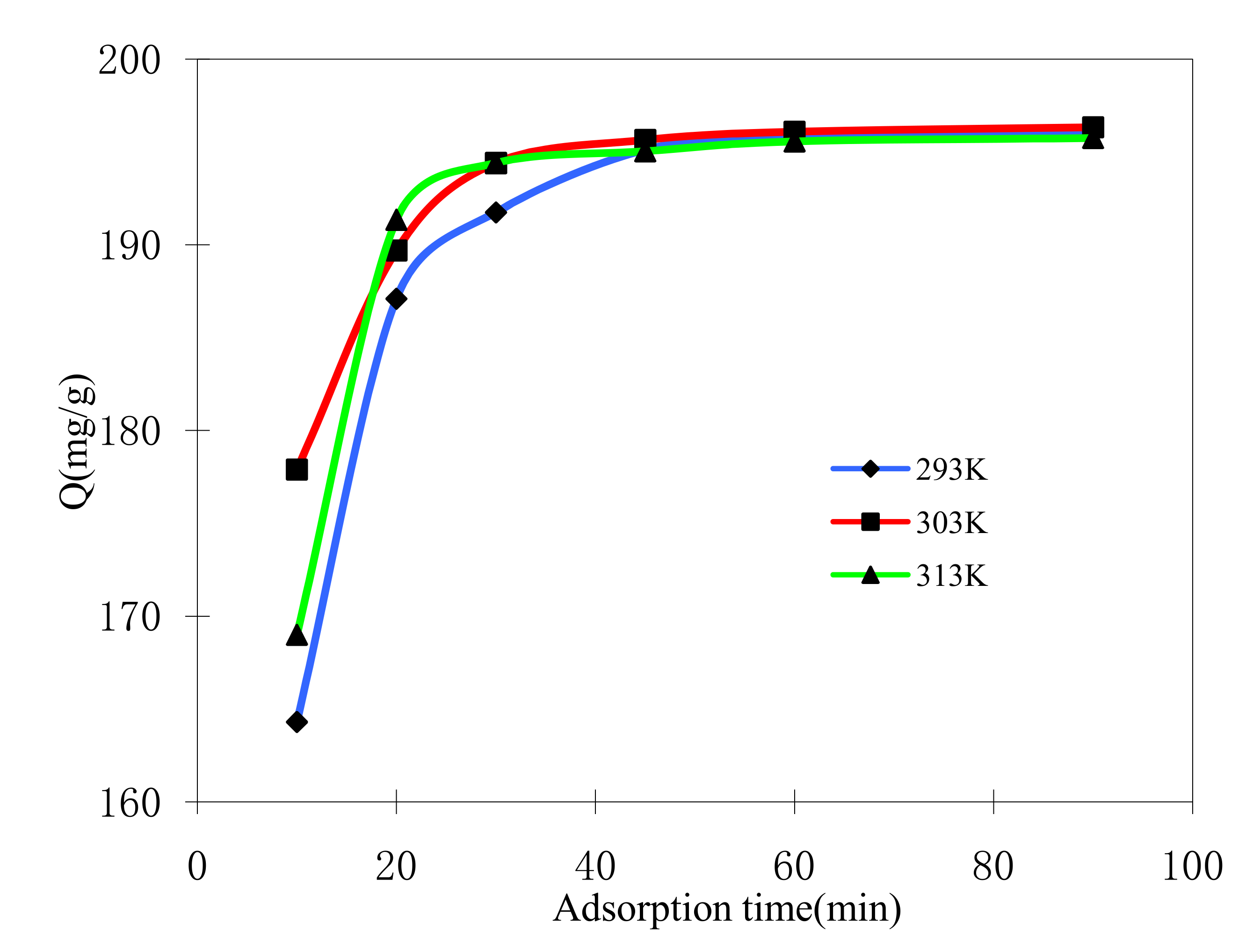
2.4.2. Effect of Adsorption Time
2.4.3. Effect of Adsorption Temperature
2.5. Comparison with other adsorbents
3. Experimental
3.1. Materials and Reagents
3.2. Synthesis of Anionic Starch Microspheres (ASMs)
3.2.1. Preparation of Neutral Starch Microspheres (NSMs)
3.2.2. Preparation of Anionic Starch Microspheres (ASMs)
3.3. Measurements
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. Fourier Transform InfraRed (FTIR) spectroscopy
3.3.3. Particle size analysis
3.3.4. Batch equilibrium studies
3.3.5. Batch kinetic studies
4. Conclusions
Acknowledgements
References and Notes
- Wang, S.; Zhua, Z.; Coomes, A. The physical and surface chemical characteristics of activited carbons and the adsorption of methylene blue from wastewater. J. Colloid Interf. Sci. 2005, 284, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Juang, L.C.; Hsu, T.C.; Lee, C.K.; Lee, J.F.; Huang, F.C. Adsorption of basic dyes onto montmorillonite. J. Colloid Interf. Sci. 2004, 273, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T.; Chang, Y.M.; Lai, C.W.; Lo, C.C. Adsorption of basic dyes in aqueous solution by clay adsorbent from regenerated bleaching earth. Appl. Clay Sci. 2005, 29, 149–154. [Google Scholar] [CrossRef]
- Bilgic, C. Investigation of the factors affecting organic cation adsorption on some silicate minerals. J. Colloid Interf. Sci. 2005, 281, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.M.; Wang, J.L.; Wu, R.L.; Wang, J.D.; Li, H. Adsorption behaviors of acid and basic dyes on crosslinked amphoteric starch. Chem. Eng. J. 2006, 117, 161–167. [Google Scholar] [CrossRef]
- Inthorn, D.; Singhtho, S.; Thiravetyan, P.; Khan, E. Decolorization of basic direct and reactive dyes by pre-treated narrow-leaved cattail (Typha angustifolia Linn.). Bioresource Technol. 2004, 94, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kum ar, K.V.; Ramamurthi, V.; Sivanesan, S. Modeling the mechanism involved during the sorption of methylene blue onto fly ash. J. Colloid Interf. Sci. 2005, 284, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Janos, P.; Buchtova, H.; Ryznarova, M. Sorption of dyes from aqueous solutions onto fly ash. Water Res. 2003, 37, 4938–4944. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.K.; Amita, M.; Kumar, R. Basic dye (methylene blue) removal from simulated wastewater by adsorption using Indian Rosewood sawdust: a timber industry waste. Dyes Pigments 2004, 63, 243–250. [Google Scholar] [CrossRef]
- Chiou, M.S.; Li, H.Y. Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J. Hazard. Mater. 2002, B93, 233–248. [Google Scholar] [CrossRef]
- Delval, F.; Crini, G.; Bertini, S.; Filiatre, C.; Torri, G. Preparation, characterization and sorption properties of crosslinked starch-based exchangers. Carbohyd. Polym. 2005, 60, 67–75. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.J. Characteristics of crosslinked potato starch and starch filled linear low-density polyethylene films. Carbohyd. Polym. 2002, 50, 331–337. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Shelke, N.B.; Rokhade, A.P.; Patil, S.A.; Aminabhavi, T.M. Formulation and in-vitro evaluation of novel starch-based tableted microspheres for controlled release of ampicillin. Carbohyd. Polym. 2008, 71, 42–53. [Google Scholar] [CrossRef]
- Mao, S.R.; Chen, Z.M.; Wei, Z.P.; Liu, H.; Bi, D.Z. Intranasal administrationof melatonin starch microspheres. Inter. J. Pharm. 2004, 272, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Kulkarni, A.R.; Aminabhavi, T.M. Encapsulation of antihypertensive drugs in cellulose-based matrix microspheres: characterization and release kinetics of microspheres and tableted microspheres. J. Microencaps. 2001, 18, 397–409. [Google Scholar]
- Aminabhavi, T.M.; Patil, G.V.; Balundgi, R.H.; Harlapur, S.F.; Manvi, F.V.; Bhaskar, C. A review on the sustained release of cardiovascular drugs through hydroxypropyl methylcellulose and sodium carboxymethylcellulose polymers. Des. Monomers Polym. 1998, 1, 347–372. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Adebowale, K.O.; Lawal, O. S. Functional properties and retrogradation behaviour of native and chemically modified starch of mucuna bean (Mucuna pruriens). J. Sci. Food Agric. 2003, 83, 1541–1546. [Google Scholar] [CrossRef]
- Zhan, G.P.; Huang, K.L.; Zhang, F.W. The study on synthesis of anion starch microspheres. New Chem. Mater. 2005, 33, 44–46. [Google Scholar]
- Khalil, M.I.; Abdel-Halim, M.G. Preparation of anionic starch containing carboxyl groups and its utilization as chelating agent. Starch Stärke 2001, 53, 35–41. [Google Scholar] [CrossRef]
- Li, B.Z.; Wang, L.J.; Li, D.; Chiu, Y.L.; Zhang, Z.J.; Shi, John; Chen, X.D.; Mao, Z.H. Physical properties and loading capacity of starch-based microparticles crosslinked with trisodium trimetaphosphate. J. Food Eng. 2009, 92, 255–260. [Google Scholar] [CrossRef]
- van Soest, J.J.G.; de Wit, H.; Tournois, D.; Vliegenthart, J.F.G. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohyd. Res. 1995, 279, 201–214. [Google Scholar] [CrossRef]
- Chang, J.H.; Dong, Q.G. Spectrum Mechanism and Analysis, second ed.; Science Press: Beijing, China, 2006; pp. 88–89. [Google Scholar]
- Allen, S.J.; Mckayb, G.; Porter, J.F. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interf. Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef] [PubMed]
- McKay, G.; Blair, H.S.; Gardner, J.R. Adsorption of dyes on chitin Equilibrium studies. J. Appl. Polym. Sci. 1982, 27, 3043–3057. [Google Scholar] [CrossRef]
- Guo, L.; Sun, C.M.; Li, G.Y.; Liu, C.P.; Ji, C.N. Thermodynamics and kinetics of Zn (II) adsorption on crosslinked starch phosphates. J. Hazard. Mater. 2008, 81, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 2001, 35, 613–618. [Google Scholar] [CrossRef]
- Aksu, Z.; Tezer, S. Equilibrium and kinetic modelling of biosorption of remazol black B by rhizopus arrhizus in a batch system: effect of temperature. Process Biochem. 2000, 36, 431–439. [Google Scholar] [CrossRef]
- Aksu, Z. Biosorption of reactive dyes by dried activated sludge: equilibrium and kinetic modeling. Biochem. Eng. J. 2001, 7, 79–84. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Yu, Y.; Zhuang, Y.; Xin, B.; Zou, Q. Interaction between molecules of decolorant and active dyes. Acta Sci. Natural. Univ. Nankaiensis 1999, 32, 140–145. [Google Scholar]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.A. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazard. Mater. 2009, 164, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Vadivelan, V.; Kumar, K. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interf. Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Batzias, F.A.; Sidiras, D.K. Dye adsorption by calcium chloride treated beech sawdust in batch and fixed-bed systems. J. Hazard. Mater. 2004, B114, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; El-Khaiary, M.I. Batch removal of malachite green from aqueous solutions by adsorption on oil palm trunk fibre: equilibrium isotherms and kinetic studies. J. Hazard. Mater. 2008, 154, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; El-Khaiary, M.I. Sorption kinetics and isotherm studies of a cationic dye using agricultural waste: broad bean peels. J. Hazard. Mater. 2008, 154, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Mittal, A.; Jain, R.; Mathur, M.; Sikarwar, S. Adsorption of Safranin-T from wastewater using waste materials—activated carbon and activated rice husks. J. Colloid Interf. Sci. 2006, 303, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Banat, F.; Al-Asheh, S.; Al-Makhadmeh, L. Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Proc. Biochem. 2003, 39, 193–202. [Google Scholar] [CrossRef]
- Banerjee, S.; Dastidar, M.G. Use of jute processing wastes for treatment of wastewater contaminated with dye and other organics. Bioresour. Technol. 2005, 96, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.M.; Sun, Q.Y.; Cang, C.J.; Zhou, D.; Zhao, L. Study of synthesis of starch microspheres by inverse microemulsion. Chinese J. Col. Polym. 2007, 25, 5–7. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |


| Temperature(K) | Langmuir equation | Freundlich equation | |||||
|---|---|---|---|---|---|---|---|
| b(L/mg) | Qm(mg/g) | R2 | ΔGӨ (kJ/mol) | KF | n | R2 | |
| 293 | 0.228 | 357.14 | 0.9969 | −27.65 | 104.56 | 3.19 | 0.7589 |
| 303 | 0.119 | 666.67 | 0.9709 | −26.96 | 80.6 | 1.5 | 0.9568 |
| 313 | 0.167 | 500 | 0.9909 | −28.73 | 87.9 | 1.86 | 0.9536 |
| Temperature(K) | Qe-exp | Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|---|
| K1 (min−1) | Qe-cal (mg/g) | R2 | K2 (min−1) | Qe-cal (mg/g) | R2 | ||
| 293 | 196.8 | 0.0475 | 25.81 | 0.8607 | 0.003 | 200 | 0.9998 |
| 303 | 196.56 | 0.054 | 17.24 | 0.9049 | 0.0058 | 200 | 1.0000 |
| 313 | 195.81 | 0.071 | 22.63 | 0.9323 | 0.0047 | 200 | 0.9998 |
| Adsorbents | Maximum monolayer adsorption capacity (mg/g) | References |
|---|---|---|
| Garlic peel | 82.64 | [33] |
| Rice husk | 40.50 | [34] |
| Raw beech sawdust | 9.78 | [35] |
| Oil palm trunk fibre | 149.35 | [36] |
| Broad bean peels | 192.72 | [37] |
| Activated rice husks | 0.21 | [38] |
| Date pits | 80.31 | [39] |
| Jute processing waste | 22.47 | [40] |
| ASMs | 666.67 | Present study |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, Y.; Wei, X.; Sun, P.; Wan, J. Preparation, Characterization and Adsorption Performance of a Novel Anionic Starch Microsphere. Molecules 2010, 15, 2872-2885. https://doi.org/10.3390/molecules15042872
Yang Y, Wei X, Sun P, Wan J. Preparation, Characterization and Adsorption Performance of a Novel Anionic Starch Microsphere. Molecules. 2010; 15(4):2872-2885. https://doi.org/10.3390/molecules15042872
Chicago/Turabian StyleYang, Yati, Xiuzhi Wei, Peng Sun, and Juanmin Wan. 2010. "Preparation, Characterization and Adsorption Performance of a Novel Anionic Starch Microsphere" Molecules 15, no. 4: 2872-2885. https://doi.org/10.3390/molecules15042872