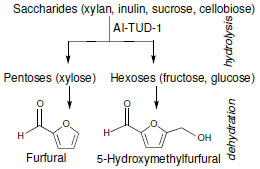
Acid-Catalysed Conversion of Saccharides into Furanic Aldehydes in the Presence of Three-Dimensional Mesoporous Al-TUD-1
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and characterisation of the catalyst




2.2. Catalysis
2.2.1. General considerations


2.2.2. Pentose-based carbohydrate feedstock
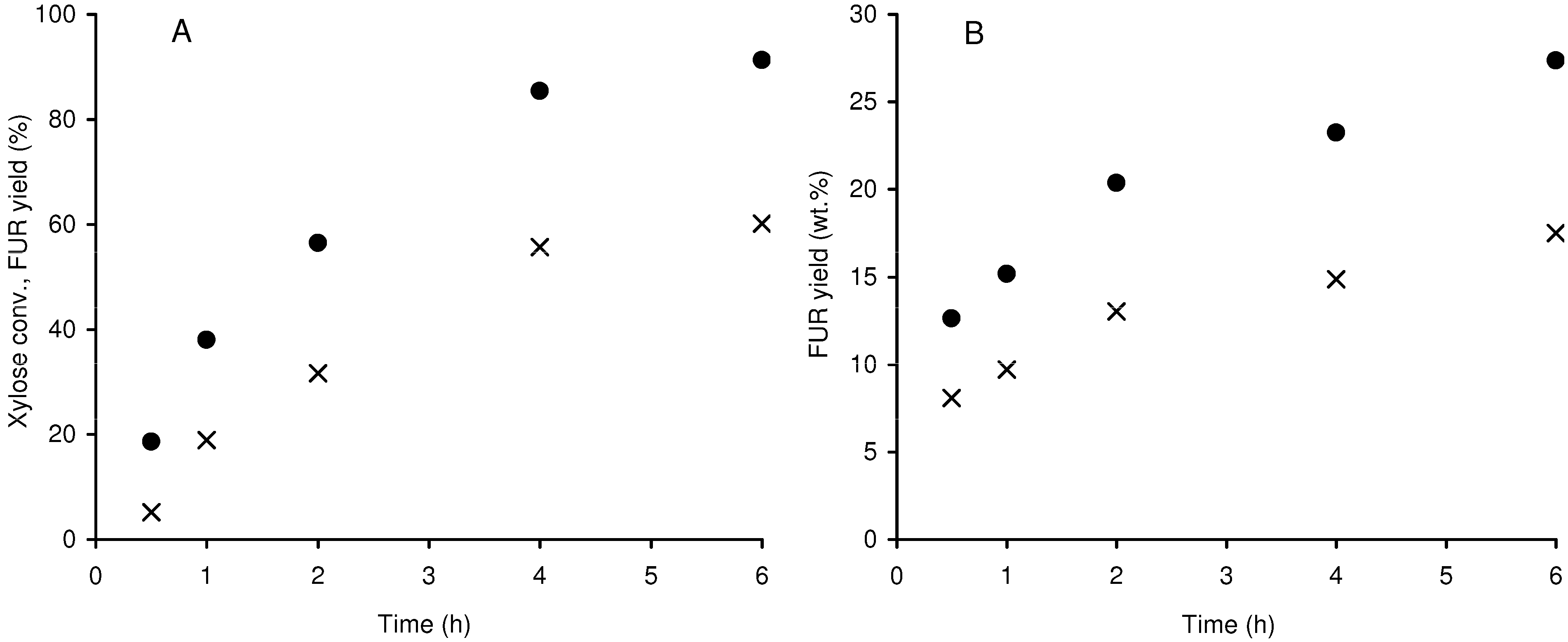
2.2.3. Hexose-based carbohydrate feedstock



3. Experimental
3.1. General
3.2. Synthesis of Al-TUD-1
3.3. Catalytic experiments
4. Conclusions
Acknowledgements
References and Notes
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef]
- Chheda, J.N.; Román-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Briens, C.; Piskorz, J.; Berruti, F. Biomass Valorization for Fuel and Chemicals Production - A Review. Int. J. Chem. Reactor Eng. 2008, 6, 1–49. [Google Scholar]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The chemistry and technology of furfural and its many by-products. In Sugar Series, 1st ed; Elsevier: Amsterdam, The Netherlands, 2000; Volume 13. [Google Scholar]
- Mamman, A.S.; Lee, J.-M.; Kim, Y.-C.; Hwang, I.T.; Park, N.-J.; Hwang, Y.K.; Chang, J.-S.; Hwang, J.-S. Furfural: Hemicellulose/xylose-derived biochemical. Biofuels, Bioprod. Bioref. 2008, 2, 438–454. [Google Scholar] [CrossRef]
- Lourvanij, K.; Rorrer, G.L. Reactions of aqueous glucose solutions over solid-acid Y-zeolite catalyst at 110–160 ºC. Ind. Eng. Chem. Res. 1993, 32, 11–19. [Google Scholar] [CrossRef]
- Moreau, C.; Durand, R.; Pourcheron, C.; Razigade, S. Preparation of 5-hydroxymethylfurfural from fructose and precursors over H-form zeolites. Ind. Crops Prod. 1994, 3, 85–90. [Google Scholar] [CrossRef]
- Moreau, C. Zeolites and related materials for the food and non food transformation of carbohydrates. Agro-Food Ind. Hi-Tech. 2002, 13, 17–26. [Google Scholar]
- Dias, A.S.; Lima, S.; Brandão, P.; Pillinger, M.; Rocha, J.; Valente, A.A. Liquid-phase dehydration of D-xylose over microporous and mesoporous niobium silicates. Catal. Lett. 2006, 108, 179–186. [Google Scholar] [CrossRef]
- Lima, S.; Fernandes, A.; Antunes, M.M.; Pillinger, M.; Ribeiro, F.; Valente, A.A. Dehydration of xylose into furfural in the presence of crystalline microporous silicoaluminophosphates. Catal. Lett. 2010, 135, 41–47. [Google Scholar] [CrossRef]
- Carlini, C.; Patrono, P.; Galletti, A.M.R.; Sbrana, G. Heterogeneous catalysts based on vanadyl phosphate for fructose dehydration to 5-hydroxymethyl-2-furaldehyde. Appl. Catal. A: General 2004, 275, 111–118. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, S.; Carriazo, D.; Rives, V.; Pillinger, M.; Valente, A.A. Exfoliated titanate, niobate and titanoniobate nanosheets as solid acid catalysts for the liquid-phase dehydration of D-xylose into furfural. J. Catal. 2006, 244, 230–237. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schüth, F. Design of solid catalysts for the conversion of biomass. Energy Environ. Sci. 2009, 2, 610–626. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, S.; Pillinger, M.; Valente, A.A. Furfural and Furfural-Based Industrial-Chemicals. In Ideas in Chemistry and Molecular Sciences: Advances in Synthetic Chemistry; Pignataro, B., Ed.; Wiley-VCH: Weinheim, Germany, 2010; pp. 167–186. [Google Scholar]
- Netrabukkana, R.; Lourvanij, K.; Rorrer, G.L. Diffusion of glucose and glucitol in microporous and mesoporous silicate aluminosilicate catalysts. Ind. Eng. Chem. Res. 1996, 35, 458–464. [Google Scholar]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Telalović, S.; Ramanathan, A.; Mul, G.; Hanefeld, U. TUD-1: synthesis and application of a versatile catalyst, carrier, material. J. Mater. Chem. 2010, 20, 642–658, and references cited therein. [Google Scholar] [CrossRef]
- Shan, Z.; Jansen, J.C.; Zhou, W.; Maschmeyer, Th. Al-TUD-1, stable mesoporous aluminas with high surface areas. Appl. Catal. A: General 2003, 254, 339–343. [Google Scholar] [CrossRef]
- Simons, C.; Hanefeld, U.; Arends, I.W.C.E.; Sheldon, R.A.; Maschmeyer, T. Noncovalent anchoring of asymmetric hydrogenation catalysts on a new mesoporous aluminosilicate: application and solvent effects. Chem. Eur. J. 2004, 10, 5829–5835. [Google Scholar] [CrossRef]
- Anand, R.; Maheswari, R.; Hanefeld, U. Catalytic properties of the novel mesoporous aluminosilicate AlTUD-1. J. Catal. 2006, 242, 82–91. [Google Scholar]
- Zhang, Z.-X.; Bai, P.; Xu, B.; Yan, Z.F. Synthesis of mesoporous alumina TUD-1 with high thermostability. J. Porous Mater. 2006, 13, 245–250. [Google Scholar] [CrossRef]
- Telalović, S.; Ng, J.F.; Maheswari, R.; Ramanathan, A.; Chuah, G.K.; Hanefeld, U. Synergy between Brønsted acid sites and Lewis acid sites. Chem. Commun. 2008, 4631–4633. [Google Scholar]
- Telalović, S.; Hanefeld, U. Noncovalent immobilization of chiral cyclopropanation catalysts on mesoporous TUD-1: Comparison of liquid-phase and gas-phase ion-exchange. Appl. Catal. A: Chem. 2010, 372, 217–223. [Google Scholar] [CrossRef]
- Jansen, J.C.; Shan, Z.; Marchese, L.; Zhou, W.; Puil, N.v.d.; Maschmeyer, Th. A new templating method for three-dimensional mesopore networks. Chem. Commun. 2001, 713–714. [Google Scholar]
- Sing, K.S.W. Characterization of adsorbents. In Adsorption, Science and Technology; Rodrigues, A.E., LeVan, M.D., Tondeur, D., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 3–14. [Google Scholar]
- Sangwichien, C.; Aranovich, G.L.; Donohue, M.D. Density functional theory predictions of adsorption isotherms with hysteresis loops. Colloids Surf., A 2002, 206, 313–320. [Google Scholar] [CrossRef]
- Blin, J.L.; Léonard, A.; Su, B.L. Well-ordered spherical mesoporous materials CMI-1 synthesized via an assembly of decaoxyethylene cetyl ether and TMOS. Chem. Mater. 2001, 13, 3542–3553. [Google Scholar] [CrossRef]
- Shan, Z.; Jansen, J.C.; Marchese, L.; Maschmeyer, Th. Synthesis, characterization and catalytic testing of a 3-D mesoporous titanosilica, Ti–TUD-1. Microporous Mesoporous Mater. 2001, 48, 181–187. [Google Scholar] [CrossRef]
- Zhou, J.; Hua, Z.; Shi, J.; He, Q.; Guo, L.; Ruan, M. Synthesis of a hierarchical micro/mesoporous structure by steam-assisted post-crystallization. Chem. Eur. J. 2009, 15, 12949–12954. [Google Scholar] [CrossRef]
- Webster, C.E.; Drago, R.S.; Zerner, M.C. Molecular dimensions for adsorptives. J. Am. Chem. Soc. 1998, 120, 5509–5516. [Google Scholar] [CrossRef]
- Luan, Z.; Fournier, J.A. In situ FTIR spectroscopic investigation of active sites and adsorbate interactions in mesoporous aluminosilicate SBA-15 molecular sieves. Microporous Mesoporous Mater. 2005, 79, 235–240. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Bisio, C.; Gatti, G.; Marchese, L.; Pastore, H.O. Physicochemical characterization and surface acid properties of mesoporous [Al]-SBA-15 obtained by direct synthesis. Langmuir 2010, 26, 5791–5800. [Google Scholar]
- Van-Bekkum, H.E.; Kieboom, A.P.G.; Van-Bekkum, H. The conversion of fructose and glucose in acid media. Formation of hydroxymethylfurfural. Starch/Stärke 1986, 95–101, and references therein. [Google Scholar]
- Moreau, C.; Durand, R.; Peyron, D.; Duhamet, J.; Rivalier, P. Selective preparation of furfural from xylose over microporous solid acid catalysts. Ind. Crops Prod. 1998, 7, 95–99. [Google Scholar] [CrossRef]
- Lichenthaler, F.W. The key sugars of biomass: Availability, non-food uses and future development lines. In Biorefineries - Industrial Processes and Products: Status Quo and Future Directions; Kamm, B., Grober, P.R., Kamm, M., Eds.; Wiley-VCH: New York, NY, USA, 2006; Volume 2, pp. 3–59. [Google Scholar]
- Moreau, C.; Belgacem, M.N.; Gandini, A. Recent catalytic advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Top. Catal. 2004, 27, 11–30. [Google Scholar]
- Lima, S.; Pillinger, M.; Valente, A.A. Dehydration of d-xylose into furfural catalysed by solid acids derived from the layered zeolite Nu-6(1). Catal. Commun. 2008, 9, 2144–2148. [Google Scholar] [CrossRef]
- Antal, M.J.; Leesomboon, T.; Mok, W.S.; Richards, G.N. Mechanism of formation of 2-furaldehyde from D-xylose. Carbohydr. Res. 1991, 217, 71–85. [Google Scholar] [CrossRef]
- Asghari, F.S.; Yoshida, H. Acid-Catalyzed Production of 5-hydroxymethylfurfural from D-fructose in subcritical water. Ind. Eng. Chem. Res. 2006, 45, 2163–2173. [Google Scholar] [CrossRef]
- Antal, M.J.; Mok, W.S.L. Kinetic studies of the reactions of ketoses and aldoses in water at high temperature. 1. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from D-fructose and sucrose. Carbohydr. Res. 1990, 199, 91–109. [Google Scholar] [CrossRef]
- Aida, T.M.; Sato, Y.; Watanabe, M.; Tajima, K.; Nonaka, T.; Hattori, H.; Arai, K. Dehydration of D-glucose in high temperature water at pressures up to 80 MPa. J. Supercrit. Fluids 1997, 40, 381–388. [Google Scholar]
- Kuster, F.M.; Temmink, H.M.G. The influence of pH and weak acid anions on the dehydration of D-fructose. Carbohydr. Res. 1977, 54, 185–191. [Google Scholar] [CrossRef]
- Moreau, C.; Durand, R.; Razigade, S.; Duhamet, J.; Faugeras, P.; Rivalier, P.; Ross, P.; Avignon, G. Dehydration of fructose to 5-hydroxymethylfurfural over H-Mordenites. Appl. Catal. A: General 1996, 145, 211–224. [Google Scholar] [CrossRef]
- Moreau, C.; Durand, R.; Duhamet, J.; Rivalier, P. Hydrolysis of fructose and glucose precursors in the presence of H-form zeolites. J. Carbohydr. Chem. 1997, 16, 709–714. [Google Scholar] [CrossRef]
- Moreau, C.; Durand, R.; Aliès, F.; Cotillon, M.; Frutz, T.; Théoleyre, M.-A. Hydrolysis of sucrose in the presence of H-form zeolites. Ind. Crops Prod. 2000, 11, 237–242. [Google Scholar] [CrossRef]
- Buttersack, C.; Laketic, D. Hydrolysis of sucrose by dealuminated Y-zeolites. J. Mol. Catal. 1994, 94, L283–L290. [Google Scholar] [CrossRef]
- Campelo, J.M.; Lafont, F.; Marinas, J.M. Pt/SAPO-5 and Pt/SAPO-11 as Catalysts for the Hydroisomerization and Hydrocracking of n-Octane. J. Chem. Soc. Faraday Trans. 1995, 91, 1551–1555. [Google Scholar] [CrossRef]
- Anand, R.; Maheswari, R.; Hanefeld, U. Catalytic properties of the novel mesoporous aluminosilicate AlTUD-1. J. Catal. 2006, 242, 82–91. [Google Scholar]
- Sample Availability: Small quantities of the catalyst Al-TUD-1 are available from the authors on request.
© 2010 by the authors;
Share and Cite
Lima, S.; Antunes, M.M.; Fernandes, A.; Pillinger, M.; Ribeiro, M.F.; Valente, A.A. Acid-Catalysed Conversion of Saccharides into Furanic Aldehydes in the Presence of Three-Dimensional Mesoporous Al-TUD-1. Molecules 2010, 15, 3863-3877. https://doi.org/10.3390/molecules15063863
Lima S, Antunes MM, Fernandes A, Pillinger M, Ribeiro MF, Valente AA. Acid-Catalysed Conversion of Saccharides into Furanic Aldehydes in the Presence of Three-Dimensional Mesoporous Al-TUD-1. Molecules. 2010; 15(6):3863-3877. https://doi.org/10.3390/molecules15063863
Chicago/Turabian StyleLima, Sérgio, Margarida M. Antunes, Auguste Fernandes, Martyn Pillinger, Maria Filipa Ribeiro, and Anabela A. Valente. 2010. "Acid-Catalysed Conversion of Saccharides into Furanic Aldehydes in the Presence of Three-Dimensional Mesoporous Al-TUD-1" Molecules 15, no. 6: 3863-3877. https://doi.org/10.3390/molecules15063863